Abstract
Background
Epilepsy is a complex neurologic disorder with abnormal electrical impulses in the brain. A crucial role of purinergic signalling in the proper working of the nervous system has been reported but much less is known about the modulation of P2X3 purinergic receptors in epilepsy. This study investigated the effect of NF110, a potent P2X3 receptor antagonist, in the rat epilepsy model of pentylenetetrazole (PTZ)-induced kindling.
Material/Methods
The mean kindling score, motor activity, locomotion, emotional tension, anxiety, discrimination ability, learning, memory, serum neuron-specific enolase (sNSE), hippocampal IL-1β and TNF-α, thiobarbituric acid-reactive substance (TBARS), catalase (CAT) and reduced glutathione (GSH), and mitochondrial complex I, II, and IV levels of PTZ-kindling animals were assessed.
Results
The PTZ-kindling animals have shown impaired motor activity, locomotion, discrimination ability, learning, and memory, along with increased emotional tension, anxiety, neuronal damage (increased sNSE), hippocampal pro-inflammatory mediators (increased IL-1β and TNF-α), oxidative stress (increased TBARS, decreased GSH and CAT), and mitochondrial dysfunction. The administration of NF110 in 3 different doses has significantly and dose-dependently corrected PTZ-kindling-induced impaired behavior, learning, memory, locomotion, motor activity, discrimination ability, neuronal damage, hippocampal inflammation, oxidative stress, and mitochondrial dysfunction. These beneficial effects of NF110 in PTZ-kindling animals were significantly abolished by the administration of the P2X agonist α, β methylene-ATP.
Conclusions
P2X3 receptors play a very important role in kindling epilepsy and further research should be done to design P2X3 modulators for their possible therapeutic benefits in epileptic disorders.
MeSH Keywords: Hippocampus; Inflammation; Oxidative Stress; Receptors, Purinergic P2X
Background
Epilepsy is a kind of chronic clinical syndrome of brain function caused by abnormal discharge of neurons. Epilepsy is the most common neurological disorder after stroke and Alzheimer’s disease, and more than 1% of the world population has epilepsy [1]. In the clinical manifestations of epilepsy, 30~40% patients with epilepsy have memory impairment, attention dispersion, and other cognitive dysfunctions. Several studies suggest a role of oxidative stress and mitochondrial dysfunction in the pathophysiology of epilepsy [2]. It is an intractable and sometimes a fatal neurological disease. Excitotoxicity, oxidative stress, mitochondrial damage, and neuronal death have been reported as important causes of the sequelae of chronic seizures [3].
Kindling results due to repeated seizures, which induces a permanent and progressive enhancement of epilepsy. It is an activity-dependent neural circuit plasticity phenomenon which involves a permanent structural and functional modifications due to induction of various, molecular, cellular, and functional alterations in neural circuits [4]. Pentylenetetrazol (PTZ) is widely used to induce seizures and to assess the effectiveness of antiepileptic drugs [5]. Kindling induced by repeated PTZ administration has helped researchers to identify the pathophysiological pathways of epilepsy. This model has also helped researcher to discover new as well as effective therapeutic options to manage this condition [1,5].
P2X receptors are trimeric ligand-gated ion channels, and adenosine triphosphate (ATP) is the endogenous ligand which activates these receptors. The 3 P2X subunits out of 7 complements (P2X1-7) get assembled to create homomeric or heteromeric forms of these receptors [6]. ATP and various P2X receptors have been identified as potential targets for many diseases. The P2X receptors along with its ligand ATP have been reported to play important roles during various inflammatory conditions such as nociception and neuroinflammation. Much research has been done on P2X7 followed by P2X3 subtype of these receptors and they are now a mainstay of drug research and development [7].
The P2X3 receptor increases the membrane permeability to potassium (K+) and calcium (Ca2+). The P2X3R is a tripolymer that is broadly distributed in the peripheral as well as central nervous systems, including the hippocampus, which is the main brain region involved in epilepsy, the dorsal root ganglion, and other brain regions [8]. Furthermore, these purinergic receptors produce a positive current and play key roles in neuropathic pain, inflammatory mediation, and increased neuronal excitability [9]. Very recently it has been reported that P2X3Rs were located at the cell bodies and dendrites of neurons with significantly increased expression in temporal lobe epilepsy, and P2X3R activation accelerated sustained repetitive firing in vitro [10], but not much is known about the utility of P2X3 receptors modulators in kindling epilepsy-induced behavioral changes, motor activity, locomotor activity, discrimination, learning, memory, anxiety, emotional tension, mitochondrial dysfunction, oxidative stress, or inflammation. Thus, we designed this study which provides the first proof of concept of the broad-spectrum utility of a potential P2X3 antagonist, NF110, in the rat epilepsy model of pentylenetetrazole (PTZ)-induced kindling.
Material and Methods
Animals
Estrogen has been reported to interfere with neuroinflammation, microglial activation, learning, and memory. Thus, we used male rats to avoid possible estrogen-mediated alterations in higher brain functions [11]. Adult male Sprague-Dawley (SD) rats weighing 260 to 300 g were maintained in the animal house of Qingdao University, where controlled experimental conditions of temperature (25°C), light (12 h dark and 12 h light cycle), and humidity (50–60%) were monitored, with food and water available ad libitum. All the experiments are conducted as per the guidelines of the National Institute of Health Guide for the Care and Use of Laboratory Animals, and the experimental protocol was approved by the Animal Experimentation Committee. Every possible care was taken to minimize animal use and suffering.
