Abstract
Introduction
We investigated the effects of mesenchymal stem cells (MSCs) on cutaneous wound healing in pigs in order to develop new therapies to enhance wound healing in humans.
Methods
We cultured bone marrow cells from the femurs of male pigs, and the multipotency of these cells were then confirmed. The characteristics of the cultured cells were determined by flow cytometric analyses. The MSCs were injected intradermally into the skin of pigs as auto-transplantation, and linear full-thickness incisional wounds were made through the injected area immediately afterward.
Results
The MSCs were found to be positive for SWC3a, CD44, SLA class I, CD29, CD44H, and CD90. At 28 days post-surgery, wounds treated with MSCs had healed well, with only very fine scars visible macroscopically. Histologically, collagen architecture was thick and elastic fibers appeared in the wounds. Histomorphologic scale analysis demonstrated that the wounds treated with MSCs scored better than the controls. Significantly larger fibroblasts were observed in the wounds treated with MSCs than controls.
Conclusion
These results indicate that transplantation of MSCs causes wounds to heal almost completely, possible indicating regeneration to normal skin. We hypothesize that the transplantation protocol described in this study may also be applicable to human wound healing.
Keywords: Mesenchymal stem cells, Bone marrow, Pig, Cutaneous wound healing, Wound regeneration, Collagen fiber
Highlights
-
•
MSCs contribute to skin regeneration in acute cutaneous wounds of pigs.
-
•
Cutaneous wounds of pig transplanted with bone marrow-derived MSCs healed with very fine scars, and collagen architectures were similar to normal dermis.
-
•
We hypothesize that the transplantation of MSCs may also be applicable to human wound healing, because cutaneous of pigs are an excellent model for human skin.
1. Introduction
Recent reports have shown that bone marrow-derived mesenchymal stem cells (MSCs) are multipotent cells, and can be induced in vitro and in vivo for the regeneration and repair of damaged tissue in almost all major organs of the body, including the heart, brain, lung, liver, kidney and eyes [1], [2]. MSCs have also been investigated as a potential therapy in cutaneous wound healing. For stem cell transplantation therapy, MSCs and embryonic stem cells (ESCs) have been most common cell types used, and they have been effective in promoting wound healing when injected into wound sites, alone or in combination with biological materials [3], [4], [5]. However, the objectives of most of these studies were to increase acceleration of wound closure for chronic non-healing wounds, such as diabetic ulcers or burns.
The biological response to wounds in higher organisms falls into two categories: regeneration and wound repair [6]. We have studied regeneration in wounds with the aim of suppressing scar formation after surgery. It is known that the fetal skin of mice possesses a regenerative activity before embryonic day 13, and that dermal mesenchymal cells are vital in this process [7]. Accordingly, the local application of multipotent cells such as fetal mesenchymal cells on cutaneous wounds may result in wounds that heal without scars.
In a previous study, we transplanted rat MSCs into the incisional cutaneous wounds of rats [8] and found that wounds transplanted with rat MSCs had healed well, with very fine scars macroscopically. Significant differences were observed between the controls and the wounds transplanted with MSCs. These results indicate that wounds transplanted MSCs healed and resulted in skin that appeared to be essentially normal; thus, the process we induced was essentially regeneration of the structure of the skin.
In order to test the clinical application of our skin regeneration process, we investigated cutaneous wound healing in pigs. Pigs are often chosen to evaluate the biology of cutaneous wounds because they are an excellent model for human skin; they possess high similarity with humans in terms of anatomy, physiology, the wound healing response and chromosomal structural homology [9].
In the present study, we determined that acute cutaneous wounds in pigs treated with MSCs healed leave only very fine scars, and that MSCs can contribute to skin regeneration in such wounds. We hypothesize that further research may show similar effects in humans.
2. Materials and methods
2.1. Bone marrow cell preparation
This study was conducted according to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health. The experimental protocols used for pigs were approved by the Animal Care and Use Committee of Showa University School of Medicine. Surgical treatments were performed under general anesthesia. Bone marrow tissue was aspirated from the femurs of two 4-month-old male L.W.D. pigs. Bone marrow tissue samples were plated in plastic dishes. The nonadherent cell population was removed after 24 h and the adherent layer was washed once with fresh media; the cells were then continuously cultured for 4 weeks. Medium was completely replaced every 3 days. When the dishes became nearly confluent, the adherent cells were released from the dishes with 0.25% trypsin-EDTA (Sigma, USA), at 37 °C for 5 min. Trypsin digestion was stopped by the addition of fetal bovine serum (FBS; Sigma, USA). Cells were collected, centrifuged, resuspended in complete medium, split 1:3, and seeded onto fresh plates. Three to four passages were performed. The cells were routinely cultured in a complete medium consisting of Dulbecco's modified Eagle's medium (DMEM; Sigma, USA) containing 20% FBS and 100 U/mL penicillin-streptomycin (Sigma, USA) at 37 °C in a humidified atmosphere of 5% CO2.
2.2. Quantitation of adipogenesis and osteogenesis
To induce adipocyte differentiation, cells were cultured in a medium consisting of DMEM plus 10% FBS supplemented with 5 μg/ml insulin and 10−9 M dexamethasone for 21 days. To induce an osteochondrogenic phenotype, cells were cultured in a medium consisting of DMEM containing 10% FBS, 10 mM α-glycerophosphate, 50 μg/ml ascorbic acid, and 10−7 M dexamethasone [10]. Cells were cultured for 14 days.
The cultures were examined daily using a microscope (Power BX-51, Olympus, Japan). For quantitation of adipocytes, cultures were fixed with formalin for 10 min at 4 °C and stained with oil red O. For quantitation of osteocytes, cultures were fixed with acetone for 10 min at room temperature, and Von Kossa staining was performed.
