Abstract
Despite the excellent success rates of modern implants, unicompartmental knee arthroplasty (UKA) continues to show relatively high failure and revision rates, especially when compared with total knee arthroplasty (TKA).
These higher rates of failure and revision are mainly observed during the early (< 5 years) post-operative period and are often due to incorrect indications and/or surgical errors.
The correct clinical and radiological indications for UKA have therefore been analysed and correlated as far as possible with the principal mechanisms and timing of failures of UKA.
Cite this article: EFORT Open Rev 2018;3:442-448. DOI: 10.1302/2058-5241.3.170060
Keywords: unicompartmental knee arthroplasty, polyethylene, loosening, bearing dislocation, progression of osteoarthritis, unexplained pain, early failure, revision
Introduction
Unicompartmental knee arthroplasty (UKA) is a worldwide-recognized procedure for the treatment of unicompartment femoro-tibial degeneration. Over the last decade, the advent of the concept of minimally invasive surgery, together with the development and refinement of surgical techniques and implant design, has led to a favourable evolution of clinical results and, consequently, renewed interest in UKA. In selected patients, UKA has been shown to be a satisfactory and less invasive alternative to total knee arthroplasty (TKA) for specific indications.1 Several reports have recently demonstrated survival rates > 90% at 10 years or even 93% at 15 years and 90% at 20 years after UKA.2-4
Despite this, results of knee replacement registries still show a relatively high revision and failure rate of UKA, especially if compared with TKA.5 In particular, many of these failures occur in the early post-operative period (< 5 years).6 The higher incidence of failure in unicompartmental knee replacement has been often associated with indications that are not strictly correct, and with surgical technical errors when performing UKA, therefore leading to early revision.7 Furthermore, the relative simplicity of converting a UKA to a TKA seems to lower the threshold for revision of a UKA in comparison with TKA, even if there is no obvious technical failure or incorrect indication present. Concerns still exist about age, activity level, body mass index (BMI), presence of chondrocalcinosis and anterior knee pain as possible indications or contraindications for UKA.8 Similarly, the radiological criteria for determining the suitability for UKA implantation are still debated.9
All these arguments explain why, unlike TKA, complications following UKA have distinctive characteristics. The causes of UKA failures can be different depending on the type and design of implant (mobile versus fixed), timing of failure (early or late) and the surgeon who performed the surgery.10
With the clear potential advantages of UKA, critical evaluation of the correct clinical and radiological indications and corresponding causes of UKA failure is necessary. The purpose of this review was therefore to analyse the literature to elucidate the correct clinical indications and the precise radiological criteria, and the main causes of UKA failure.
Indications for UKA
Current indications for UKA implantation are: unicondylar osteoarthritis (OA) or osteonecrosis; frontal deformity < 15°; flexion contracture < 15°; functional integrity of the anterior cruciate ligament (ACL) and peripheral ligaments of the knee as well as the absence of an inflammatory arthropathy.11 UKA implantation for anteromedial OA requires medial bone-on-bone arthritis, a functionally normal ACL, a functionally normal medial collateral ligament, intact full-thickness lateral cartilage and a patellofemoral joint (PFJ) with no lateral grooving and bone loss.10
ACL integrity
The role of the ACL functional integrity has been strongly debated among UKA surgeons, as ACL insufficiency has been often associated with implant subluxation, polyethylene (PE) wear and early failure after UKA.12 However, two different patterns of ACL insufficiency should be considered when approaching a patient with unicompartmental tibio-femoral degeneration. They are patients with primary ACL deficiency (usually traumatic ACL rupture) or those with secondary ACL deficiency (usually degenerative ACL rupture), as both groups can develop a posteromedial OA. The first group typically presents with symptoms of instability and pain and tends to be young and active. The second group shows symptoms of pain and joint swelling but not instability, which is associated to secondary ACL deficiency.13 In this regard, patients with primary traumatic ACL deficiency and secondary posteromedial OA should be not considered candidates for UKA because of the increased risk of higher failure rates from wear and tibial loosening due to the knee instability.13,14 On the contrary, patients with primary anteromedial OA and secondary degenerative ACL deficiency are nowadays accepted as possible candidates for UKA, as compensatory mechanisms of the knee prevent instability symptoms in most cases, and recent papers have begun to confirm good short- to mid-term outcome without increased risk of prosthesis failure.15,16 A special subgroup of patients is the young individuals with a rotatory ACL instability in combination with an anteromedial OA. Here, combined UKA with ACL reconstruction should be considered.17
Deformity