Drugs and chemicals
All the chemicals used in this study were of analytical grade (AR) grade and were prepared freshly before use. NF110 and α, β methylene-ATP were purchased from Tocris Bioscience, China. The enzymatic ELISA kits for Neuron-Specific Enolase (NSE), IL-1β, and TNF-α were procured from Biosynthysis Company, China. All other chemicals were purchased from Sigma-Aldrich, Hong Kong, China.
Surgical procedure
The animals were anesthetized with a 40 mg/kg dose of sodium pentobarbital. A steel guide cannula (10 mm) was implanted in the right lateral ventricle using a stereotaxic apparatus and the Atlas of Paxinos and Watson. A single injection of 3% xylocaine and 2% epinephrine was given and the skull was exposed, the position of cannula was implanted 0.9 mm backside to the bregma, 1.6 mm adjacent from midline, and 3.4 mm ventral from the skull’s surface. The dentate acrylate was used to fix the cannula to the skull. To avoid obstruction, the cannula was sealed with a stainless-steel wire. A 10-day recovery period was allowed for each animal.
PTZ-induced kindling
To induce kindling, PTZ (30 mg/kg, i.p.; by dissolving in ACSF) was administered to the animals on alternate days for a maximum of up to 35 days. Then, animals were observed singly in an isolated transparent box for 30 min. The modified Racine scale was used to assess the convulsions intensity: 0 was given for no response; 1 was given for ear and facial cramping; 2 was given for myoclonic jerk without rearing; 3 was given for myoclonic jerk with rearing; 4 was given for change into the side position i.e. clonic-tonic seizures; and 5 was given for change into the supine position i.e. generalized tonic-clonic convulsions [12]. Once the animal exhibited a seizure score of 4 or 5 on at least 3 consecutive trials, it was considered kindled. No further PTZ injection is given to those animals and the maximum score of 5 is given to such animals for all the remaining trials for comparison purpose. During the re-challenge test at the end of the study, irrespective of the seizure score, all the kindling animals were again injected with the subconvulsive dose of PTZ (30 mg/kg) to confirm the kindling persistence.
Behavioral parameters
Assessment of PTZ-induced kindling
To assess the PTZ-induced kindling, we noted the mean kindling score in relation to the number of days and number of days taken for stimulation of each stage in each group. The mean kindling score was also calculated on day 42 after PTZ re-challenge [5].
Motor function and grip strength
The Rotarod apparatus was used to assess motor function and grip strength of the rats. The rats were placed individually on the Rotarod (25 rpm speed) on day 0 (before the first injection of PTZ) and on day 35 (on the last day of PTZ injection). The rat was exposed to 3 trials with a 5-min gap and the cut-off time of 3 min, and the average fall-off time was recorded for each rat [13].
The ppen-field test
A wooden box (100L×100W×40H) with its floor divided into 25 equal squares was used for this test. On day 35, each rat was kept in the center of the box and absolute values of the following variables for each animal were scored for 5 min: the total number of crossed squares, the number of crossed central squares, rearings, and the total number of fecal boli. The floor of the apparatus was cleaned after every use and before exposure of the next animal to avoid any olfactory clues [14].
Elevated plus maze (EPM)
The apparatus was made of wood with 4 arms arranged in (2 closed and 2 open) elevated 100 cm from the floor with 50 cm length and 10 cm width. The 2 opposite closed arms were also provided with walls 40 cm tall, whereas the other 2 opposite arms remained open and instead of walls, a raised ledge of 1 mm thickness and 5 mm height was provided to prevent the rats from falling off the open arms. All 4 arms were linked with a central open platform of 10×10 cm dimensions. On day 35, each rat was kept in the central platform facing an open arm and the number of entries and the time spent inside each arm were recorded. The amount of time spent in the open arms and number of open arm entries were interpreted as anti-anxiety behavior [14].
Object recognition task (ORT)
The above open-field apparatus was also used for the object recognition task on day 36. The objects made of wood and plastic and of different shapes, colors, and textures were provided to the animals. Initially, animals were allowed a 2-min session of exploration for apparatus habituation was given to the animals. After 24 h, first sample trial (T1) with 2 identical objects (FO1 and FO2) for 3 min was initiated. After 60 min, the second choice trial (T2) with 1 familiar (FO1) and 1 novel object (NO) was conducted for 3 min. To prevent potential bias of locations or objects, various combinations of the 2 were utilized. The floor of the apparatus was cleaned after every use and before exposure of the next animal to avoid any olfactory clues. The time spent by each animal in exploring 2 given objects in each trial was noted. Exploration was considered as the touching of the object with the nose or directing the nose towards the object at a distance not more than 2 cm. The discrimination between the familiar and the novel object during T2 was noted by comparing the time spent exploring FO1 and NO [15].
Morris water maze (MWM) test
The MWM apparatus used in this study was a circular water tank of 180 cm in diameter and 50 cm in height, filled with water maintained at 22±1°C. The pool was considered to be divided into 4 equal quadrants labelled as N, W, S, and E. A colorless escape platform 10 cm in diameter was submerged 2 cm below the surface in the E quadrant, which was considered as the target quadrant. The acquisition trials were performed daily for 4 days (day 37 to day 40). Each acquisition session consisted of 4 trials with an inter-trial interval of 5 min. In each trial, the rat was randomly and gently placed in a quadrant and was allowed to find the escape platform. If a rat did not find the platform within 120 s (cut-off time), the escape latency was recorded as 120 s and the rat was gently guided and placed on the platform. The rat was allowed to remain on the platform for 20 s. On the 5th day (day 41), a retrieval trial was conducted in which the escape platform was removed and the time spent by the animal in the target quadrant and the number of crossings over the former platform location was noted [16].