2.3. Flow cytometric analysis of MSCs
Flow cytometric analysis was performed with an FC 500 cytometer (Beckman-Coulter, Hialeah, FL, USA). MSCs were harvested by brief trypsinization, washed twice in ice-cold blocking buffer (PBS with 1% FBS), and then incubated with primary antibodies (2 μg/5 × 105 cells/0.2 ml) for 30 min in ice-cold blocking buffer and immunofluorescent secondary antibodies. Antibodies against SWC3a (monocyte/granulocyte; BD PharMingen, San Diego, CA), CD44 (Pgp-1; BD PharMingen), SLA-class I (MHC class I; BD PharMingen), SLA-DR (MHC class II), CD29 (integrin β1; BD PharMingen), SLA-DQ (MHC class II; BD PharMingen), CD31 (PECAM-1; BD PharMingen), and CD90 (Thy-1; PE-labeled, BD PharMingen) were used. The antibody against CD44H (Pgp-1, Hermes antigen; BD PharMingen) was FITC-labeled, and FITC-labeled nonimmune isotype-matched antibody was used as a negative control [11].
2.4. Cell transplantation into incisional wounds of pig
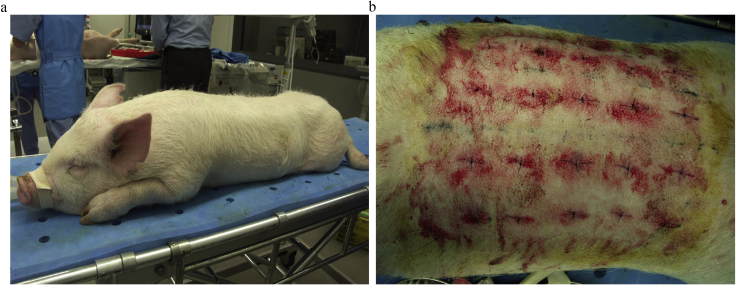
Two of the five-month-old male L.W.D. pigs that had been subjected to bone marrow aspiration 28 days previously were anesthetized with propofol. Their dorsal hair was shaved, and 20 injection points were chosen 7 cm apart (Fig. 1a). The bone marrow-derived, plastic-adhesive cells that had been passaged 3–4 times were suspended, collected, and centrifuged, and 1.5 × 107 cells were resuspended in 200 μl of PBS. These MSCs were injected intradermally into the 20 points of the dorsal skin of each pig as auto-transplantation. One-mL syringes with 23-gauge needles were used for injection. The same volume of PBS was injected intradermally as controls. Linear full-thickness incisions, each 30 mm long, were made in the dorsal skin of pigs through the injection points immediately after injection. Injection was performed before surgical incision in order to prevent cell leakage out of the wounds. The wounds were closed with 4-0 nylon sutures; no wound dressing was applied and no antibiotics were used (Fig. 1b).
Fig. 1.
Cell transplantation into incisional wounds of pigs. a. Bone marrow tissue was aspirated from the pelvises of two 4-month-old male L.W.D. pigs under general anesthesia. b. Cultured MSCs were suspended and injected intradermally into 20 points of the dorsal skin of each pig. Linear full-thickness incisions were created through the cell injected points. The wounds were closed with 4-0 nylon sutures.
Macroscopic observation of the wounds was documented on day 28, and the wounds were harvested on the same day.
2.5. Macroscopic findings and scoring of incisional wounds
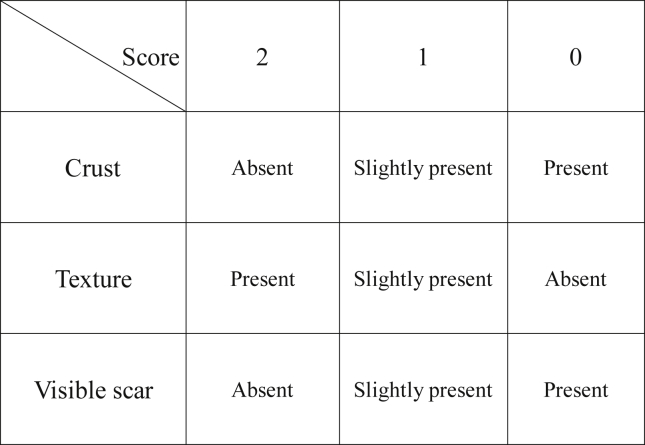
The wounds were photographed with a digital camera (Konica Minolta DiMAGE A1, Japan) and a dermascope (USB microscopeM2, Scalar, Japan). Based on the images, scoring of wounds was performed according to the presence of crust, texture and visible scarring by three specialists in plastic surgery (Fig. 2). The maximum score is 6 points, which indicates that the wound healed very well.
Fig. 2.
Macroscopic assessment scale. Based on the images of digital camera and dermascope, scoring of wounds was performed according to the presence of crust, texture and visible scarring by three specialists in plastic surgery.
2.6. Sample processing
Each wound sample was harvested for examination as a square of 4 × 3 × 1.5 cm. The harvested tissues were bisected and half of each sample was fixed with 10% neutral buffered formalin solution. Briefly, 3-μm paraffin sections were prepared from the fixed tissue in a standard fashion. The tissues were observed in detail using Masson trichrome and Elastica van Gieson staining. The remainder of each sample was dipped into OCT compound (Sakura, Japan) and frozen in liquid nitrogen. Cryosections 7 μm in thickness were made using a cryostat (Leica, Germany), and immunostaining was performed. The sections were incubated for 1 h at 37 °C with the primary antibodies of anti-human muscle actin (α-SMA) mouse monoclonal antibody (1:200, DAKO North America, Inc., USA) or anti-human vimentin rabbit polyclonal antibody (1:200, Bethyl Laboratories Inc., USA). The samples were incubated with the secondary antibody for 30 min at 37 °C with Simple stain MAX PO MULTI (for mouse and rabbit primary antibodies; Nichirei, Japan). The sections were stained with the Liquid DAB+ Substrate Chromogen System (DAKO). A microscope was used for observation of the slides.
