Abstract
Background
Osteosarcoma is the most common primary bone malignancy and often presents at an early age. Calycosin is a phytoestrogen isoflavone, which has previously been reported to inhibit tumor cell growth. The aim of this study was to investigate the effects of calycosin on apoptosis of estrogen receptor (ER)-positive and ER-negative human osteosarcoma cell lines and tumor xenografts in mice.
Material/Methods
Cultured ER-positive MG-63 human osteosarcoma cells and ER-negative U2-OS human osteosarcoma cells were treated with increasing doses of calycosin (0, 25, 50, and 100 μm). Cell viability and apoptosis were studied by an MTT assay and flow cytometry. Western blot measured the expression levels of the apoptosis-related protein p-PI3K, p-Akt, and p-mTOR in MG-63 cells, with and without pretreatment with the PI3K inhibitor, LY294002, the AKT inhibitor, MK-2206, or the mTOR inhibitor, rapamycin. MG-63 tumor-bearing nude mice were used to evaluate the effects of treatment with calycosin.
Results
Calycosin treatment inhibited proliferation and induced apoptosis in MG-63 cells, but had no effect on U2-0S cells. In MG-63 cells, calycosin treatment increased the expression of the PI3K/AKT/mTOR pathway proteins; inhibitor assays showed that expression of the PI3K protein was most strongly associated with the antitumor effects of calycosin. In the nude mouse MG-63 tumor xenografts, calycosin inhibited tumor growth and regulated the expression levels of apoptosis-related PI3K/AKT/mTOR pathway proteins.
Conclusions
The phytoestrogen, calycosin, induced apoptosis of cells of the ER-positive osteosarcoma cell line, MG-63, via the PI3K/AKT/mTOR pathway, with these effects being mainly due to PI3K.
MeSH Keywords: Apoptosis, Estrogen Receptor alpha, Osteosarcoma
Background
Osteosarcoma is the most common primary malignancy of bone and often presents at an early age [1]. In recent decades, improvements have been made in the diagnosis and in the surgical and medical treatment of osteosarcoma. Current therapeutic strategies have evolved with the addition of neoadjuvant chemotherapy and more precise local surgical resection. However, there remains a high incidence of side effects from therapy, early tumor metastasis, tumor chemoresistance, and relapse following treatment, the clinical course for patients can be painful, and the prognosis remains poor [2–5]. There is still a need for treatment approaches for patients with osteosarcoma that combine effectiveness with minimal side effects. Malignant tumors are characterized by alterations in the cell cycle and tumor cell apoptosis, knowledge of these pathogenic tumor pathways can provide the basis for drug discovery and development [6]. Treatments that induce apoptosis in tumor cells currently include chemotherapy and radiotherapy [7]. The remaining challenges for drug treatment in malignancy are to provide rapid treatment response by increasing tumor cell apoptosis, combined with minimal side effects, or adverse events.
Calycosin is a bioactive phytoestrogen isoflavone that is extracted from Trifolium pratense or red clover. Previous studies have shown calycosin can act as a pharmacological estrogen analog [8,9]. Calycosin has also been shown to have anti-tumor effects on several types of cancer cells when studied in vitro and in vivo [10–12]. However, previous studies have shown that, in tumors, the effects of calycosin are specific estrogen receptor (ER)-positive tumor cells [11–13]. To our knowledge, although ER-positive osteosarcoma cell lines are available for in vitro studies, no previous studies have been undertaken on the effects of calycosin on ER-positive osteosarcoma. The MG-63 human osteosarcoma cell line has been reported to be ER-positive, and the U2-OS cell line is reported to be ER-negative [14,15]. Both these cell lines can be studied in vitro in cell culture, and in vivo when used to form tumor xenografts in animal models.
Therefore, the aim of this study was to investigate the effects of calycosin on cell proliferation and apoptosis of the ER-positive MG-63 human osteosarcoma cell and the ER-negative U2-OS human osteosarcoma cell line in vitro and on their tumor xenografts in nude mice in vivo.
Material and Methods
Calycosin
Calycosin (purity 98%) was purchased from Tianjin JAHE Science and Technology Co. Ltd., China. Original solution was processed into a 250 μg/ml stock solution with dimethyl sulfoxide (DMSO).