In UKA, the final limb alignment is determined by the thickness of the implant relative to the bone excised. The aim of UKA is and should be to restore knee kinematics by restoring ligament tension to normal and not the correction of the axis.18 Many patients have varus alignment before they develop medial OA because of constitutional tibia vara (extra-articular deformity), therefore resulting in varus alignment post-operatively.19 Therefore, it might be thought that patients with pre-operative frontal deformity (or flexion contracture) > 15° are not ideal candidates for UKA since the residual post-operative axis will exceed 8° to 10°, leading to possible failure due to polyethylene wear and implant loosening.20-22
Age
Activity level, age, BMI, anterior knee pain and a previous history of high tibial osteotomy are no longer considered as factors for or against UKA in themselves, although they can influence its mid- to long-term final survivorship.6,8 The classic criteria defining the ideal and non-ideal patient for UKA, as suggested by Kozinn and Scott,23 are widely used as indications and contraindications for UKA but are clearly ungrounded. Only 5% of knees would be regarded as suitable for UKA if those criteria were strictly adhered to.24,25 In particular, the criterion that only patients aged > 65 years should be considered for UKA is misleading as, on the contrary, younger patients around the age of 50 years perform better and are considered ideal candidates for UKA.11 Furthermore, there are reports that patient satisfaction was significantly low among patients aged < 55 years who had undergone TKA,26 and Parvizi reported that residual symptoms and functional deficits were more prevalent in younger patients after TKA.27 While high morbidity and mortality could be associated with TKA in elderly patients, UKA may provide lower morbidity, faster recovery and a more physiological return to function in these patients, with reduced complications and excellent survivorship.28
BMI
The influence of BMI on outcome of UKA is controversial. An increasing number of studies, however, show that BMI does not have a negative correlation with the results after UKA.29,30 In the absence of severe osteoporosis, a high BMI should not be considered a contraindication to UKA. Obesity should be considered a relative, if not absolute, contraindication to the implantation of a UKA. In a previous study from 2007 by Berend et al, it was concluded that a BMI > 32 kg/m2 significantly increased failure rates of UKA.31 In patients with BMI ⩾ 35 kg/m2, Bonutti et al32 found, in 2011, higher UKA failure rates (12.5%) whereas Knee Society scores were lower in the surviving high-BMI knees. In a retrospective study on 471 failed medial UKAs, Citak et al33 found that approximately 60% of the failed UKA patients had a BMI < 30 kg/m2, whereas < 40% were obese. Additionally, BMI showed no significant relationship to time of revision, i.e. within five years and after ten years following primary implantation. Certainly, other papers showing no adverse correlation between high BMI and clinical outcome and final survivorship of UKA have also been reported in the literature, but most of these studies had short follow-up.34
Activity level
The activity level of younger patients is in general higher than in elderly ones; wear could represent one of the major concerns in these patients.35 However, most of the studies have failed to demonstrate a significantly negative correlation between high activity level and/or younger age and final outcome of UKA.11,20,36
Chondrocalcinosis
Chondrocalcinosis is considered to be a contraindication for UKA as it is thought to be associated with more rapid progression of contralateral OA. In several studies this was not shown, as no statistical significant differences in the clinical or functional outcome, failure or survival rate between patients with chondrocalcinosis and others could be shown.37
Anterior knee pain
Nowadays, it is universally accepted that anterior knee pain and moderate degenerative changes of PFJ should no longer be considered absolute contraindications to UKA implantation, as centring of the PFJ after UKA may even decrease stress in the PFJ. Most of the recent reports38-41 clearly demonstrate that neither anterior knee pain nor radiologically demonstrated medial PFJ degeneration significantly affect clinical outcome and survivorship of UKA (both fixed and mobile). However, management of severe arthritis of the lateral facet of the patella remains controversial and UKA is not usually considered an appropriate choice of surgery in this setting. Less severe damage to the lateral side of the PFJ and damage to the medial side, however severe, do not compromise the overall function or survival, so should not be considered to be contraindications.39 Pre-operative anterior knee pain also does not compromise the functional outcome or survival and should not be considered a contraindication to UKA.39
Radiological suitability for UKA implantation
Pre-operative radiographs, including stress views, are necessary to confirm suitability for UKA implantation.9 On the other hand, the role of pre-operative MRI is still debated, since MRI interpretations can over-estimate the degree of knee pathology.42
A complete radiograph study including weight-bearing long-leg anteroposterior (AP) view radiographs as well as Rosenberg, Merchant and lateral projections are performed to determine the appropriateness of UKA. Weight-bearing long-leg radiographs are used to calculate the pre-operative femoro-tibial mechanical alignment and the proximal tibial epiphyseal axis in both the affected and healthy sides. In this manner, the surgeon can know in advance the real correctability of the pre-operative deformity and predict approximately the post-operative residual varus (or valgus) resulting from the UKA implantation.20