Collection of samples
On day 43 (after the PTZ re-challenge on day 42), animals were killed and the hippocampus of each rats was carefully dissected out of the brain. At the same time, 2 ml of blood was also collected from each anesthetized animal by cardiac puncture prior to decapitation.
Estimation of IL-1β and TNF-α
The dissected hippocampus was homogenized in T-PER buffer containing protease inhibitors (BioSource International, Inc, USA). The lysates were centrifuged at 100 000 g, and the supernatant was collected and used for ELISA analysis. The concentrations of IL-1β and TNF-α were measured by ELISA kit (Biosynthysis Company, China) according to the manufacturer’s instructions.
Estimation of serum neuron-specific enolase (NSE)
The 2 ml of blood was collected from each anesthetized rat by cardiac puncture. Blood coagulation were allowed for 2 h at room temperature or overnight at 4°C before centrifugation for 15 min at 1000×g. Serum was removed and the samples were stored at −20°C or −80° to prevent damage from repeated freeze-thaw cycles. NSE of the samples were measured by ELISA kit (Biosynthysis Company, China) according to the manufacturer’s instructions.
Estimation of thiobarbituric acid-reactive substance (TBARS)
According to the method of Ohokawa et al. [17], the extent of lipid peroxidation was estimated in homogenate of the hippocampus. Tissue homogenate was prepared in a ratio of 1g of wet tissue to 9 ml of phosphate buffer (pH 7.2) using a homogenizer. To 0.1 ml of the homogenate we added 0.2 ml of 8.1% sodium dodecyl sulfate, 1.5 ml of 20% acetic acid solution, and 1.5 ml of a 0.8% aqueous solution of thiobarbituric acid. The mixture was finally made up to 4.0 ml with distilled water, and heated at 95°C for 60 min. After cooling, we added 1.0 ml of distilled water and 5.0 ml of the mixture of n-butanol and pyridine (15: 1, v/v), and the mixture was shaken vigorously. After centrifugation at 4000 rpm for 10 min, the absorbance of the organic layer (upper layer) was measured at 532 nm in a spectrophotometer against a blank containing all the reagents except the homogenate. The malondialdehyde (MDA) equivalents of the samples were calculated using the extinction coefficient 1.56×105 M−1 cm−1.
Estimation of catalase (CAT) activity
According to the method of Luck [18], the activity of CAT was measured. A 10% w/v homogenate of the hippocampus was prepared in phosphate buffer. The homogenate was centrifuged and the supernatant was used for the enzyme assay. In short, the reaction mixture contained Tris (50 mM) – EDTA (5 mM) buffer, pH 7.0, 10 mM H2O2 (in 0.1 M KH2PO4 buffer, pH 7.0) in a test cuvette. The reference cuvette contained Tris-EDTA solution and distilled water only. The contents of both cuvettes were incubated at 37°C for 10 min. The reaction was started by the addition of tissue homogenate to the reference and the test cuvettes. The rate of elimination of H2O2 by catalase was measured by recording the rate of change of absorbance per min at 240 nm for 4 min. Catalase activity was expressed as μmol of H2O2 consumed/min/mg protein using a molar extinction coefficient of 43.6 mM−1 cm−1.
Estimation of reduced glutathione (GSH)
According to the method of Moron et al. [19], assay of GSH was performed in homogenates of hippocampus tissue. We added 100 μl of 25% trichloroacetic acid (TCA) to 500 μl homogenate. The precipitated proteins were separated by centrifugation at 2000×g for 15 min. Supernatants were diluted with 0.2 M sodium phosphate buffer, pH 8.0. Then, we added 2.0 ml of 0.6 mM 5,5 Dithiobis (2-Nitrobenzoic acid). The colored complex formed by DTNB and GSH was measured spectrophotometrically at 412 nm against a reference cuvette containing 0.1 or 0.2 ml of 5% TCA. A standard curve of GSH was plotted with each assay. All the assays were done in duplicate. The levels of GSH were expressed as μg of GSH/mg protein.
Estimation of mitochondrial complex I, II and IV
The hippocampus regions were homogenized in the isolation buffer with EGTA (215 mM Mannitol, 75 mM sucrose, 0.1% BSA, 20 mM HEPES, 1 mM EGTA, and pH-7.2). Homogenate was centrifuged at 13 000×g for 5 min at 4°C. Pellets were resuspended in the isolation buffer with EGTA and centrifuged again at 13 000×g for 5 min. The resulting supernatant was transferred to new tubes and topped off with isolation buffer with EGTA and again spun at 13 000×g for 10 min. Pellets containing purified mitochondria were resuspended in the isolation buffer without EGTA. Lastly, rat brain mitochondria were isolated [20].
Assessment of complex I (NADH dehydrogenase) activity
The NADH dehydrogenase was measured spectrophotometrically. The method involves the catalytic oxidation of NADH to NAD+ with subsequent reduction of cytochrome-C. The reaction mixture contained 0.2 M glycyl glycine buffer, pH 8.5, 6 mM NADH in 2 mM glycyl glycine buffer and 10.5 mM cytochrome-C. The reaction was initiated by the addition of a requisite amount of solubilized hippocampal mitochondrial sample. The absorbance change at 550 nm was followed for 2 min [21].
Assessment of complex II (succinate dehydrogenase-SDH) activity
The SDH was measured spectrophotometrically. The method involves the oxidation of succinate by an artificial electron acceptor, potassium ferricyanide. The reaction mixture contained 0.2 M phosphate buffer pH 7.8, 1% BSA, 0.6 M succinic acid, and 0.03 M potassium ferricyanide. The reaction was initiated by the addition of the solubilized hippocampal mitochondrial sample, and the absorbance change at 420 nm was followed for 2 min [22].