2.7. Evaluation with Masson trichrome staining and Elastica van Gieson staining
The samples were examined histologically, and the appearance of the rate ridges and collagen fibers were assessed after Masson trichrome staining. In order to compare the orientation of the collagen fibers, one field of 640,000 μm2 was observed in each wound. Histologic assessment was done based on the method of Beausang et al. [12] (Fig. 3). For evaluating epidermis, the presence of rate ridge was scored. For evaluating dermis, three collagen fiber characteristics were determined: collagen fiber orientation, density, and maturity. The mean scores were calculated and compared between the control and the MSCs. Scores range from 0 to 32, and lower scores indicate nearly normal skin histology. The elastic fibers of the samples were examined after Elastica van Gieson staining.
Fig. 3.
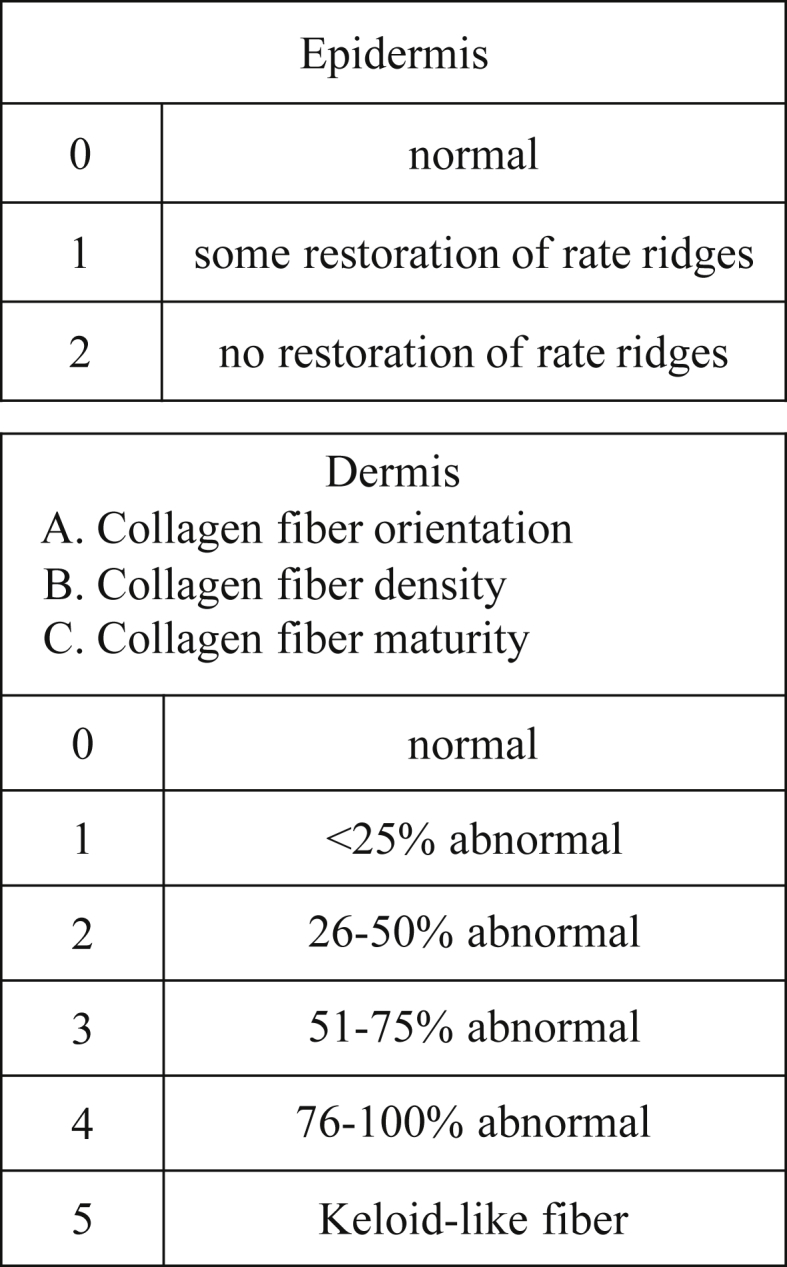
Histologic assessment scale. Histologic assessment was done based on the method of Beausang et al. [12]. For evaluating epidermis, the presence of rate ridge was scored. For evaluating dermis, collagen fiber orientation, density, and maturity were determined.
2.8. Evaluation with immunostaining
Anti-vimentin antibody staining was performed to evaluate fibroblasts, and anti-αSMA antibody staining was performed to evaluate myofibroblasts. Four fields (1 field = 200 μm2) in each wound was observed, and the number of fibroblasts and myofibroblasts were counted. The mean value of cell counts for each specimen in the four fields was calculated and compared between the control and the MSC-treated tissue.
2.9. Statistical analysis
Statistical significance of scoring of macroscopic and histologic findings was determined by t-test between the control and the MSC-treated animals. We used the Mann–Whitney U test to compare the score values for myofibroblast and fibroblast count between the control and the MSC-treatment groups. All statistical analyses were performed using Graph Pad Prism 5 (GraphPad Software, La Jolla, CA, USA) SPSS 20.0 (IBM Corp, NY, USA). Data are expressed as mean ± standard deviation (mean ± SD). For all cases, a p-value of <0.05 (one-sided) was considered as statistically significant.