The Rosenberg view helps to confirm most precisely the total-thickness cartilage loss of the affected femoro-tibial compartment which is important in confirming bone-on-bone arthritis, regarded as a mandatory condition for implantation of a UKA. It has been reported how patients with partial-thickness cartilage loss can do badly with UKA and have worse outcomes compared with those with bone-on-bone anteromedial OA, resulting in higher revision rates.43,44 Optimal outcome can therefore be achieved for patients with full-thickness cartilage loss on both the femur and tibia.44 Although some patients with partial-thickness loss achieve a good result, orthopaedic surgeons cannot currently always identify pre-operatively which knees these will be; in this situation, MRI findings can mislead the surgeon with regard to his decision to perform UKA. The Rosenberg view is also very useful to determine the real amount of cartilage loss of the contralateral (unaffected) side of the knee. The presence of osteophytes alone on the unaffected side should be not considered a contraindication to UKA.45 Lateral knee projection, performed with perfect overlapping of the femoral condyles, is primarily used to analyse the state of the PFJ and to detect the presence of a trochlear dysplasia that could imply an incipient PFJ arthritis. On the other hand, in patients with primitive traumatic ACL rupture and instability, a lateral view of the knee can reveal posteromedial OA of the tibiofemoral joint due to anterior translation of the tibia in relation to the femur. Merchant axial view of the patella provides information as to the state of the PFJ, which is important because severe damage to the lateral side of the PFJ with bone loss and grooving might represent a contraindication to UKA. Less severe damage to the lateral side of the PFJ and damage to the medial side do not compromise the overall function or survival, so should not be considered contraindications.38-40
Radiographic valgus and varus stress views have been advocated to evaluate, respectively, the actual status of the lateral and of the medial femoro-tibial compartments9 in order to determine the potential of progression of OA after UKA implantation. The relative lack of lateral compartment disease progression as a mode of failure was particularly notable in the series of 1000 consecutive medial UKAs reported by Bergeson et al.6 However, possibly due in part to the early follow-up interval, the authors highlighted the utility of the valgus-stress radiograph, obtained for all patients in that study. Therefore, they strongly suggested the use of the valgus-stress radiograph, in the pre-operative evaluation, to ensure full-thickness preservation of the unaffected compartment.
MRI has not been traditionally recommended as the primary means of determining candidacy for UKA as many of these MRI interpretations can over-estimate the degree of knee pathology or ACL deficiency and can therefore falsely contraindicate a UKA.42,46 However, when the clinical presentation is not clear, an MRI can be very useful in assessing other conditions such as avascular necrosis and neoplasm which otherwise might have gone undetected.47
Failure modes
Described failure modes of UKAs are related to the chosen implants, e.g. mobile or fixed, progression of the disease or loosening being the most common. Less frequent failures are infection, peri-prosthetic fractures, persistent pain or ankylosis. Between these two groups, there is a major difference: in one, failures are due to technical problems with the implantation, whereas in the other, failure seems to be independent of surgeon or technique.48
Mobile-bearing UKA
The overall re-operation rate and incidence of failures are similar for both mobile and fixed UKAs. However, bearing dislocation is still reported as the predominant mechanism of failure in mobile-bearing UKAs.49
Mechanical loosening, lateral OA and unexplained pain are other mechanisms of failure, with revision for patellofemoral problems and PE wear exceedingly rare.6,50,51
Most failures from bearing dislocation can be caused by surgical errors: component malalignment; PE impingement with adjacent bone or prosthetic components; unbalanced flexion-extension gap; instability due to medial collateral ligament injury or secondary to femoral and/or tibial component loosening.48 Dislocations are particularly common in lateral mobile UKA as the lateral collateral ligament is slack in flexion, in contrast to the medial side in which the MCL is tight.52
Progression of disease and aseptic loosening are the second and third most common reasons for failure after UKA.49-51,53,54 One of the problems lies in overcorrection after UKA. Particularly with the use of mobile bearings, it has been theorized that a surgeon may tend to choose a tight knee and risk slight valgus overcorrection to avoid bearing dislocation in mobile UKA. This results in greater contact stresses and over-loading of the lateral compartment, accelerating the progression of OA (see Figs 1 and 2).55 This happens regardless of the implant and should therefore be avoided. While overcorrection of the pre-degeneration deformity may cause degenerative changes in the contralateral compartment,20 degeneration of PFJ may occur in the presence of an oversized femoral component with possible impingement with patellar cartilage.56
Fig. 1.