Assessment of complex IV (cytochrome oxidase) activity
The cytochrome oxidase activity was assayed in hippocampus mitochondria. The assay mixture contained 0.3 mM reduced cytochrome-C in 75 mM phosphate buffer. The reaction was initiated by the addition of the solubilized hippocampal mitochondrial sample, and absorbance change at 550 nm was measured spectrophotometrically for 2 min [23].
Experimental protocol
In total, 8 groups were used in this study and each group consisted of 12 (n=12) animals.
Group I – Sham control: The cannula was surgically implanted in the animals as per the procedure and these animals were handled daily by the same person who administered various treatments to animals of other groups. No treatment was given to these animals and from day 35 onwards these animals were assessed for behavioral parameters, similar to the animals of other groups.
Group II – Vehicle control (ACSF): 5 μl of artificial cerebrospinal fluid was administered to the animals through the surgically implanted cannula from day 1 to until the end of study (day 42). From day 35 onwards, these animals were exposed to the behavioral parameters.
Group III – NF110 per se: 5 μl of NF110 (60 nM prepared by dissolving in ACSF, daily) was administered to the animals through the surgically implanted cannula from day 1 to until the end of study (day 42). From day 35 onwards, these animals were exposed to the behavioral parameters.
Group IV – Pentylene tetrazole (PTZ)-kindling: The animals were treated with PTZ (30 mg/Kg, i.p.) on alternate days from day 1 until animals showed kindling or for a maximum of up to 35 days. From day 35 onwards, these animals were exposed to the behavioral parameters.
Group V, VI, and VII – NF110 (dose 1, 2 and 3 respectively) + PTZ: The PTZ (30 mg/Kg, i.p., on alternate days from day 1 to until animals have shown kindling or maximum up to 35 days)-treated animals were administered 5 μl of NF110 in 3 different doses (20 nM, 40 nM, and 60 nM) daily through the surgically implanted cannula from day 1 to until the end of the study (day 42). NF110 was administered at least 20 min before the administration of PTZ. From day 35 onwards, these animals were exposed to the behavioral parameters.
Group VIII – α, β methylene-ATP + NF110 (dose 3) + PTZ: PTZ and NF110 (60 nM) treatment was the same as in group VII above, but these animals were also treated with 5 μl of 0.5 mM α, β methylene-ATP (by dissolving in ACSF, daily, 10 min before NF110 dose 3–60 nM treatment), from day 27 onwards to until the end of the study (day 42). From day 35 onwards, these rats were exposed to the behavioral parameters.
Statistical analysis
The results are expressed as mean ±S.D. Data were statistically analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple range test. p≤0.05 was considered to be statistically significant.
Results
The sham control, vehicle control, and NF110 did not shown any significant effect on the results of any of the parameters.
Effect of the P2X3 antagonist NF110 on mean kindling score of PTZ-kindling animals
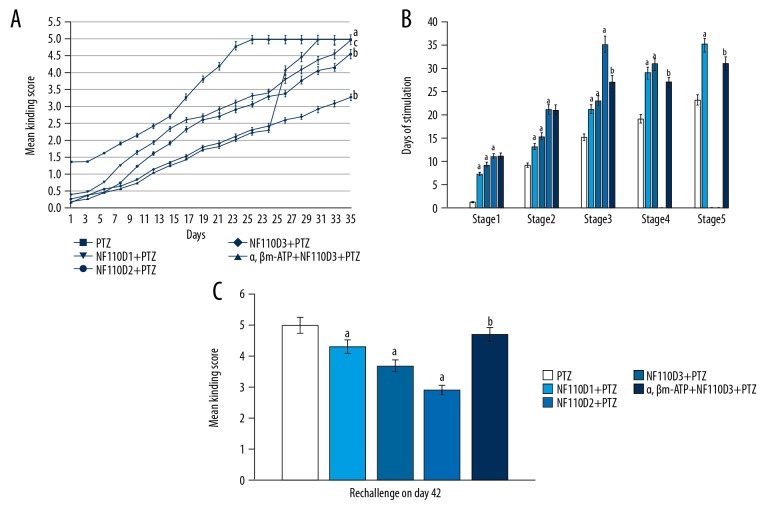
The PTZ-kindling animals achieved stage 5 seizure by day 23 and these showed induction of generalize tonic-clonic seizure (GTCS). When compared to the PTZ-kindling group, NF110 dose 1 (20 nM)-treated PTZ-kindling animals achieved stage 5 seizure by day 35, and NF110 dose 2 (40 nM)-treated PTZ-kindling animals achieved only stage 4 seizure by day 35, whereas NF110 dose 3 (60 nM)-treated PTZ-kindling animals did not achieve stage 4 or stage 5 by day 35. NF110 significantly attenuated the mean kindling score of the PTZ-kindling group. The PTZ-kindling animals treated with all 3 doses of NF110 had significantly increased number of days required for stimulation for various stages of PTZ-kindling. In contrast, treatment with NF110 dose 3 significantly prevented PTZ-induced generalize tonic-clonic seizure, which was significantly abolished by the treatment of α, β methylene-ATP (0.5 mM). Similar results were obtained after re-challenge test on day 42 (Figure 1).
Figure 1.
Effect of the P2X3 antagonist NF110 on mean kindling score of PTZ-kindling animals. (A) The seizure stage for each group. (B) Days of stimulation for various stages of each group. (C) Mean kindling score for each group.
Effect of P2X3 antagonist, NF110, on motor performance of PTZ-kindling animals, as assessed on Rotarod test
The PTZ-kindling animals showed significant reduction of motor activity when compared to sham control animals. The daily administration of NF110 significantly increased the motor activity of PTZ-kindling animals, which was significantly abolished by the treatment of α, β methylene-ATP (Figure 2A)
Figure 2.