The culture of the MG-63 and U2-OS human osteosarcoma cell lines
Human osteosarcoma cell lines, MG-63 and U2-OS, were obtained from the Cell Bank of the Chinese Academy of Sciences. The two cell lines were incubated in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS) (Cell Bank of the Chinese Academy of Sciences), with 100 units/ml penicillin and 100 mg/ml streptomycin, and cultivated in a humidified atmosphere of 5% CO2 at 37°C. The two cultured cell lines, MG-63 and U2-OS, were divided into four groups, according to their treatment doses of calycosin (0, 25, 50, and 100 μM). In some experiments, MG-63 cells were pretreated with the PI3K inhibitor, LY294002 (5 mM) (Beyotime, China), the AKT inhibitor, MK-2206 (10 μM) (Sigma, St. Louis, MO, USA), or the mTOR inhibitor, rapamycin (5 mM) (Beyotime, China).
MTT cell proliferation assay
The MTT colorimetric assay was used to detect the effect of calycosin treatment on cell proliferation of MG-63 cells and U2-OS osteosarcoma cells. Cells were harvested using trypsinase, and then seeded into 96 wells plates at about 3×104 cells/well for 24 h. Then, the MG-63 and U2-OS cells were treated with different concentrations of calycosin (0, 25, 50, and 100 μM) for 72 h. MTT solution (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Dojindo, Kumamoto, Japan) at 10 μl was added and the cells were incubating for 4 h. Calycosin and the MTT supernatant mixture was replaced with 100 μl of 100% DMSO (Sigma-Aldrich, USA) to dissolve the formazan crystals. A full-wavelength microplate reader (Bio-Rad Laboratories, CA, USA) was applied to detect the optical density (OD) value at a wavelength of 490 nm. Fresh DMEM was used as a control. The results were presented as the proliferation ratio from three independent experiments. The proliferation ratio was calculated according to following formula: proliferation ratio (%)=OD administrated/OD control×100%.
Flow cytometry assay
A flow cytometry assay was used to study the effects of calycosin treatment on apoptosis of the osteosarcoma cell lines. After MG-63 cells and U2-OS cells were incubated with calycosin (0, 25, 50 and 100 μM) for 72 h, cells were harvested and washed twice in PBS. Cells were then stained with Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) for 40 min at room temperature. A FACS Aria flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) was used to determine the percentage of apoptotic cells.
Animal care and treatment
The study protocol, including the care of the animals used in the study were reviewed and approved by the Animal Care and Use Committee of Nanjing University of Traditional Chinese Medicine. All animal procedures were conducted according to guidelines of the local Experimental Research Institute.
BALB/c nude mouse osteosarcoma xenografts
Fifty female BALB/c nude mice, eight weeks-of-age, weighing between 17–22 gm (Vital River Laboratories, Beijing, China) were raised in sterile conditions at an ambient temperature of between 20–21°C, at 40–50% humidity, and alternating 12 h periods of light and darkness. A 0.2 ml volume of logarithmic phase MG-63 cell suspension (4×106 per ml) was injected subcutaneously into the dorsal area of each mouse. Ten days after implantation of the GM-63 cells, when tumors were seen to develop, mice were randomly divided into five groups: a control group (n=10), an ifosfamide-treated group (4mg/kg) (n=10), and three calycosin-treated groups (2, 4, and 8 mg/kg) (n=10) treated by intraperitoneal injection. Calycosin and ifosfamide were injected daily for 15 days. Body weight (W) and tumor volume (V) were determined every two days.
The formulae used were: V (mm3)=ab2/2, in which ‘a’ represents the largest superficial diameter and ‘b’ represents the shortest. The tumor inhibitory rate (%)=(TWcontrol−TWtreated)/TWcontrol×100%.
Twenty days after tumor cell transplant, all mice were sacrificed and all the transplanted tumors were aseptically removed and weighed (weight of tumor was recorded as TW). Tumor samples were stored at –80°C for further experiments.
Western blot assay
Western blot was used to assess the effect of calycosin treatment on protein expression levels in the osteosarcoma cell lines. Osteosarcoma U2-OS cells and MG-63 cells were pre-treated with increasing concentrations of calycosin (0, 25, 50, and 100μM) for 72 h. Total protein was extracted using lysis buffer, containing 50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS) on ice, and then the lysates were centrifuged at 10,000 rpm for 15 min. The concentration of total protein in the collected supernatant was determined using the Bio-Rad assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using bovine serum albumin (BSA) as a standard control. Total proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto 0.2 μm polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were blocked for 1 hour in Tris-buffered saline, pH 7.6, with 0.05% Tween20 (TBST), and 5% dried non-fat milk powder, and sequentially incubated with the primary antibodies for 24 hours at 4°C. After washing three times in TBS-T, the membranes were was incubated in horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature for 1 h. The protein bands were extracted by enhanced chemiluminescence (ECL) (WBKLS0100, Millipore) and and analyzed with an ECL detection system (Thermo Scientific, Beijing, China). The primary antibodies used for Western blotting included rabbit anti-p-AKT (1: 1000), AKT (1: 1000), PI3K (1: 1000), p-PI3K (1: 1000), mTOR (1: 1000), p-mTOR (1: 1000), caspase-3 (1: 1000), cleaved caspase-3 (1: 1000), PARP (1: 1000), cleaved PARP (1: 1000), Bax (1: 250), Bcl-2 (1: 250), Bad (1: 250), and mouse-anti-GAPDH (1: 1000) (Santa Cruz, CA, USA). The relative expression levels of these proteins were presented as relative intensities of protein bands compared with the control (GAPDH), and analyzed by the National Institutes of Health (NIH) ImageJ software.