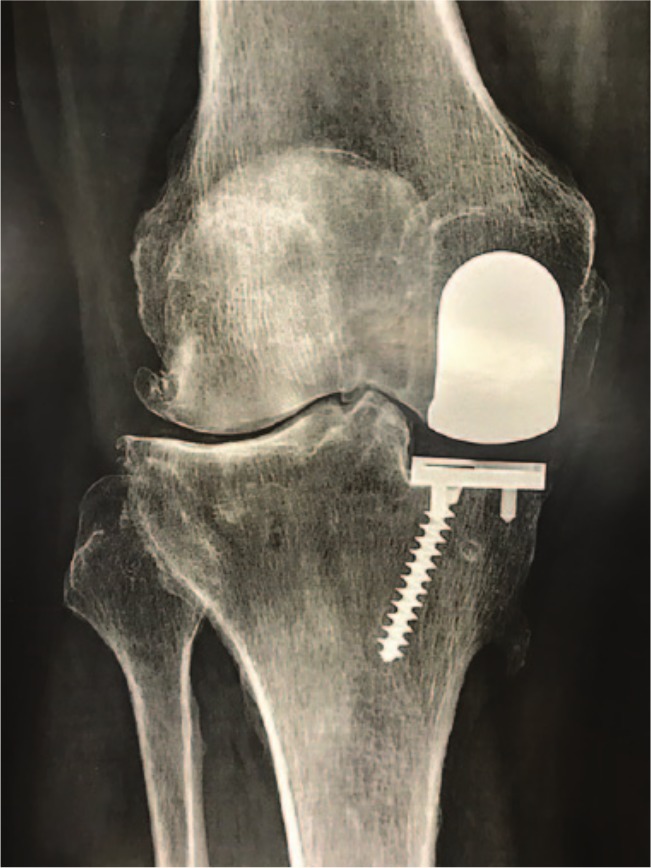
An example showing that the tibial component has not been implanted in the optimal varus position.
Fig. 2.
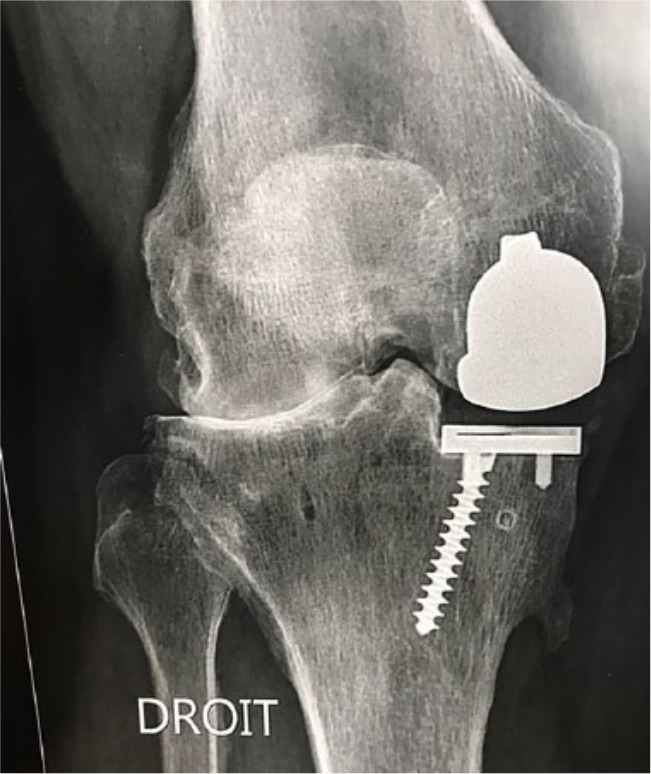
At three years after surgery, rapid progression of disease on the contralateral compartment is present.
A crucial factor for implant survival which should be taken into consideration is the fact that, in contrast to TKA, the coronal (varus/valgus) contact angle of femoral to tibial component is defined by how the components are surgically implanted and therefore can be highly variable in UKA.57 Diezi showed that there can be a relevant relative malpositioning of the femoral and tibial component independent of the general limb axis.57 His numbers showed that an unfavourable angle of implantation could produce a situation where the round smooth centre of the femoral component was disturbed so that the contact area may be reduced by > 70%, and this could potentially increase the local stress by a factor of 3 to 4.57 This is a key factor for implant survival which is often neglected or underestimated, especially if only the general limb axis is taken into account.