Effect of the P2X3 antagonist NF110 on motor performance, locomotion and emotional tension of PTZ-kindling animals. (A) Motor activity of day 0 and day 35, assessed on Rotarod test. (B) Locomotor activity and emotional tension of each group.
Effect of P2X3 antagonist, NF110, on locomotion and emotional tension of PTZ-kindling animals, as assessed on open-field test
The PTZ-kindling animals showed a significant reduction of locomotor activity (noted as reduction in number of crossings of central and other squares) and an increase of emotional tension (noted as increase in defecations) when compared to sham control animals. The daily administration of NF110 significantly increased the locomotor activity and reduced the emotional tension of PTZ-kindling animals, which was significantly abolished by the treatment of α, β methylene-ATP (Figure 2B).
Effect of the P2X3 antagonist NF110 on anxiety activity of PTZ-kindling animals, as assessed by elevated plus maze
The PTZ-kindling animals showed a significant reduction in open arm time spent and open arm entries along with a significant increase in closed arm time spent and closed arm entries when compared to sham control animals, suggesting anxiety activity of PTZ-kindling animals. The daily administration of NF110 has significantly increased the open arm time spent and open arm entries along with reduction in the closed arm time spent and closed arm entries of PTZ-kindling animals, which was significantly abolished by the treatment of α, β methylene-ATP (Figure 3).
Figure 3.
Effect of the P2X3 antagonist NF110 on anxiety activity of PTZ-kindling animals. (A) Time spent in open arm and closed arm. (B) Frequency in open arm and closed arm.
Effect of P2X3 antagonist, NF110, on discrimination ability of PTZ-kindling animals, as assessed on object recognition task
In test 1 (T1), in which both objects were similar or familiar (FO1 and FO2), the exploration time was similar in all groups. On the other hand, in test 2 (T2), when animals were exposed to a familiar object (FO1) and a novel object (NO), then discrimination was observed. The PTZ-kindling animals were not able to discriminate between the FO1 and NO as they have spent almost the same amount of time with both objects, when compared to sham control animals. The daily administration of NF110 significantly increased the discrimination between FO1 and NO and more time was spent with the novel objects by these NF110 PTZ-kindling animals, which was significantly abolished by the treatment of α, β methylene-ATP (Figure 4).
Figure 4.
Effect of the P2X3 antagonist NF110 on discrimination ability of PTZ-kindling animals. (A) In test 1 (T1) where both the objects were similar or familiar (FO1 and FO2), the exploration time was similar in all the groups. (B) NF110 significantly increased the discrimination between FO1 and NO.
Effect of P2X3 antagonist, NF110, on learning and memory of PTZ-kindling animals, as assessed on Morris water maze
The sham control animals showed a significant reduction of day 4 (day 40 of the study) escape latency time (ELT) when compared to day 1 (day 37 of the study) ELT, which suggests normal learning. The PTZ-kindling animals showed a significant increase in day 4 ELT when compared to the day 4 ELT of control animals, which suggests the impairment of learning. The daily administration of NF110 significantly reduced the day 4 ELT of PTZ-kindling animals, which was significantly abolished by the treatment of α, β methylene-ATP (Figure 5A). Furthermore, the sham control animals showed significantly higher day 5 (day 41 of the study) mean time spent in the target quadrant (TSTQ) when compared to the other quadrants, which suggests normal memory. The PTZ-kindling animals showed a significant reduction in day 5 TSTQ and number of crossings in the platform area when compared to the control animals, which suggests the impairment of memory. The daily administration of NF110 significantly increased the day 5 TSTQ and number of crossings in the platform area of PTZ-kindling animals, which was significantly abolished by the treatment of α, β methylene-ATP (Figure 5B, 5C).
Figure 5.
Effect of the P2X3 antagonist NF110 on learning and memory of PTZ-kindling animals, as assessed on Morris water maze. (A) PTZ impaired learning of rats and NF110 mitigated this damage in PTZ-kindling animals. (B, C) NF110 significantly increased the day 5 TSTQ and number of crossings in the platform area of PTZ-kindling animals in a dose-dependent manner.
Effect of the P2X3 antagonist NF110 on hippocampal IL-1β and TNF-α levels of PTZ-kindling animals
The PTZ-kindling animals showed a significant increase in hippocampal IL-1β and TNF-α levels when compared to sham control animals. The daily administration of NF110 significantly reduced the hippocampal IL-1β and TNF-α levels of PTZ-kindling animals, which were significantly abolished by the treatment of α, β methylene-ATP (Figure 6).
Figure 6.
Effect of the P2X3 antagonist NF110 on hippocampal IL-1β and TNF-α levels of PTZ-kindling animals. (A) NF110 significantly reduced the hippocampal IL-1β of PTZ-kindling animals. (B) NF110 significantly reduced the hippocampal TNF-α levels of PTZ-kindling animals.
Effect of the P2X3 antagonist NF110 on hippocampal serum neuron-specific enolase (sNSE), thiobarbituric acid-reactive substance (TBARS), catalase (CAT), and reduced glutathione (GSH) levels of PTZ-kindling animals
The PTZ-kindling animals showed a significant increase in sNSE and hippocampal TBARS along with significant reduction in hippocampal CAT and GSH when compared to sham control animals. The daily administration of NF110 significantly reduced the sNSE and hippocampal TBARS levels along with significant increase in hippocampal CAT and GSH of PTZ-kindling animals, which were significantly abolished by the treatment of α, β methylene-ATP (Figure 7).
Figure 7.