3. Results
3.1. Quantitation of adipogenesis and osteogenesis
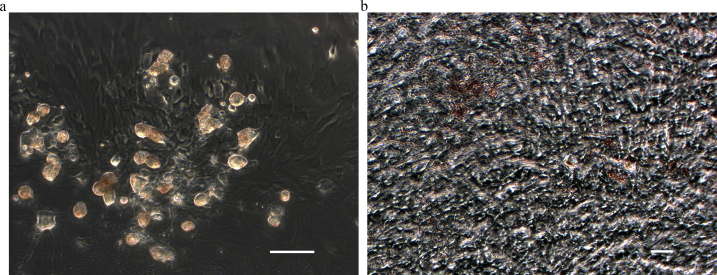
To determine whether the cells that adhered to the plastic plates could be induced to differentiate into adipocytes, they were cultured as described in Materials and Methods and stained with oil red O. A large number of cells, each with several clear droplets were observed, and the droplets in the cytoplasm of these cells stained positive with oil red O (Fig. 4a). These results indicate the presence of lipid, and that the cells with these droplets are adipocytes.
Fig. 4.
Quantitation of adipogenesis and osteogenesis. Bone marrow-derived plastic-adhesive cells that had been passaged 3–4 times were induced to differentiate for quantitation of adipocytes and osteocytes. a. The droplets in the cytoplasm were positive for oil red O staining: these are adipocytes (Magnification ×400; scale bar = 10 μm). b. Diffuse deposits of calcium were found by Von Kossa staining: these are osteocytes (Magnification ×400; scale bar = 10 μm).
The cells were also tested for osteogenetic by Von Kossa staining differentiation after the treatment described in Materials and Methods. Diffuse deposits of calcium were found in almost all cells, suggesting that osteocytes were present (Fig. 4b).
Together, these results confirm the multipotency of the cells we prepared from bone marrow-derived cells of pigs.
3.2. Phenotype of MSCs from pigs
The characteristics of the cultured MSCs were confirmed by flow cytometric analyses using purified or conjugated antibodies. The cells were positive for SWC3a, CD44, SLA class I, CD29, CD44H, and CD90, and negative for CD31 and SLA-DQ (Table 1).
Table 1.
Flow cytometric analysis of cultured MSCs.
| MSCs | MSCs | MSCs | |||
|---|---|---|---|---|---|
| SWC3a-PE | + | CD44-FITC | + | SLAClassI-PE | ++ |
| CD29-PE | ++ | CD44H-PE | ++ | SLA DR-PE | − |
| CD31-PE | − | CD90-PE | ++ | SLA DQ-PE | − |
MSCs are positive for SWC3a, CD44, SLA class I, CD29, CD44H, and CD90, and negative for CD31 and SLA-DQ. Scores of peak intensity, compared with isotype controls. “++”: strongly positive (10-fold and above of the isotype control), “+”: weakly positive (less than 10 times, and twice and above of the isotype control), “−”: negative (less than twice of the isotype control).
3.3. Macroscopic findings and scoring of incisional wounds
After MSCs were transplanted, full-thickness incisional wounds were made and sutured. At 28 days after surgery, wounds were photographed with a digital camera and dermascope.
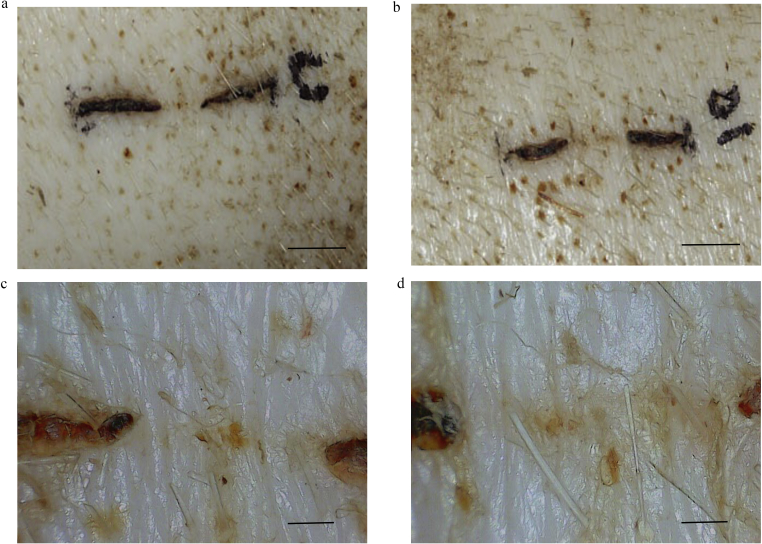
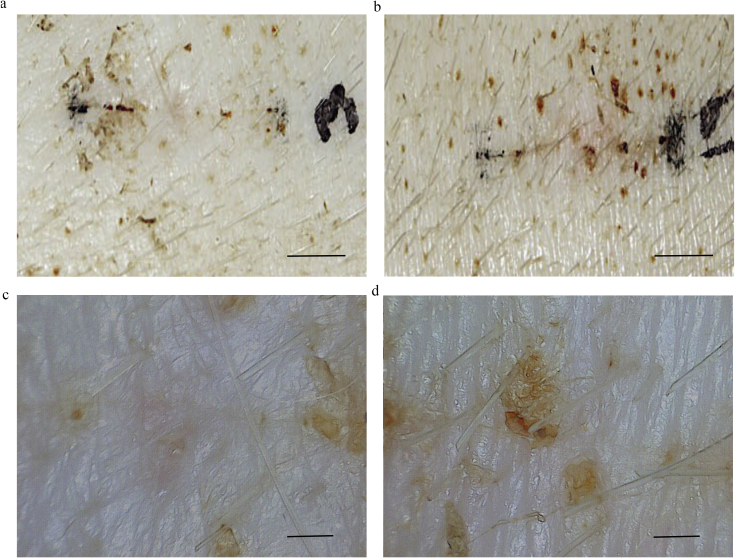
The macroscopic appearance of the wounds of the controls were examined 28 days after wounds were made (Fig. 5a and b). In the 30× dermascope images, crust remained and epithelization progressed only to the center of wounds (Fig. 5c and d). In contrast, wounds with MSCs had already healed very well, and epithelization was almost complete (Fig. 6a and b). In dermascope images, the skin texture was re-created and the wounds were difficult to distinguish from the surrounding normal skin (Fig. 6c and d).