Inhibitor assays
The inhibitors of AKT, PI3K, and mTOR including MK-2206, LY294002, and rapamycin, respectively (Beyotime, China) and were applied to MG-63 cells to explore further the key proteins expressed following treatment with calycosin of osteosarcoma MG-63 cells. Specifically, 106 MG-63 cells were cultured in a 100 mm dish and incubated with each inhibitor for 2 h. Cells were then treated with increasing concentrations of calycosin (0, 25, 50, and 100 μM) for 72 h. Cells were then harvested and protein expression was analyzed by Western blot.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD). Differences were tested using one-way analysis of variance (ANOVA), two-way ANOVA, and by the least significant difference (LSD) t-test. All statistical analysis was performed by SPSS software. A p-value <0.05 was regarded as statistically significant.
Results
Calycosin treatment suppressed cell viability and enhanced apoptosis of estrogen receptor (ER)-positive osteosarcoma cells
The effects of calycosin treatment on osteosarcoma xenografts, and estrogen receptor (ER)-positive osteosarcoma MG-63 cells and ER-negative osteosarcoma U2-OS cells, which were incubated in increasing concentrations of calycosin (0, 25, 50, and 100 μM) for 24, 48 and 72 h before the MTT assay were evaluated. The results showed that calycosin treatment resulted in significantly higher cell viability inhibition in MG-63 cells than U2-OS cells in a time-dependent and dosage-dependent manner (Figure 1A, 1B). This result suggested that calycosin suppressed osteosarcoma cell growth by ER-related mechanisms.
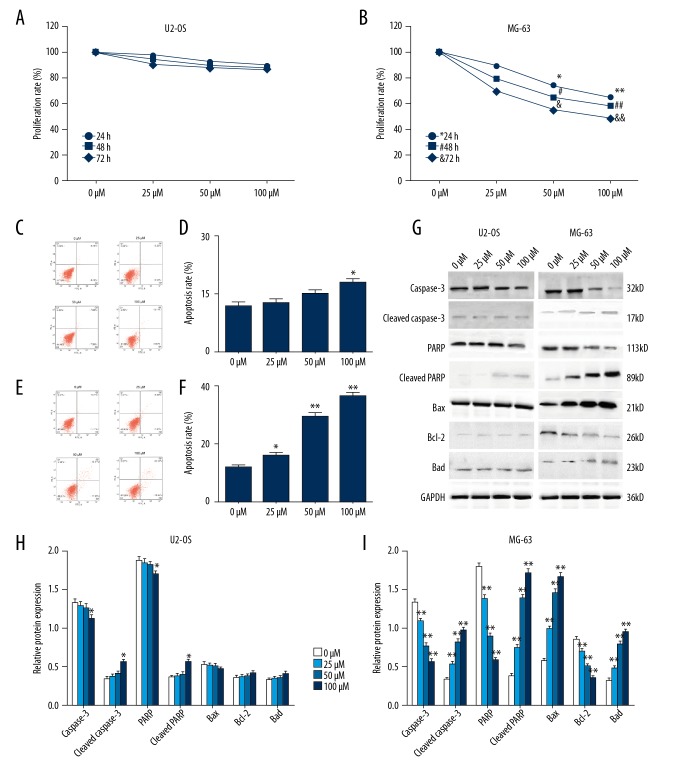
Figure 1.