Furthermore, in a series of 559 medial UKAs Chatellard identified a number of factors that were associated with decreased prosthesis survival rate.58 In particular, factors that were associated with a decreased prosthesis survival rate were: a joint space height > 2 mm; a change in tibial component obliquity > 3°; a slope value > 5° or a change in slope > 2°; and > 6° divergence between the tibial and femoral components.58
Progression of OA in the un-replaced compartments could be also enhanced by incorrect indications, i.e. pre-operative presence of bi- or tri-compartmental OA, or systemic inflammatory diseases such as rheumatoid or psoriatic arthritis. While overcorrection of the pre-degeneration deformity may cause degenerative changes in the contralateral compartment,20 degeneration of the PFJ may occur in the presence of an oversized femoral component with possible impingement with patellar cartilage.56
Failure of fixed UKA
Aseptic mechanical loosening and PE wear remain the main causes of failure reported in fixed UKA, although the incidence of these has significantly reduced with the introduction of new implants and designs.51,56 At the same time, careful patient selection and newer instrumentation has significantly reduced the incidence of mechanical loosening and progression of OA in contemporary UKA, leaving PE wear as the predominant mechanism failure of fixed implants in some reports.11 Mechanical loosening following UKA could be determined by: component malalignment; under-correction of the pre-degeneration deformity; ACL deficiency; and excessive tibial slope. Tibial component loosening is particularly observed in all-poly UKAs.59 PE wear is a complication inherent in the design of fixed bearings, secondary to higher surface deformation and delamination in comparison with mobile bearings.60 Fixed-bearing UKA results in greater contact-stress on the PE insert (when compared with mobile designs) due to low conformity, which may eventually lead to wear and peri-prosthetic osteolysis with potential failure associated with component subsidence and/or loosening.51 However, as mentioned above, most studies suggest that higher wear rates are constantly seen in association with fixed-bearing UKA, in spite of advancements in PE processing and implant design.49 Furthermore, it is worth mentioning the research being done in improving PE biomechanics, and vitamin E stabilized PE is recognized as a potential technology offering advantages in terms of fatigue strength and improvement and reduction of wear debris.61
Several recent studies have suggested that PE wear, progressive OA loosening or subsidence may now be the most common reasons for failure.7,49,54,59
Limitation of failures
UKAs mainly fail due to bearing dislocation, loosening and progression of disease. It could be demonstrated that the surgeon performing this intervention might be the main factor for the failure. From the National Joint Registry for England and Wales, it is known that very low-volume surgeons obtain the worst results whereas high-volume surgeons were found to have similar revision rates to TKA.62 By considering these conditions, the Registry suggests that knee surgeons should consider modifying their practice in terms of patient selection by adopting an extended indication spectrum that would increase their caseload and potentially significantly improve their results. On the other hand, very low-volume surgeons, who cannot substantially increase their patient numbers even by adopting more indications, should consider referring their patients to a high-volume clinic.62
The question arises as to why high-volume surgeons have fewer revisions compared with others. A potential explanation might be that those have a better component orientation and superior surgical technique which is crucial for the survival of the UKA. Therefore, in addition to correct patient selection, optimal implant positioning might be crucial to reduce the potential for surgical errors. With the use of computer navigation (CAS), it has been shown that the position of the implant can be improved.63 A study using patient-specific instrumentation (PSI) showed even better results compared with CAS. In their work, the margin of error was < 1° in slope and varus/valgus inclination.18 Additionally, in that series, no overcorrection occurred. It therefore seems that the use of CAS or PSI has the potential to further improve component orientation and thus reduce failures.64
Limitations of the study
The greatest limitation of this paper is that it was not a full meta-analysis but a pooled analysis of collected study-series. Therefore, sampling bias, confounders affected by unmeasured variables, and selection bias from the literature search with certain inclusion and exclusion criteria may have influenced our analysis, producing limited generalized applicability. However, the focus of this research was not on the statistical comparisons but on the overall understating of the correct clinical and radiological indications for UKA and the overall aspect of failures following UKA. This paper may, however, be of clinical relevance for clinicians considering unicompartmental knee replacement surgery since all the currently accepted indications for UKA are largely explained as well as all the possible complications (for mobile and fixed implants) and failure mechanisms (both early and late) that may result when the clinician fails to follow them closely.
Conclusions
UKA is an excellent treatment option for patients with unicondylar knee degeneration with optimal survivorship rates of up to 20 years. Most failures after UKA occur in the first five years after the index surgery. The higher incidence of failure and revision rate in UKA, especially in the early period, has been often attributed to incorrect clinical and/or radiological indications in combination with surgical errors when performing a UKA. An ultimate goal in UKA surgery, therefore, is to reduce surgical error by performing a certain number of cases per year and, ideally, to combine it with technology such as CAS or PSI to reduce malpositioning of the implants.