Fig. 5.
Macroscopic findings of control wounds. PBS was injected for the control wounds, and the wounds were photographed with a digital camera and dermascope (×30) 28 days after operation. The crust remained and epithelialization progressed only in the center of wound. a. Image from digital camera (Scale bar = 1 cm). b. Image from digital camera (Scale bar = 1 cm). c. Image from dermascope of 5a. (Scale bar = 1 mm). d Image from dermascope of 5b. (Scale bar = 1 mm).
Fig. 6.
Macroscopic findings of wounds transplanted MSCs. The wounds transplanted with MSCs were photographed with a digital camera and dermascope (×30) 28 days after operation. The wounds had already healed very well and epithelialization had almost completed. a. Image from digital camera (Scale bar = 1 cm). b. Image from digital camera (Scale bar = 1 cm). c. Image from dermascope of 6a. (Scale bar = 1 mm). d. Image from dermascope of 6b. (Scale bar = 1 mm).
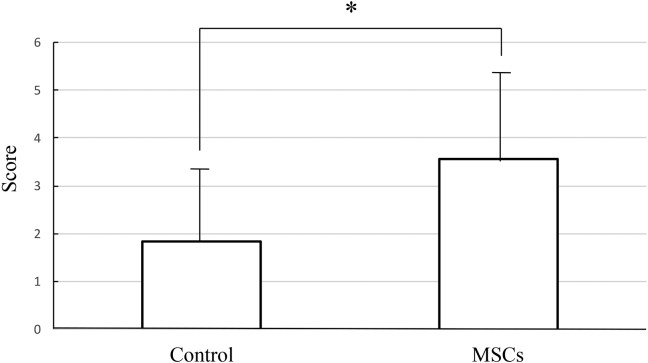
Based on the results described above, each wound was scored. Fig. 7 shows the results of macroscopic assessment of the wounds at 28 days post-surgery. The mean score of controls was 1.84 and MSC-treated animals was 3.55. The MSC group had significantly higher scores than the controls. These results show that wounds transplanted with MSCs healed rapidly and appeared similar to normal skin.
Fig. 7.
Macroscopic scores of incisional wounds. Macroscopic assessment of the wounds at 28 days after surgery. The mean score of control was 1.84 and MSCs was 3.55. The MSCs group had significantly higher scores than the control. Results show that MSC-transplanted wounds healed rapidly and similar to normal skin. (Control: n = 17, MSCs: n = 17, mean ± SE, *p < 0.01, paired t-test).
3.4. Histomorphologic evaluation of wounds
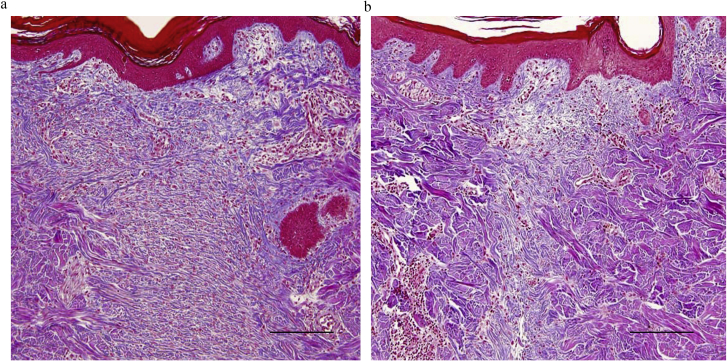
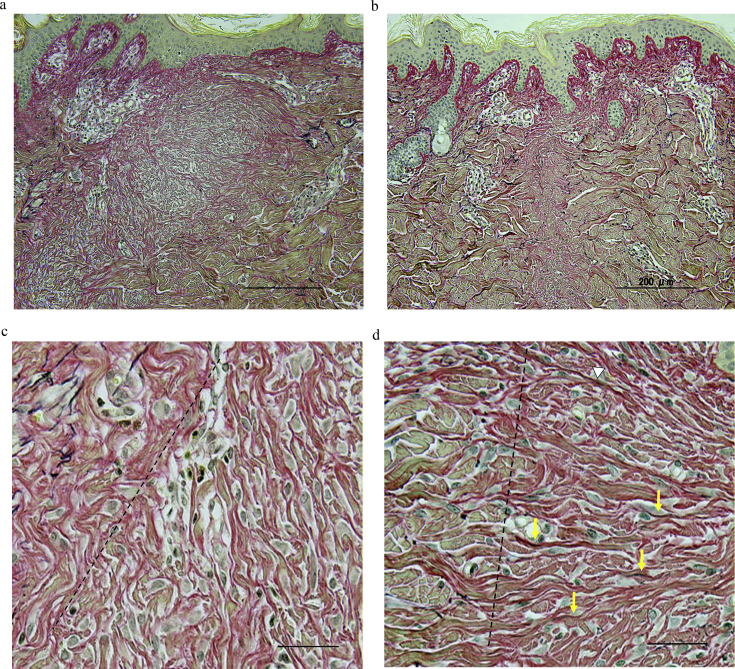
The samples were examined histologically after Masson trichrome staining. In the control group, the wounds were wide and collagen fibers were thin, compact, and disoriented (Fig. 8a). The MSC-treatment group showed thicker and well-oriented collagen architecture in the wounded site compared to the control (Fig. 8b). There was no significant difference between the groups when the rate ridges were examined.