Effect of the P2X3 antagonist NF110 on hippocampal serum neuron-specific enolase (sNSE), thiobarbituric acid-reactive substance (TBARS), catalase (CAT) and reduced glutathione (GSH) levels of PTZ-kindling animals. (A) sNSE levels in each group. (B) Hippocampal TBARS levels in each group. (C) GSH levels in each group. (D) CAT levels in each group.
Effect of the P2X3 antagonist NF110 on hippocampal mitochondrial complex I, II, and IV levels of PTZ-kindling animals
The PTZ-kindling animals showed a significant reduction in hippocampal mitochondrial complex I, II, and IV levels when compared to sham control animals. The daily administration of NF110 significantly increased the hippocampal mitochondrial complex I, II, and IV levels of PTZ-kindling animals, which were significantly abolished by the treatment of α, β methylene-ATP (Figure 8).
Figure 8.
Effect of the P2X3 antagonist NF110 on hippocampal mitochondrial complex I, II, and IV levels of PTZ-kindling animals. NF110 significantly increased the hippocampal mitochondrial complex I (A), II (B), and IV (C) levels of PTZ-kindling animals.
Discussion
In this study, PTZ (30 mg/kg i.p., on alternate days) significantly impaired motor activity, locomotion, discrimination ability, learning, and memory along with increased emotional tension, anxiety, neuronal damage (increased sNSE), hippocampal pro-inflammatory mediators (increased TNF-α and IL-1β), oxidative stress (increased TBARS, decreased GSH and CAT), and mitochondrial dysfunction, which is in line with previous studies [1,4,5,24]. The administration of NF110 in 3 different doses significantly and dose-dependently corrected PTZ-kindling-induced impaired behavior, learning, memory, locomotion, motor activity, discrimination ability, neuronal damage, hippocampal inflammation, oxidative stress, and mitochondrial dysfunction. These beneficial effects of NF110 in PTZ-kindling animals were significantly abolished by the administration of P2X agonist, α, β methylene-ATP.
For the assessment of progression of kindling, mean kindling score was assessed. Our results suggest that PTZ administration induced GTCS (stage 5) in 23 days, which is in line with the previous findings that PTZ administration induced kindling at 23–27 days (5, 24). PTZ-kindling is widely used for induction of epilepsy similar to clinical symptoms of generalized epilepsy. Administration of a potent antagonist of P2X3 receptors such as NF110 has significantly increased the time of induction of kindling in PTZ-treated rats. The dose 3 (60 nM, the highest dose of NF110 used in this study) of NF110 prevented kindling induction, even after 35 days. The beneficial effect of NF110 on mean kindling score was significantly abolished by the administration of P2X agonist, α, β methylene-ATP, which further emphasizes that NF110 may provide benefits specifically through P2X types of receptors. It is also reported that, for NF110, the Ki values are 36, 82 and 4144 nM for P2X3, P2X1, and P2X2 receptors, respectively [25], suggesting that the doses of NF110 used in this study may have preferential action on P2X3 receptors. Zhou et al. [10] reported that P2X3 receptors were located at the cell bodies and dendrites of neurons with significantly increased expression in temporal lobe epilepsy. They also found that P2X3 receptors activation accelerated sustained repetitive firing, whereas their inhibition led to relatively low-frequency discharges in epilepsy. They suggested that with upregulated P2X3 receptors, expression exists in both epileptic humans and rats and may aggravate the epileptic state. Our study clearly supports the notion of Zhou et al. [10], as the central administration of the potent inhibitor of P2X3 receptor, NF110, significantly attenuated the PTZ-kindling.
The Rotarod test have been performed in many studies to assess the motor function and grip strength of rats [13]. Open-field tests have been utilized for the assessment of locomotion and emotional tension of animals [14]. Elevated plus maze has been utilized by many researchers for assessment of anxiety in animals [14]. Morris water maze has been used for assessment of learning and memory of animals [16]. The administration of NF110 corrected the PTS-kindling impaired motor function, grip strength, locomotion, emotional status, anxiety, learning, and memory of the animals in this study.
Little is known about the effect of P2X3 receptor modulators on the motor function, grip strength, locomotion, emotional status, anxiety, learning, and memory of rats. P2X receptors are widely expressed and distributed throughout the different brain regions. These receptors are involved in synaptic co-transmission, trophic signalling, and neuromodulation. Purinergic signalling utilizes these receptors and controls learning, memory, sleep, arousal, locomotion, and feeding activities. P2X receptors have been reported to be involved in various conditions like injury, inflammation, neuropathic pain, Alzheimer’s disease, depression, anxiety, Parkinson’s diseases, multiple sclerosis, and amyotrophic lateral sclerosis [8]. To the best of our knowledge, the present study is the first to assess the effect of a P2X3 receptor modulator, NF110, on motor function, grip strength, locomotion, emotional status, anxiety, learning, and memory of rats.
The pro-inflammatory cytokines TNF-α and IL-1β are known to participate in the induction and maintenance of epilepsy [26,27]. The inflammatory mediators have pro-epileptogenic potential as they have the ability to reduce the seizure threshold and thus have been linked with the sporadic spontaneous epilepsy condition [27]. It has been previously reported that activation of P2X3 receptors stimulate inflammatory mechanisms which involve bradykinin, prostaglandins, sympathetic amines, the pro-inflammatory cytokines TNF-α-a and IL-1β, and neutrophil migration [28]. This suggests that NF110 significantly inhibits PTZ-induced hippocampal inflammation by inhibiting the P2X3 receptors.