Footnotes
ICMJE Conflict of interest statement: M. Vasso declares a grant from the EFORT Foundation Visiting Fellowship, activity relating to the submitted work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Siman H, Kamath AF, Carrillo N, et al. Unicompartmental knee arthroplasty vs total knee arthroplasty for medial compartment arthritis in patients older than 75 years: comparable reoperation, revision, and complication rates. J Arthroplasty 2017;32(6):1792-1797. [DOI] [PubMed] [Google Scholar]
- 2. Koshino T, Sato K, Umemoto Y, et al. Clinical results of unicompartmental arthroplasty for knee osteoarthritis using a tibial component with screw fixation. Int Orthop 2015;39(6):1085-1091. [DOI] [PubMed] [Google Scholar]
- 3. Vasso M, Del Regno C, Perisano C, et al. Unicompartmental knee arthroplasty is effective: ten year results. Int Orthop 2015;39(12):2341-2346. [DOI] [PubMed] [Google Scholar]
- 4. Yoshida K, Tada M, Yoshida H, et al. Oxford phase 3 unicompartmental knee arthroplasty in Japan—clinical results in greater than one thousand cases over ten years. J Arthroplasty 2013;28(9)(suppl):168-171. [DOI] [PubMed] [Google Scholar]
- 5. Niinimäki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee 2011;18(6):432-435. [DOI] [PubMed] [Google Scholar]
- 6. Bergeson AG, Berend KR, Lombardi AV, Jr, et al. Medial mobile bearing unicompartmental knee arthroplasty: early survivorship and analysis of failures in 1000 consecutive cases. J Arthroplasty 2013;28(9)(suppl):172-175. [DOI] [PubMed] [Google Scholar]
- 7. Epinette JA, Brunschweiler B, Mertl P, Mole D, Cazenave A; French Society for Hip and Knee. Unicompartmental knee arthroplasty modes of failure: wear is not the main reason for failure: a multicentre study of 418 failed knees. Orthop Traumatol Surg Res 2012;98(6)(suppl):S124-S130. [DOI] [PubMed] [Google Scholar]
- 8. Emerson RH, Jr, Higgins LL. Unicompartmental knee arthroplasty with the oxford prosthesis in patients with medial compartment arthritis. J Bone Joint Surg [Am] 2008;90-A(1):118-122. [DOI] [PubMed] [Google Scholar]
- 9. Hamilton TW, Pandit HG, Lombardi AV, et al. Radiological decision aid to determine suitability for medial unicompartmental knee arthroplasty: development and preliminary validation. Bone Joint J 2016;98-B(10)(suppl B):3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamilton TW, Rizkalla JM, Kontochristos L, et al. The interaction of caseload and usage in determining outcomes of unicompartmental knee arthroplasty: a meta-analysis. J Arthroplasty 2017;32(10): 3228-3237.e2. [DOI] [PubMed] [Google Scholar]
- 11. Panni AS, Vasso M, Cerciello S, Felici A. Unicompartmental knee replacement provides early clinical and functional improvement stabilizing over time. Knee Surg Sports Traumatol Arthrosc 2012;20:579-585. [DOI] [PubMed] [Google Scholar]
- 12. Tinius M, Hepp P, Becker R. Combined unicompartmental knee arthroplasty and anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 2012;20(1):81-87. [DOI] [PubMed] [Google Scholar]
- 13. Mancuso F, Dodd CA, Murray DW, Pandit H. Medial unicompartmental knee arthroplasty in the ACL-deficient knee. J Orthop Traumatol 2016;17(3):267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tian S, Wang B, Wang Y, et al. Combined unicompartmental knee arthroplasty and anterior cruciate ligament reconstruction in knees with osteoarthritis and deficient anterior cruciate ligament. BMC Musculoskelet Disord 2016;17(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boissonneault A, Pandit H, Pegg E, et al. No difference in survivorship after unicompartmental knee arthroplasty with or without an intact anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc 2013;21(11):2480-2486. [DOI] [PubMed] [Google Scholar]
- 16. Engh GA, Ammeen DJ. Unicondylar arthroplasty in knees with deficient anterior cruciate ligaments. Clin Orthop Relat Res 2014;472(1):73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weston-Simons JS, Pandit H, Jenkins C, et al. Outcome of combined unicompartmental knee replacement and combined or sequential anterior cruciate ligament reconstruction: a study of 52 cases with mean follow-up of five years. J Bone Joint Surg [Br] 2012;94-B(9):1216-1220. [DOI] [PubMed] [Google Scholar]
- 18. Dao Trong ML, Diezi C, Goerres G, Helmy N. Improved positioning of the tibial component in unicompartmental knee arthroplasty with patient-specific cutting blocks. Knee Surg Sports Traumatol Arthrosc 2015; 23(7):1993-1998. [DOI] [PubMed] [Google Scholar]
- 19. Levine B, Rosenberg AG. The simple unicondylar knee: extramedullary technique. Clin Sports Med 2014;33(1):77-85. [DOI] [PubMed] [Google Scholar]
- 20. Vasso M, Del Regno C, D’Amelio A, et al. Minor varus alignment provides better results than neutral alignment in medial UKA. Knee 2015;22(2):117-121. [DOI] [PubMed] [Google Scholar]