Fig. 8.
Histology images of Masson trichrome staining of the wounds. a. In the control group, the wounds are wide and collagen fibers are thin, compact, and disoriented (Thickness of sections, 10 μm; magnification ×100; scale bar = 200 μm). b. The MSCs group showed thicker and well-oriented collagen architecture compared to the control (Thickness of sections, 10 μm; magnification ×100; scale bar = 200 μm).
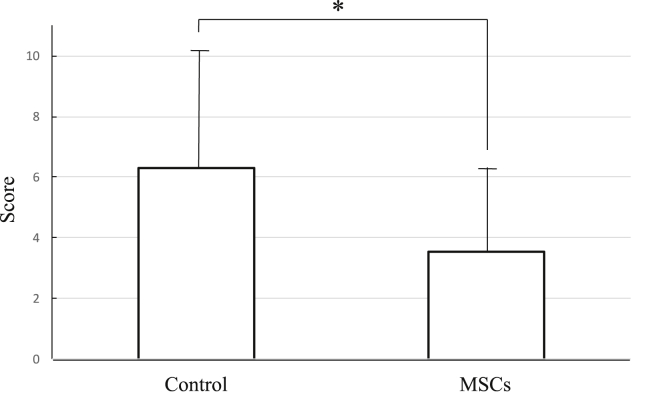
Based on the pathological findings, we developed a histologic assessment scale for all specimens. Fig. 9 shows the histologic scales of the wounds 28 days after surgery. The mean score of the controls was 6.31, and for the MSC-treated tissue, the mean score was 3.75. The score of the MSC-treated wounds was significantly lower, meaning that they were similar to normal tissue.
Fig. 9.
Histologic scores of incisional wounds. Histologic scales of the wounds at 28 days after operation. The mean score of the control was 6.31, and the MSCs was 3.75. Statistical significance was determined by t-test between the control and the MSCs. The score of the MSCs was significant lower, it meant that the wounds transplanted MSCs were similar to normal tissue. (Control: n = 17, MSCs: n = 17, mean ± SE, *p < 0.01, paired t-test).
The elastic fibers in the samples were examined after Elastica van Gieson staining, in which collagen fibers stain red and elastic fibers are dark purple. The controls showed thin, compact, and disoriented collagen fibers in the wound site (Fig. 10a). In the MSC-treated tissue, collagen fibers were thicker, and well organized (Fig. 10b). Higher-power images are shown in Fig. 6c and d. On the right side of the dotted lines, the ranges of the wounds are shown. We observed few elastic fibers in the control (Fig. 10c); but in contrast, elastic fibers (pointed by arrows) are obvious in wound sites of the tissue treated with MSCs (Fig. 10d), which resemble normal skin.
Fig. 10.
Histology images by Elastica van Gieson staining of the wounds. Collagen fibers stain red and elastic fibers are dark purple. a. In the control group, collagen fibers are thin and disoriented (Thickness of sections, 10 μm; magnification ×100; scale bar = 200 μm). b. The MSCs group showed well-oriented collagen architecture (Thickness of sections, 10 μm; magnification ×100; scale bar = 200 μm). c. Enlarged image of Fig. 6a. The right side of the dotted line is the area of the wound. In the control group, we hardly observed elastic fibers in the control (Magnification ×200; scale bar = 30 μm). d. Enlarged image of Fig. 6b. The right side of the dotted line is the area of the wound. In the MSCs group, elastic fibers (arrows) are obvious in wound sites (Magnification × ×200; scale bar = 30 μm).
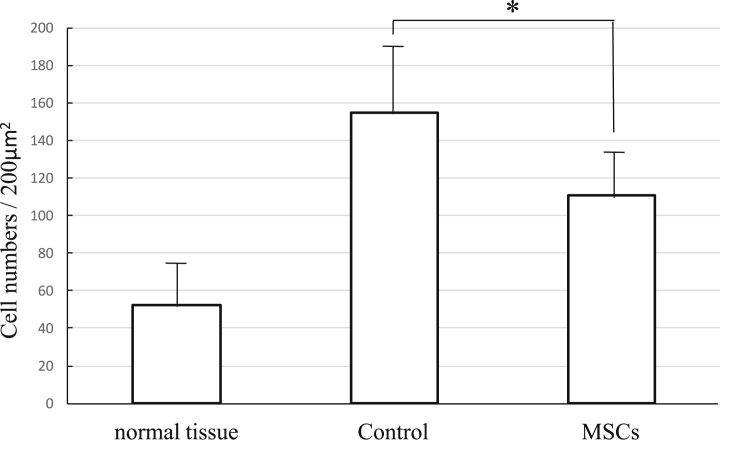
Anti-vimentin staining was performed to evaluate the presence of fibroblasts. Positive cells at the wound site were counted, and the average of one visual field was determined and compared between samples (Fig. 11). The mean number for the controls was 154.5, and for the MSC-treated animals, 110.75. There was a significant difference between the control and the MSC-treated groups, and for the treated animals, the number of fibroblasts was similar to normal tissue.
Fig. 11.
Histologic scale of anti-vimentin staining. Vimentin positive cells of the wounded site were counted. The mean number of the control was 154.5, and the MSCs was 110.75. The number of fibroblasts of the MSCs was strongly similar to normal tissue (mean ± SE, *p < 0.05, Mann–Whitney U test).