Inhibition of cell viability and increased apoptosis in estrogen receptor (ER)-positive osteosarcoma cell proliferation by calycosin. Cell viability was detected by the MTT assay after estrogen receptor (ER)-negative U2OS cells (A), and ER-positive MG-63 cells (B), were incubated with increasing concentrations of calycosin (0, 25, 50, and 100 mM) for 24, 48, and 72 h respectively. Flow cytometry determined the cell apoptosis rate after U2-OS cells (C), and MG-63 cells (E), were treated with calycosin (0, 25, 50, and 100 μM) for 72h. Apoptosis rates of U2-OS cell (D), and MG-63 cells (F), are summarized in the bar graph (right). The expression of apoptosis-related proteins by U2-OS cells and MG-63 cells was detected by Western blot (G) after 72h of treatment of calycosin (0, 25, 50, and 100 μM). Variations of protein expression in U2-OS cells (H) and MG-63 cells (I) are summarized in the histograms. Expression levels of proteins are shown as the relative intensities of bands compared to the control (GADPH). All results are representative of three independent experiments. All the data are expressed as the mean ±SD and analyzed with one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) t-test. P<0.05 is shown by * and P <0.01 is shown by **.
Flow cytometry performed after the MG-63 cells and U2-OS cells were treated with calycosin (0, 25, 50, and 100 μM) demonstrated that U2-OS cells showed no significant change of apoptotic rate except in the subgroup of MG-63 cells treated with 100 μM calycosin (Figure 1C, 1D), while a significant increase in the rate of apoptosis was observed in MG-63 cells after treatment with calycosin at all concentrations (Figure 1E, 1F). These findings showed that calycosin enhanced the apoptosis rate of MG-63 cells in a dose-dependent manner.
These results support the view that ER-associated mechanisms were involved in the effects of calycosin on the regulation of osteosarcoma cell growth. The results of the Western blot supported this conclusion. After the U2-OS cells and MG-63 cells had been treated with calycosin (0, 25, 50, and 100 μM) for 72h, the expression levels of apoptosis-related proteins were detected, including caspase-3, cleaved caspase-3, PARP, p-PARP, Bax, Bcl-2, and Bad. The MG-63 cells expressed a dose-dependent increase in levels of cleaved caspase-3, p-PARP, Bax, and Bad, and a dosage-dependent decrease in levels of Bcl-2 in all dose concentrations. However, in U2-OS cells, only caspase-3, PARP, cleaved caspase-3 and p-PARP showed significant expression decrease in the subgroup treated with 100 μM calycosin (Figure 1G–1I). These findings support the role of calycosin-induced apoptosis of MG-63 cells osteosarcoma cells being by ER-related-mechanisms.
Calycosin-induced apoptosis in osteosarcoma MG-63 cells via the PI3K/AKT/mTOR pathway
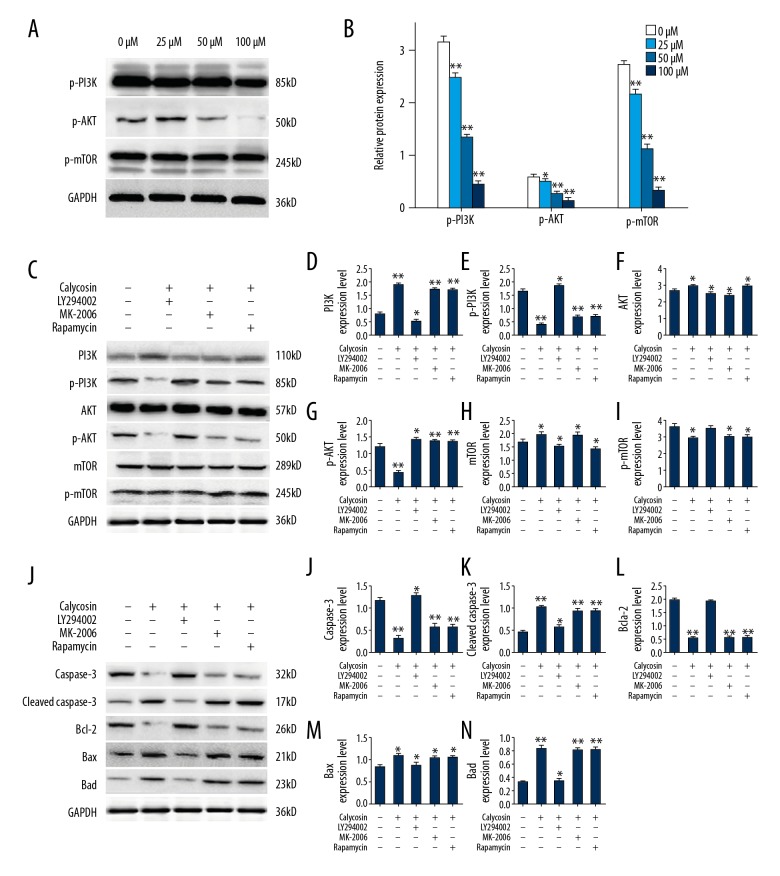
The Western blot assay was conducted to examine the molecular mechanism of calycosin-induced apoptosis in osteosarcoma MG-63 cells. After 72 h of exposure to increasing concentrations of calycosin (0, 25, 50, and 100 μM), the expression levels of phosphorylated- PI3K, AKT, and mTOR in MG-63 cells were significantly decreased in a dosage-dependent manner (Figure 2A, 2B). These results showed that calycosin-induced apoptosis in MG-63 was regulated by the PI3K/AKT/mTOR pathway. When inhibitors of these proteins were applied to clarify the role of calycosin treatment on MG-63 cells further, the results showed that the inhibitor of PI3K (LY294002) reduced phosphorylation of PI3K, AKT, and mTOR (Figure 2C–2I), but also the expression of apoptosis-related proteins (Figure 2J–2O). However, inhibitors of AKT (MK-2206) and mTOR (rapamycin) could only affect expression levels of phosphorylation of AKT or mTOR without changing activities of upstream and downstream proteins. Therefore, these findings support that calycosin-induced apoptosis in MG-63 cells mainly depend on suppressing the activation of PI3K.