- 21. Deschamps G, Chol C. Fixed-bearing unicompartmental knee arthroplasty. Patients’ selection and operative technique. Orthop Traumatol Surg Res 2011;97(6):648-661. [DOI] [PubMed] [Google Scholar]
- 22. Valenzuela GA, Jacobson NA, Geist DJ, Valenzuela RG, Teitge RA. Implant and limb alignment outcomes for conventional and navigated unicompartmental knee arthroplasty. J Arthroplasty 2013;28(3):463-468. [DOI] [PubMed] [Google Scholar]
- 23. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg [Am] 1989;71-A(1):145-150. [PubMed] [Google Scholar]
- 24. Stern SH, Becker MW, Insall JN. Unicondylar knee arthroplasty. An evaluation of selection criteria. Clin Orthop Relat Res 1993;(286):143-148. [PubMed] [Google Scholar]
- 25. Ritter MA, Faris PM, Thong AE, et al. Intra-operative findings in varus osteoarthritis of the knee. An analysis of pre-operative alignment in potential candidates for unicompartmental arthroplasty. J Bone Joint Surg [Br] 2004;86-B(1):43-47. [PubMed] [Google Scholar]
- 26. Williams DP, Price AJ, Beard DJ, et al. The effects of age on patient-reported outcome measures in total knee replacements. Bone Joint J 2013;95-B(1):38-44. [DOI] [PubMed] [Google Scholar]
- 27. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res 2014;472(1):133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iacono F, Raspugli GF, Akkawi I, et al. Unicompartmental knee arthroplasty in patients over 75 years: a definitive solution? Arch Orthop Trauma Surg 2016;136(1):117-123. [DOI] [PubMed] [Google Scholar]
- 29. Murray DW, Pandit H, Weston-Simons JS, et al. Does body mass index affect the outcome of unicompartmental knee replacement? Knee 2013;20(6):461-465. [DOI] [PubMed] [Google Scholar]
- 30. Thompson SA, Liabaud B, Nellans KW, Geller JA. Factors associated with poor outcomes following unicompartmental knee arthroplasty: redefining the “classic” indications for surgery. J Arthroplasty 2013;28(9):1561-1564. [DOI] [PubMed] [Google Scholar]
- 31. Berend KR, Lombardi AV., Jr Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int 2007;16:193-197. [PubMed] [Google Scholar]
- 32. Bonutti PM, Goddard MS, Zywiel MG, et al. Outcomes of unicompartmental knee arthroplasty stratified by body mass index. J Arthroplasty 2011;26(8):1149-1153. [DOI] [PubMed] [Google Scholar]
- 33. Citak M, Dersch K, Kamath AF, et al. Common causes of failed unicompartmental knee arthroplasty: a single-centre analysis of four hundred and seventy one cases. Int Orthop 2014;38(5):961-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Woo YL, Chen YQ, Lai MC, et al. Does obesity influence early outcome of fixed-bearing unicompartmental knee arthroplasty? J Orthop Surg (Hong Kong) 2017;25(1):2309499016684297. [DOI] [PubMed] [Google Scholar]
- 35. Collier MB, Engh CA, Jr, McAuley JP, Engh GA. Factors associated with the loss of thickness of polyethylene tibial bearings after knee arthroplasty. J Bone Joint Surg [Am] 2007;89-A(6):1306-1314. [DOI] [PubMed] [Google Scholar]
- 36. Ali AM, Pandit H, Liddle AD, et al. Does activity affect the outcome of the Oxford unicompartmental knee replacement? Knee 2016;23(2):327-330. [DOI] [PubMed] [Google Scholar]
- 37. Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res 2004;423:161-165. [DOI] [PubMed] [Google Scholar]
- 38. Adams AJ, Kazarian GS, Lonner JH. Preoperative patellofemoral chondromalacia is not a contraindication for fixed-bearing medial unicompartmental knee arthroplasty. J Arthroplasty 2017;32(6):1786-1791. [DOI] [PubMed] [Google Scholar]
- 39. Hamilton TW, Pandit HG, Maurer DG, et al. Anterior knee pain and evidence of osteoarthritis of the patellofemoral joint should not be considered contraindications to mobile-bearing unicompartmental knee arthroplasty: a 15-year follow-up. Bone Joint J 2017;99-B(5):632-639. [DOI] [PubMed] [Google Scholar]
- 40. Konan S, Haddad FS. Does location of patellofemoral chondral lesion influence outcome after Oxford medial compartmental knee arthroplasty? Bone Joint J 2016;98-B(10)(suppl B):11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Song EK, Park JK, Park CH, et al. No difference in anterior knee pain after medial unicompartmental knee arthroplasty in patients with or without patellofemoral osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2016;24(1):208-213. [DOI] [PubMed] [Google Scholar]
- 42. Hurst JM, Berend KR, Morris MJ, Lombardi AV., Jr Abnormal preoperative MRI does not correlate with failure of UKA. J Arthroplasty 2013;28(9)(suppl):184-186. [DOI] [PubMed] [Google Scholar]
- 43. Maier MW, Kuhs F, Streit MR, et al. Unicompartmental knee arthroplasty in patients with full versus partial thickness cartilage loss (PTCL): equal in clinical outcome but with higher reoperation rate for patients with PTCL. Arch Orthop Trauma Surg 2015;135(8):1169-1175. [DOI] [PubMed] [Google Scholar]