It has been reported that neuron-specific enolase generally increases in various neuronal damage conditions like brain injury, cerebral hypoxia, and epilepsy and thus it is a sensitive marker of these conditions [29]. It has been reported that PTZ kindling increases the levels of sNSE in PTZ-kindling rats [30]. Our study also shows that PTZ-kindling rats have higher levels of sNSE, which suggests PTZ induced neuronal damage. Chen et al. [31] reported that during nociception and neuronal damage, P2X3 receptors are uploaded and the inhibitory control of P2X3 receptors was sufficient for reduction of neuronal damage. In our study, NF110 reduced the sNSE level, which suggests the reduction of neuronal damage in PTZ-kindled rats and shows that NF110 provided neuroprotection.
Free radicals have been considered as the products of normal cellular metabolism and they have been found to be involved in the development of seizures [32]. The increase in the production of free radicals and the decrease in the endogenous antioxidant systems of the body may attack phospholipids of the cellular membrane, thereby inducing lipid peroxidation and cellular dysfunction. In the present study, PTZ kindling increased the level of MDA and decreased the level of GSH as well as CAT in the rat hippocampus. PTZ thus caused an imbalance between antioxidant and oxidant defense systems, which may be responsible for seizures and cognitive impairment [24].
In previous studies, a significant decrease in mitochondrial complex I, II, and IV was observed in PTZ-treated animals. It has been suggested that mitochondrial complexes are inhibited due to the generation of reactive oxygen species following PTZ treatment [33]. The inhibition of the components of the mitochondrial cascade contributes to the incomplete electron transport and reduction in the intracellular ATP production. This alteration may disturb calcium homeostasis, thereby affecting neuronal excitability and synaptic transmission [34]. The administration of NF110 significantly reduced the lipid peroxidation in the hippocampus of the PTZ-kindling rats, which suggest the anti-oxidative stress property of P2X3 inhibitor, due to which NF110 also provides a protective effect in PTZ-kindling-induced mitochondrial dysfunction. Thus, in this study, NF110, a potent P2X3 antagonist, provided behavioral as well as biochemical benefits in PTZ-kindling epilepsy due to its anti-inflammatory, anti-oxidative stress, neuroprotective, and mitochondrial-protective effects.
Conclusions
Our results show that PTZ kindling significantly impaired motor activity, locomotion, discrimination ability, learning, and memory, and increased emotional tension, anxiety, neuronal damage, hippocampal pro-inflammatory mediators, oxidative stress, and mitochondrial dysfunction. The administration of NF110, a potent P2X3 antagonist, in 3 different doses significantly and dose-dependently corrected PTZ-kindling-induced impaired behavior, learning, memory, locomotion, motor activity, discrimination ability, neuronal damage, hippocampal inflammation, oxidative stress, and mitochondrial dysfunction. These beneficial effects of NF110 in PTZ-kindling animals were significantly abolished by the administration of a P2X agonist, α, β methylene-ATP. Thus, P2X3Rs may represent a novel therapeutic target for antiepileptic drugs. Further research should be targeted towards understanding the various pharmacological mechanisms that may be involved in the antiepileptic effects of P2X3 modulators.
Acknowledgements
The authors thank the Department of Cardiology, Henan University Huaihe Hospital, Henan, China for providing us the facilities for conducting this research.
Footnotes
Source of support: This project was supported by a grant from the Natural Science Foundation of Hubei, China (No. 2016CFB345)
Conflict of interest
None.
References
- 1.Beheshti Nasr SM, Moghimi A, Mohammad-Zadeh M, et al. The effect of minocycline on seizures induced by amygdala kindling in rats. Seizure. 2013;22:670–74. doi: 10.1016/j.seizure.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Lalitha S, Mishra J. Hesperidin potentiates the neuroprotective effects of diazepam and gabapentin against pentylenetetrazole-induced convulsions in mice: Possible behavioral, biochemical and mitochondrial alterations. Indian J Pharmacol. 2014;46:309–15. doi: 10.4103/0253-7613.132180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kernan CL, Asarnow R, Siddarth P, et al. Neurocognitive profiles in children with epilepsy. Epilepsia. 2012;53:2156–63. doi: 10.1111/j.1528-1167.2012.03706.x. [DOI] [PubMed] [Google Scholar]
- 4.Langberg T, Dashek R, Mulvey B, et al. Distinct behavioral phenotypes in novel “fast” kindling-susceptible and “slow” kindling-resistant rat strains selected by stimulation of the hippocampal perforant path. Neurobiol Dis. 2016;85:122–29. doi: 10.1016/j.nbd.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Chen Y, Lü Y, et al. Effects of JIP3 on epileptic seizures, Evidence from temporal lobe epilepsy patients, kainic-induced acute seizures and pentylenetetrazole-induced kindled seizures. Neuroscience. 2015;300:314–24. doi: 10.1016/j.neuroscience.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Ralevic V. P2X receptors in the cardiovascular system and their potential as therapeutic targets in disease. Curr Med Chem. 2015;22:851–65. doi: 10.2174/0929867321666141215094050. [DOI] [PubMed] [Google Scholar]
- 7.Müller CE. Medicinal chemistry of P2X receptors, allosteric modulators. Curr Med Chem. 2015;22:929–41. doi: 10.2174/0929867322666141210155610. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G. Physiopathological roles of P2X receptors in the central nervous system. Curr Med Chem. 2015;22:819–44. doi: 10.2174/0929867321666140706130415. [DOI] [PubMed] [Google Scholar]