Figure 2.
Calycosin-induced apoptosis regulation by the PI3K/AKT/mTOR pathway. Expression levels of p-PI3K, p-AKT and p-mTOR were detected by Western blot (A), after 72 h of treatment with increasing concentrations of calycosin (0, 25, 50, and 100 μM) and the results are summarized in the bar graph (right) (B). Expression of PI3K/AKT/mTOR pathway proteins (C), and apoptosis-related proteins (J), were determined after inhibitor assays. Specifically, expression levels of PI3K (D), AKT (F), mTOR (H), p-PI3K (E), p-AKT (G), p-mTOR (I), caspase-3 (K), cleaved caspase-3 (L), Bcl-2 (M), Bax (N), and Bad (O), are summarized in the histograms. All protein expression levels are presented as relative intensities of bands compared with the control band (GADPH). Variations of protein expression are summarized in the histograms. Results are representative of three independent experiments. All the data are expressed as the mean ±SD and analyzed with one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) t-test. P<0.05 is shown by * and P <0.01 is shown by **.
Calycosin affected tumor growth in MG-63 tumor-bearing nude mice
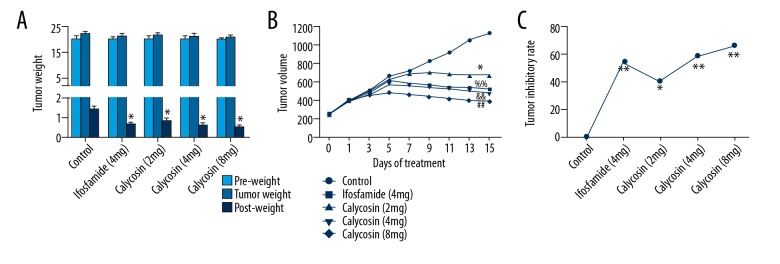
The effect of calycosin in MG-63 tumor-bearing nude mice after treatment with calycosin or ifosfamide did not result in a significant change in body weight of the mice, but the tumor volume significantly decreased in the treatment groups after four day of treatment. However, tumor volume declined in a time-dependent and dose-dependent manner in the calycosin treatment groups after five days of treatment (Figure 3A, 3B). Also, the inhibition ratio of tumor growth also showed a dose-dependent increase in the calycosin treatment groups, and 8 mg/kg calycosin treatment was more effective than 4 mg/kg ifosfamide treatment, according to the calculated tumor inhibitory ratio (Figure 3C). These finding showed that calycosin could inhibit MG-63 mouse xenograft tumor growth in vivo.
Figure 3.
The effect of calycosin treatment on body weight and tumor growth in MG-63 tumor bearing nude mice. The average body weight of each group of mice was significantly reduced following treatment with calycosin (A). The calculated tumor volume significantly declined after 5 days of treatment (B). Calculated inhibition ratios of tumor growth were significantly increased by all treatments (C). Results are representative of three independent experiments. All the data are expressed as the mean ±SD and analyzed with one-way analysis of variance (ANOVA) or two-way ANOVA. P<0.05 is shown by * and P<0.01 is shown by **, &&, %%, or ##.