- 44. Hamilton TW, Pandit HG, Inabathula A, et al. Unsatisfactory outcomes following unicompartmental knee arthroplasty in patients with partial thickness cartilage loss: a medium-term follow-up. Bone Joint J 2017;99-B(4):475-482. [DOI] [PubMed] [Google Scholar]
- 45. Waldstein W, Kasparek MF, Faschingbauer M, Windhager R, Boettner F. Lateral-compartment osteophytes are not associated with lateral-compartment cartilage degeneration in arthritic varus knees. Clin Orthop Relat Res 2017;475(5):1386-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Waldstein W, Jawetz ST, Farshad-Amacker NA, et al. Assessment of the lateral patellar facet in varus arthritis of the knee. Knee 2014;21(5):920-925. [DOI] [PubMed] [Google Scholar]
- 47. Berend KR, Lombardi AV, Jr, Jacobs CA. The combination of preoperative bone marrow lesions and partial-thickness cartilage loss did not result in inferior outcomes after medial unicompartmental knee arthroplasty. J Arthroplasty 2017;32(10):3000-3003. [DOI] [PubMed] [Google Scholar]
- 48. Kim SJ, Postigo R, Koo S, Kim JH. Causes of revision following Oxford phase 3 unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2014;22(8):1895-1901. [DOI] [PubMed] [Google Scholar]
- 49. Ko YB, Gujarathi MR, Oh KJ. Outcome of unicompartmental knee arthroplasty: a systematic review of comparative studies between fixed and mobile bearings focusing on complications. Knee Surg Relat Res 2015;27(3):141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pandit H, Jenkins C, Gill HS, et al. Minimally invasive Oxford phase 3 unicompartmental knee replacement: results of 1000 cases. J Bone Joint Surg [Br] 2011;93-B(2):198-204. [DOI] [PubMed] [Google Scholar]
- 51. Kim KT, Lee S, Lee JI, Kim JW. Analysis and treatment of complications after unicompartmental knee arthroplasty. Knee Surg Relat Res 2016;28(1):46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Price AJ, Svard U. A second decade lifetable survival analysis of the Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res 2011;469(1):174-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Choy WS, Lee KW, Kim HY, et al. Mobile bearing medial unicompartmental knee arthroplasty in patients whose lifestyles involve high degrees of knee flexion: A 10-14year follow-up study. Knee 2017;24(4):829-836. [DOI] [PubMed] [Google Scholar]
- 54. Peersman G, Stuyts B, Vandenlangenbergh T, Cartier P, Fennema P. Fixed- versus mobile-bearing UKA: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc 2015;23(11):3296-3305. [DOI] [PubMed] [Google Scholar]
- 55. Smith TO, Hing CB, Davies L, Donell ST. Fixed versus mobile bearing unicompartmental knee replacement: a meta-analysis. Orthop Traumatol Surg Res 2009;95(8):599-605. [DOI] [PubMed] [Google Scholar]
- 56. Foran JR, Brown NM, Della Valle CJ, Berger RA, Galante JO. Long-term survivorship and failure modes of unicompartmental knee arthroplasty. Clin Orthop Relat Res 2013;471(1):102-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Diezi C, Wirth S, Meyer DC, Koch PP. Effect of femoral to tibial varus mismatch on the contact area of unicondylar knee prostheses. Knee 2010;17(5):350-355. [DOI] [PubMed] [Google Scholar]
- 58. Chatellard R, Sauleau V, Colmar M, et al. ; Société d’Orthopédie et de Traumatologie de l’Ouest (SOO). Medial unicompartmental knee arthroplasty: does tibial component position influence clinical outcomes and arthroplasty survival? Orthop Traumatol Surg Res 2013;99(4)(suppl):S219-S225. [DOI] [PubMed] [Google Scholar]
- 59. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty 2016;31(5):1016-1021. [DOI] [PubMed] [Google Scholar]
- 60. Bhattacharya R, Scott CE, Morris HE, Wade F, Nutton RW. Survivorship and patient satisfaction of a fixed bearing unicompartmental knee arthroplasty incorporating an all-polyethylene tibial component. Knee 2012;19(4):348-351. [DOI] [PubMed] [Google Scholar]
- 61. Yamamoto K, Tateiwa T, Takahashi Y. Vitamin E-stabilized highly crosslinked polyethylenes: the role and effectiveness in total hip arthroplasty. J Orthop Sci 2017;22(3):384-390. [DOI] [PubMed] [Google Scholar]
- 62. Liddle AD, Pandit H, Judge A, Murray DW. Effect of surgical caseload on revision rate following total and unicompartmental knee replacement. J Bone Joint Surg [Am] 2016;98-A(1):1-8. [DOI] [PubMed] [Google Scholar]
- 63. Grant AL, Doma KD, Hazratwala K. Determination of the accuracy of navigated kinematic unicompartmental knee arthroplasty: a 2-year follow-up. J Arthroplasty 2017;32(5):1443-1452. [DOI] [PubMed] [Google Scholar]
- 64. Antoniadis A, Bergadano D, Helmy N. Accuracy of tibial cuts with patient-specific instrumentation is not influenced by the surgeon’s level of experience KSSTA 2018. doi: 10.1007/s00167-018-4992-5 [epub ahead of print]. [DOI] [PubMed] [Google Scholar]