- 9.Bele T, Fabbretti E. P2X receptors: sensory neurons and pain. Curr Med Chem. 2015;22:845–50. doi: 10.2174/0929867321666141011195351. [DOI] [PubMed] [Google Scholar]
- 10.Zhou X, Ma LM, Xiong Y, et al. Upregulated P2X3 receptor expression in patients with intractable temporal lobe epilepsy and in a rat model of epilepsy. Neurochem Res. 2016;41:1263–73. doi: 10.1007/s11064-015-1820-x. [DOI] [PubMed] [Google Scholar]
- 11.Bjorling DE, Wang ZY. Estrogen and neuroinflammation. Urology. 2001;57:40–46. doi: 10.1016/s0090-4295(01)01124-4. [DOI] [PubMed] [Google Scholar]
- 12.Racine R. Modification of seizure activity by electrical stimulation. I. After discharge threshold. Electroencephalogr Clin Neurophysiol. 1972;32:269–79. doi: 10.1016/0013-4694(72)90176-9. [DOI] [PubMed] [Google Scholar]
- 13.Soni N, Koushal P, Reddy BV, et al. Effect of GLT-1 modulator and P2X7 antagonists alone and in combination in the kindling model of epilepsy in rats. Epilepsy Behav. 2015;48:4–14. doi: 10.1016/j.yebeh.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 14.Godlevsky LS, Muratova TN, Kresyun NV, et al. Anxiolytic and antidepressive effects of electric stimulation of the paleocerebellar cortex in pentylenetetrazol kindled rats. Acta Neurobiol Exp. 2014;74:456–64. doi: 10.55782/ane-2014-2008. [DOI] [PubMed] [Google Scholar]
- 15.Deshmukh R, Sharma V, Mehan S, et al. Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine – a PDE1 inhibitor. Eur J Pharmacol. 2009;620:49–56. doi: 10.1016/j.ejphar.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Zhen J, Qu Z, Fang H, et al. Effects of grape seed proanthocyanidin extract on pentylenetetrazole-induced kindling and associated cognitive impairment in rats. Int J Mol Med. 2014;34:391–98. doi: 10.3892/ijmm.2014.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–58. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Luck H. Catalase. In: Bergmeyer HW, editor. Methods of enzymatic analysis. Academic Press; New York: 1963. pp. 885–94. Section 3. [Google Scholar]
- 19.Moron MS, Kepierre JW, Mannervick B. Level of glutathione reductase and glutathione-S-transferase activities in rat lung and liver. Biochem Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 20.Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria, implications for Parkinson’s disease. J Neurochem. 1999;73:1127–37. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 21.King TE, Howard RL. Preparations and properties of soluble NADH dehydrogenases from cardiac muscle. Methods Enzymol. 1967;10:275–84. [Google Scholar]
- 22.King TE. Preparation of succinate dehydrogenase and reconstitution of succinateoxidase. Methods Enzymol. 1967;10:322–31. [Google Scholar]
- 23.Sottocasa GL, Kuylenstierna B, Ernster L, Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967;32:415–38. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taiwe GS, Moto FC, Ayissi ER, et al. Effects of a lyophilized aqueous extract of Feretia apodanthera Del. (Rubiaceae) on pentylenetetrazole-induced kindling, oxidative stress, and cognitive impairment in mice. Epilepsy Behav. 2015;43:100–8. doi: 10.1016/j.yebeh.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Hausmann R, Rettinger J, Gerevich Z, et al. The suramin analog 4.,4′.,4″.,4′″-(carbonylbis(imino-5.,1.,3-23 benzenetriylbis (carbonylimino)))tetra-kis-benzenesulfonic acid (NF110) potently blocks P2X3 receptors, subtype selectivity is determined by location of sulfonic acid groups. Mol Pharmacol. 2006;69:2058–67. doi: 10.1124/mol.106.022665. [DOI] [PubMed] [Google Scholar]
- 26.Okada K, Matsunaga K, Yuhi T, et al. The longterm high-frequency repetitive transcranial magnetic stimulation does not induce mRNA expression of inflammatory mediators in the rat central nervous system. Brain Res. 2002;957:37–41. doi: 10.1016/s0006-8993(02)03582-5. [DOI] [PubMed] [Google Scholar]
- 27.Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Schiavuzzo JG, Teixeira JM, Melo B, et al. Muscle hyperalgesia induced by peripheral P2X3 receptors is modulated by inflammatory mediators. Neuroscience. 2015;285:24–33. doi: 10.1016/j.neuroscience.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Saha L, Bhandari S, Bhatia A, et al. Anti-kindling effect of bezafibrate: A peroxisome proliferator-activated receptors alpha agonist in pentylenetetrazole-induced kindling seizure model. J Epilepsy Res. 2014;4:45–54. doi: 10.14581/jer.14011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Wang P, Ren RN, Cai SY, et al. [Neuroprotective effects of topiramate and folic acid on young rats with kindling-induced epilepsy]. Zhong guo Dang Dai Er Ke Za Zhi. 2008;10:65–69. [in Chinese] [PubMed] [Google Scholar]
- 31.Chen Y, Zhang X, Wang C, et al. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci USA. 2008;105:16773–78. doi: 10.1073/pnas.0801793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sejima H, Ito M, Kishi K, et al. Regional excitatory and inhibitory amino acid concentrations in pentylenetetrazole kindling and kindled rat brain. Brain Dev. 1997;19:171–75. doi: 10.1016/s0387-7604(96)00492-5. [DOI] [PubMed] [Google Scholar]
- 33.Kudin AP, Kudina TA, Seyfried J, et al. Seizure-dependent modulation of mitochondrial oxidative phosphorylation in rat hippocampus. Eur J Neurosci. 2002;15:1105–14. doi: 10.1046/j.1460-9568.2002.01947.x. [DOI] [PubMed] [Google Scholar]
- 34.Folbergrová J, Kunz WS. Mitochondrial dysfunction in epilepsy. Mitochondrion. 2012;12:35–40. doi: 10.1016/j.mito.2011.04.004. [DOI] [PubMed] [Google Scholar]