Calycosin caused apoptosis of MG-63 tumor cells by suppressing protein expression of PI3K, AKT, and mTOR
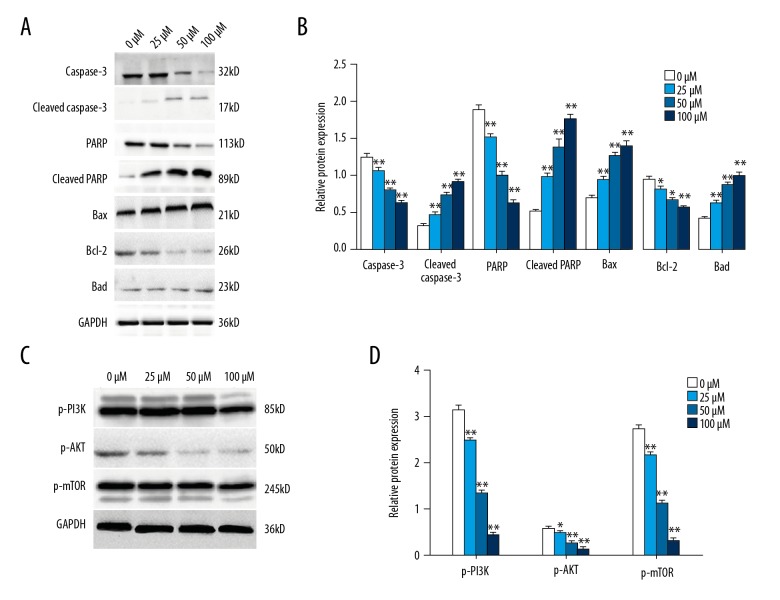
Apoptosis of cells in MG-63 osteosarcoma mouse xenografts was determined by assessing the expression of apoptosis-related proteins using Western blot. Expression levels of caspase-3, PARP, Bax, Bcl-2, and Bad in MG-63 solid tumor tissue cells of each group were detected. Cleaved caspase-3, p-PARP, Bax, and Bad were significantly overexpressed in the ifosfamide-treated group and in the calycosin-treated group in a dose-dependent manner, while the expression levels of the anti-apoptosis factor, Bcl-2 was significantly decreased in all treatment groups. This finding supported that calycosin could induce apoptosis in MG-63 solid tumor cells in a dose-dependent manner (Figure 4A, 4B). However, the results of Western blot analysis showed that expression levels of PI3K, AKT, and mTOR in MG-63 mouse xenograft tumor tissue were down-regulated after calycosin treatment in a dosage-dependent manner (Figure 4C, 4D). This finding also supported a role for calycosin treatment in inducing apoptosis in MG-63 solid tumors via PI3K/AKT/mTOR pathway.
Figure 4.
Calycosin treatment increased apoptosis-related protein expression in mouse MG-63 tumor xenografts through the PI3K/AKT/mTOR pathway. Western blot assay determined the expression levels of apoptosis-related proteins in mouse MG-63 tumor xenografts after calycosin treatment (0, 25, 50, and 100 μM) (A). Variations of protein expression are summarized as a bar graph (B). Expression levels of p-PI3K, p-AKT and p-mTOR were determined by Western blot after 72h treatment of 100 μM calycosin (C). Variations of protein expression are summarized as a bar graph (D). Expression levels of proteins are shown as the relative intensities of bands compared to the control (GADPH). Results are representative of three independent experiments. All the data are expressed as the mean ±SD and analyzed with one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) t-test. P<0.05 is shown by * and P<0.01 is shown by **.
Discussion
Osteosarcoma is the most common malignant disease of bone in adolescence [1]. Current treatments for osteosarcoma do not necessarily improve patient symptoms, quality of life, or survival [2–5]. There remains a need for more effective and less toxic therapies that induce apoptosis in tumor cells [7]. Calycosin is a Chinese herbal medicine and a bioactive phytoestrogen isoflavone that contains estrogen-like isoflavones [8,9]. It has been reported that calycosin showed estrogenic activity and anti-tumor effects on several cancers by inducing apoptosis of tumor cells in vitro and in vivo [10–12]. Therefore the aim of the present study was to investigate the effects of calycosin on apoptosis of estrogen receptor (ER)-positive and ER-negative human osteosarcoma cell lines and tumor xenografts in mice. The findings showed that calycosin induced apoptosis of cells of the ER-positive osteosarcoma cell line, MG-63, occurred via the PI3K/AKT/mTOR pathway, with these effects being mainly due to PI3K.