Anti-αSMA antibody staining was also performed to evaluate myofibroblasts. When positive cells at the wound sites were counted, the mean value for the control tissue was 46, and for the MSC treatment group, 30.5. There was no significant difference between the control and the MSC groups. However, it was considered that there were more total myofibroblasts in one wound of the control than in the MSC-treated group, because the wound area tended to be wider in the control group.
4. Discussion
Cutaneous scarring is a clinical burden in humans with serious wounds. When human fetal surgery is performed, the wounds heal without scarring, but the processes involved do not continue after birth. Several methods for reducing scarring have been developed, such as subcuticular sutures, radiotherapy, laser therapy, pressure garments, and paper tape application, but have met with limited success [13].
In vivo rodent models are often used for studying cutaneous wound healing, because they are small, easy to handle, and relatively inexpensive. In contrast, porcine models require careful handling, but they have emerged as promising models to study wound healing, with over 1500 publications on the pathophysiology of various types of wounds in these animals. Similarities between pig and human skin make pigs an appropriate model for cutaneous wound healing. Like humans, they have a relatively thick epidermis, distinct rate pegs, dermal papillae, and dense elastic fibers in the dermis [14]. However, despite the similarities in cutaneous wound healing between pigs and humans, there are critical differences. The pig dermis and its hair follicles are less vascular, and pig skin has only apocrine sweat glands, but whether these facts are relevant in cutaneous wound healing was not determined in this study. Our long-term goal is to reduce post-surgical scarring after operations performed to correct congenital diseases of childhood and after general cosmetic surgery. Therefore, we used the pig as an experimental model to simulate human wound healing after operation.
MSCs have been isolated from several types of animals such as mouse, rat, rabbit, pig dog, goat, and man [15], [16]. They exist in almost all tissues and can be easily obtained from the bone marrow, adipose tissue, and other tissues [17], [18]. MSCs are known to secrete growth factors and cytokines that have autocrine and paracrine activities. MSCs produce VEGF, SCF-1, LIF, G-CSF, IL-1, -6, -7, -8, -11, -14, and -15, and other growth factors and cytokines [19], [20], [21]. The expression of these factors may be modulated through interactions with other cell types [22], [23]. MSC homing to sites of dermal injury has been observed, particularly at the site of tail clipping wounds in animal models [24], [25]. MSCs were detected in the dermis and dermal appendages of sheep and had fibroblastic features, indicating possible participation in wound regeneration [25]. Therefore, MSC treatment is expected as a possible therapy for enhancement of the regenerative dermal wound microenvironment by cell therapy [26].
We tried to mark a part of MSCs of pigs with 0.25% DiI (Molecular Probes, Inc., Eugene, OR) before injection, but we couldn't detect the cells among harvested tissue. There are two possibilities to explain this result. One is that the fluorescent dye; DiI was disappeared 28 days after injection. The second is that transplanted MSCs didn't survive, which might mean that MSCs enhance wound healing by a paracrine effect. Likewise, Chen et al. have demonstrated that MSCs can promote the proliferation, migration, and collagen secretion of fibroblasts through a paracrine mechanism [27]. Other recent studies have also demonstrated that stem cell transplantation therapy promotes wound healing mainly through in a paracrine manner [28], [29], [30].
The biological response to wounds is divided into two categories: regeneration and wound repair [6]. Regeneration involves the gross replacement and restoration of adult tissue mass with normal architecture and function. Generally, it has been observed that the capacity for tissue regeneration in mammals is limited. Wound repair involves the migration of fibroblasts, formation of granulation tissue, and the deposition of collagen in a disorganized fashion, with the formation of scar tissue. Appendages rarely return and normal architecture and function are not fully restored. During the process of wound repair, the formation of granulation tissues provides a scaffold for the assembly of neighboring cells at wound margins, contributing to wound closure. Fibroblasts are the major cell type found in granulation tissue. They are present in the dermis and proliferate rapidly and migrate to wound sites where they can secrete Type I and III collagens and elastin, which are the central components of the extracellular matrix [28], [29]. MSCs are able to promote the proliferation and activity of fibroblasts [27].
In the present study, at 28 days post-surgery, wounds transplanted with bone marrow-derived MSCs healed with very fine scars, and collagen architectures were thick and similar to normal dermis. Elastic fibers developed, and appearance of fibroblast was similar to normal skin. This process appears to be similar to skin regeneration.
It is important to note that there are limitations to the clinical application of MSCs in humans. The procedures involved in harvesting MSCs from adult tissues are invasive, the proliferation and differentiation capacities of MSCs are limited after several passages in culture, and it takes a great deal of time to generate enough MSCs for treatment.
5. Conclusions
In this study, wounds transplanted with MSCs healed with less scarring as determined macroscopically, and the dermal structure appeared as normal skin microscopically. We predict that MSC transplantation can contribute to skin regeneration in acute cutaneous wounds of pigs. Further research may show similar effects in human post-surgical wounds.
Acknowledgement
We thank Mr. Taro Uyama for his technical assistance. This work was supported in part by Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science KAKENHI Grant Number 17591883.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Hiroko Ochiai, Email: ochiroko@gmail.com, nyagi@ntmc-hosp.jp.
Kazuo Kishi, Email: kkishi@a7.keio.jp.
Yoshiaki Kubota, Email: ykubo33@a3.keio.jp.
Aiko Oka, Email: aiken722@yahoo.co.jp.
Eri Hirata, Email: erif.x.happy@gmail.com.
Hanayo Yabuki, Email: hana.yabuki.111@gmail.com.
Yoshitaka Iso, Email: yiso@med.showa-u.ac.jp.
Hiroshi Suzuki, Email: hrsuzuki@med.showa-u.ac.jp.