In this study, calycosin treatment significantly reduced cell viability and increased the apoptosis rate in ER-positive osteosarcoma MG-63 cells as shown by the MTT assay and flow cytometry assay results, with no impact on cell proliferation or apoptosis of ER-negative osteosarcoma U2-OS cells. This finding agreed is supported by several previously published studies. Chen et al. showed that calycosin could inhibit growth and enhance apoptosis in ER-positive breast cancer cell lines, based on two ER-positive cell lines (MCF-7 and T-47D) and two ER-negative cell lines (MDA-231 and MDA-435) [11]. A further study by Chen et al. showed that calycosin-induced apoptosis in human colorectal cancer cells via the ERβ/miR-17 signaling pathway [12].
In the present study, apoptosis-related proteins were detected by Western blot. The results confirmed that calycosin could more effectively induce apoptosis in ER-positive MG-63 osteosarcoma cells compared with ER-negative U2-OS cells. These proteins included caspase-3, cleaved caspase-3, PARP, phosphorylated PARP, Bax, Bad, and Bcl-2, which have all been previously reported to be closely associated with cell apoptosis [16–18]. These results support that calycosin-induced apoptosis in osteosarcoma might occur through an ER-related mechanism. Furthermore, according to previous studies, current technology is able to transfer estrogen receptor genes to osteosarcoma cells and have shown that the expression of the transferred gene is stable [19,20]. With the development of advanced technologies, the role of calycosin and its effects on osteosarcoma could be developed further.
Previously reported studies on the mechanisms of the antitumor effects of calycosin exist [11–13,21–23]. Among these previously reported studies, the PI3K/AKT signaling pathway has been shown to have a role in the functional mechanism of the effects of calycosin. Chen et al. reported that calycosin enhanced apoptosis in ER-positive breast cancer cells via ERβ-induced inhibition of IGF-1R, as well as regulation of PI3K/AKT and MAPK pathways [11]. Zhao et al. published similar findings on the mechanism of the antitumor role of calycosin on colorectal cancer (CRC) cells [13]. The results of the Western blot assay in the present study showed similar results, which supported a possible role for calycosin-induced inhibition of tumor cell proliferation and that the increase in tumor cell apoptosis was dependent on, or involved in, the PI3K/AKT/mTOR pathway as supported by the Western blot findings, as shown in Figure 3A. The PI3K/AKT/mTOR pathway is an important survival pathway that is frequently altered in cancer, the activation of which contributes to initiation and maintenance of tumors and to the resistance to many cancer treatments [24,25].
Therefore, it is possible that suppressing this signaling pathway might be a promising approach to treat malignancy. In this study, the inhibitors of PI3K, AKT and mTOR were used in further experiments to determine the most important protein involved in the calycosin functional mechanism in osteosarcoma, which indicated that the inhibitor of PI3K (LY294002) could interfere with not only with the expression of PI3K but also the expression of downstream proteins and apoptosis-related proteins. Therefore, a further conclusion is that PI3K is the pivotal protein involved in the role of the antitumor effects of calycosin in osteosarcoma. There are other possible mechanisms of the role of calycosin on osteosarcoma cells. For example, calycosin has previously been shown to decrease the expression of IκBα, NF-κB p65 and cyclin D1 in osteosarcoma, thereby inducing apoptosis [24].
In this study, the role of MG-63 mouse osteosarcoma xenograft tumors in nude mice were studied and the findings showed that calycosin could suppress tumor growth and initiate apoptosis of osteosarcoma in vivo. These preliminary findings support that calycosin requires further study to determine whether it could be a future therapy for patients with osteosarcoma The findings from Western blot of MG-63 mouse xenograft tumors showed that treatment with calycosin exerted its antitumor effect via the PI3K/AKT/mTOR pathway, which might provide a basis for further studies on the pharmacological mechanism of calycosin.