Akihiro Umezawa, Email: umezawa-a@ncchd.go.jp.
References
- 1.Otto W.R., Wright N.A. Mesenchymal stem cells: from experiment to clinic. Fibrogenes Tissue Repair. 2011;4:20. doi: 10.1186/1755-1536-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamiya K., Fujinami Y., Hoya N., Okamoto Y., Kouike H., Komatsuzaki R. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol. 2007;171:214–226. doi: 10.2353/ajpath.2007.060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura Y., Ishikawa H., Kawai K., Tabata Y., Suzuki S. Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell-derived factor-1. Biomaterials. 2013;34:9393–9400. doi: 10.1016/j.biomaterials.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 4.Lee K.B., Choi J., Cho S.B., Chung J.Y., Moon E.S., Kim N.S. Topical embryonic stem cells enhance wound healing in diabetic rats. J Orthop Res. 2011;29:1554–1562. doi: 10.1002/jor.21385. [DOI] [PubMed] [Google Scholar]
- 5.Biazar E., Keshel S.H. The healing effect of stem cells loaded in nanofibrous scaffolds on full thickness skin defects. J Biomed Nanotechnol. 2013;9:1471–1482. doi: 10.1166/jbn.2013.1639. [DOI] [PubMed] [Google Scholar]
- 6.Clark L.D., Clark R.K., Katz E.H. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- 7.Kishi K., Katsube K., Satoh H., Imanishi N., Nakajima H., Nakajima T. The fetal dermal but not loose fascial mesenchymal cells possess regenerative activity of dermal structure. Cell Transpl. 2005;14:709–714. doi: 10.3727/000000005783982729. [DOI] [PubMed] [Google Scholar]
- 8.Satoh H., Kishi K., Tanaka T., Kubota Y., Nakajima T., Akasaka Y. Transplanted mesenchymal stem cells are effective for skin regeneration in acute cutaneous wounds. Cell Transpl. 2004;13:405–412. doi: 10.3727/000000004783983765. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan T.P., Eaglstein W.H., Davis S.C., Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 10.Phinney D.G., Kopen G., Isaacson R.L., Prockop D.J. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 11.Sato T., Iso Y., Uyama T., Kawachi K., Wakabayashi K., Omori Y. Coronary vein infusion of multipotent stromal cells from bone marrow preserves cardiac function in swine ischemic cardiomyopathy via enhanced neovascularization. Lab Investig. 2011;91:553–564. doi: 10.1038/labinvest.2010.202. [DOI] [PubMed] [Google Scholar]
- 12.Bausang E., Floyd H., Dunn K.W., Orton C.I., Ferguson M.W.J. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg. 1998;102:1954–1961. doi: 10.1097/00006534-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Bayat A., McGrouther D.A., Ferguson M.W.J. Skin scarring. BMJ. 2003;326:88–92. doi: 10.1136/bmj.326.7380.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindblad W.J. Considerations for selecting the correct animal model for dermal wound-healing studies. J Biomater Sci Polym Ed. 2008;19:1087–1096. doi: 10.1163/156856208784909390. [DOI] [PubMed] [Google Scholar]
- 15.Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 16.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 17.Torensma R., Prins H.J., Schrama E., Verwiel E.T., Martens A.C., Roelofs H. The impact of cell source, culture methodology, culture location, and individual donors on gene expression profiles of bone marrow-derived and adipose-derived stromal cells. Stem Cells Dev. 2012;22:1086–1096. doi: 10.1089/scd.2012.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierro M., Ionescu L., Montemurro T., Vadivel A., Weissmann G., Oudit G. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2012;68:475–484. doi: 10.1136/thoraxjnl-2012-202323. [DOI] [PubMed] [Google Scholar]
- 19.Haynesworth S.E., Baber M.A., Caplan A.I. Cytokine expression by human marrow derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1α. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Majumdar M.K., Thiede M.A., Mosca J.D., Moorman M., Gerson S.L. Phenotypic and functional comparison of marrow-derived mesenchymal stem cells and stromal cells. J Cell Phys. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Reese J.S., Koc O.N., Gerson S.L. Human mesenchymal stem cells provide stromal support for efficient CD34+ transduction. J Hematother Stem Cell Res. 1999;8:515–523. doi: 10.1089/152581699319966. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 23.Le Blanc K., Pittenger M.F. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y., Jahagirdar B.N., Reinhardt R.L., Shwartz R.E., Keene C.D., Ortiz-Gonzalez X.R. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 25.Liechty K.W., MacKenzie T.C., Shaaban A.M., Radu R., Moseley A.B., Deans R. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J., Guan J., Niu X., Hu G., Guo S., Li Q. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi: 10.1186/s12967-015-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L., Xu Y., Zhao J., Zhang Z., Yang R., Xie J. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014;9:e96161. doi: 10.1371/journal.pone.0096161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang X., Ding Y., Zhang Y., Tse H.F., Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transpl. 2014;23:1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 29.Shen L., Zeng W., Wu Y.X., Hou C.L., Chen W., Yang M.C. Neurotrophin-3 accelerates wound healing in diabetic mice by promoting a paracrine response in mesenchymal stem cells. Cell Transpl. 2013;22:1011–1021. doi: 10.3727/096368912X657495. [DOI] [PubMed] [Google Scholar]
- 30.Song M., Heo J., Chun J.Y., Bae H.S., Kang J.W., Kang H. The paracrine effects of mesenchymal stem cells stimulate the regeneration capacity of endogenous stem cells in the repair of a bladder-outlet-obstruction-induced overactive bladder. Stem Cells Dev. 2014;23:654–663. doi: 10.1089/scd.2013.0277. [DOI] [PubMed] [Google Scholar]