Conclusions
The findings of this study have shown that calycosin, a phytoestrogen isoflavone, when administered to the estrogen-receptor (ER)-positive human osteosarcoma cell line, MG-63, in vitro, and in mouse MG-63 xenografts in vitro, had anti-proliferative and pro-apoptotic effects. At the molecular level, calycosin achieved these effects through the PI3K/AKT/mTOR signaling pathway, in which PI3K was the key protein. Further studies are needed to determine whether calycosin may be a novel therapeutic agent that can be used individually, or as part of combined therapy, in the treatment of osteosarcoma.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari S, Serra M. An update on chemotherapy for osteosarcoma. Expert Opin Pharmacother. 2015;16(18):2727–36. doi: 10.1517/14656566.2015.1102226. [DOI] [PubMed] [Google Scholar]
- 3.Bielack SS, Hecker-Nolting S, Blattmann C, Kager L. Advances in the management of osteosarcoma. F1000Res. 2016;25(5):2767. doi: 10.12688/f1000research.9465.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47(1):283–92. doi: 10.1016/j.ocl.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Yang J, Wang Y, et al. Survival and prognostic factors in Chinese patients with osteosarcoma: 13-year experience in 365 patients treated at a single institution. Pathol Res Pract. 2017;213(2):119–25. doi: 10.1016/j.prp.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Li T, Kon N, Jiang L, et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–83. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson MA, Kouzarides T. Cancer epigenetics: From mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Booth NL, Piersen CE, Banuvar S, et al. Clinical studies of red clover (Trifolium pratense) dietary supplements in menopause: A literature review. Menopause. 2016;13:251–64. doi: 10.1097/01.gme.0000198297.40269.f7. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Zhao X, Ye Y, et al. Estrogen receptor beta-mediated proliferative inhibition and apoptosis in human breast cancer by calycosin and formononetin. Cell Physiol Biochem. 2013;32:1790–97. doi: 10.1159/000356612. [DOI] [PubMed] [Google Scholar]
- 10.Qiu R, Ma G, Zheng C, et al. Antineoplastic effect of calycosin on osteosarcoma through inducing apoptosis showing in vitro and in vivo investigations. Exp Mol Pathol. 2014;97:17–22. doi: 10.1016/j.yexmp.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Hou R, Zhang X, et al. Calycosin suppresses breast cancer cell growth via ERβ-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways. PLoS One. 2014;9(3):e91245. doi: 10.1371/journal.pone.0091245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Zhao X, Li X, Wu Y. Calycosin induces apoptosis by the regulation of ERβ/miR-17 signaling pathway in human colorectal cancer cells. Food Funct. 2015;6:3091–97. doi: 10.1039/c5fo00374a. [DOI] [PubMed] [Google Scholar]
- 13.Zhao X, Li X, Ren Q, et al. Calycosin induces apoptosis in colorectal cancer cells, through modulating the ERβ/MiR-95 and IGF-1R, PI3K/Akt signaling pathways. Gene. 2016;591(1):123–28. doi: 10.1016/j.gene.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang Z. The research progress of estrogen receptors expression and therapeutic effects in osteosarcoma. Journal of Modern Oncology. 2013;6:45–53. [Google Scholar]
- 15.Davis VL, Couse JF, Gray TK, Korach KS. Correlation between low levels of estrogen receptors and estrogen responsiveness in two rat osteoblast-like cell lines. J Bone Mineral Res. 2017;9(7):983–91. doi: 10.1002/jbmr.5650090705. [DOI] [PubMed] [Google Scholar]
- 16.Deterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2013;22(53):8543–67. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 17.Patel T, Gores GJ, Kaufmann SH. The role of proteases during apoptosis. FASEB J. 1996;10(5):587–97. doi: 10.1096/fasebj.10.5.8621058. [DOI] [PubMed] [Google Scholar]
- 18.Cory S, Adams JM. The Bcl-2 family: Regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 19.Monroe DG, Secreto FJ, Khosla S, Spelsberg TC. The classical estrogen receptor transcriptional pathway. Clin Rev Bone Miner Metab. 2016;4(2):129–40. [Google Scholar]
- 20.Clay WC, Condreay JP, Moore LB, et al. Recombinant baculoviruses used to study estrogen receptor function in human osteosarcoma cells. Assay Drug Dev Technol. 2003;1:801–10. doi: 10.1089/154065803772613435. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Liu QH, Liu CL, Lin L. Calycosin induces apoptosis in human ovarian cancer SKOV3 cells by activating caspases and Bcl-2 family proteins. Tumor Biol. 2015;36(7):5333–39. doi: 10.1007/s13277-015-3194-8. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Zhao X, Li X, Wu Y. Calycosin induces apoptosis by the regulation of ERβ/miR-17 signaling pathway in human colorectal cancer cells. Food Funct. 2015;6:3091–97. doi: 10.1039/c5fo00374a. [DOI] [PubMed] [Google Scholar]
- 23.Qiu R, Ma G, Zheng C, et al. Antineoplastic effect of calycosin on osteosarcoma through inducing apoptosis showing in vitro and in vivo investigations. Exp Mol Pathol. 2014;97:17–22. doi: 10.1016/j.yexmp.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Lopiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: Effective combinations and clinical considerations. Drug Resist Updat. 2008;11(1–2):32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgensztern D, Mcleod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2015;16:797–803. doi: 10.1097/01.cad.0000173476.67239.3b. [DOI] [PubMed] [Google Scholar]