Abstract
From the recent advances, there are growing expectations toward the mass production of induced pluripotent stem cells (iPSCs) for varieties of applications. For such type of industrial cell manufacturing, the technology which can stabilize the production efficiency is strongly required. Since the present iPSC culture is covered by delicate manual operations, there are still quality differences in produced cells from same culture protocols. To monitor the culture process of iPSCs with the quantified data to evaluate the culture status, we here introduce image-based visualization method of morphological diversity of iPSC colonies. We have set three types of experiments to evaluate the influential factors in iPSC culture technique that may disturb the undifferentiation status of iPSC colonies: (Exp. 1) technical differences in passage skills, (Exp. 2) technical differences in feeder cell preparation, and (Exp. 3) technical differences in maintenance skills (medium exchange frequency with the combination of manual removal of morphologically irregular colonies). By measuring the all existing colonies from real-time microscopic images, the heterogenous change of colony morphologies in the culture vessel was visualized. By such visualization with morphologically categorized Manhattan chart, the difference between technical skills could be compared for evaluating appropriate cell processing.
Keywords: Human induced pluripotent stem cells, Morphological analysis, Colony morphology, Culture technique, Colony morphological diversity
Highlights
-
•
Morphological clustering enabled visualization of diversity of iPSC colonies.
-
•
Morphological clustering can record and visualize the effects of culture skills.
-
•
Comparison of culture skills reveals clue for designing automation protocol.
1. Introduction
Induced pluripotent stem cells (iPSCs) are defined by their unique capacity to differentiate into multiple lineages [1]. Growing expectations are accumulating for their usage both in drug discoveries and clinical applications [2], [3], [4], [5]. For wider distribution of iPSCs for various applications, technological development to enable the industrial cell manufacturing, such as their undifferentiated expansion culture, is strongly required to satisfy massive needs [6], [7], [8]. However, the present iPSC manufacturing process is mainly covered by manual operation supported by the experience-based skills and memory-based decisions. Therefore it has been considered that the qualities of produced cells may vary [9], [10], and the quality control method for massive iPSC culture is an important technological issue.
Commonly, when cells, including iPSCs, are manufactured for further applications, the final product cells are required to be intact. Therefore, in the advancing manufacturing technologies for iPSCs, non-invasive quality monitoring technology is becoming an important enabling technology. To check and evaluate the culture process of undifferentiated iPSCs non-invasively, the manual microscopic observation is the major solution in most of the facilities. Because, it is known that morphological character of cultured iPSCs is an important signature to monitor the culture status, such as the rate and the homogeneity of their undifferentiation status. Commonly, the morphological criteria of undifferentiated iPSCs has been known as; compact colonies that have distinct borders and well-defined edges, and are comprised of cells with a large nucleus with less cytoplasm, such as called ES-cell like colony [11], [12]. Colonies that show irregular morphologies are known as indicator of disturbance of their undifferentiation status in pluripotent stem cells [13]. The disturbance of these cells can lead to consist of differentiated cells or karyotic abnormal cells [14]. Recent studies reported the quality evaluation of iPSCs by their colony morphologies [15], [16], [17]. In these works, the morphological characters are linked to some biological phenomenons. In spite of such accumulating data showing correlation between the colony morphology and its undifferentiated status, such morphological evaluation methods are not yet applied to evaluate the culture process. Especially, although “the culture skill” is the background basic factor which can affect the quality of culture process, their effect has not yet been quantitatively evaluated for the standardization of cell culture.
We here propose the evaluation method of undifferentiated iPSC culture process by visualizing the quantitatively measured morphological data of iPSC colonies (Schematic illustration of our concept is shown in Fig. 1). Practically, we measured all colonies in the phase contrast microscopic images of cultured iPSCs, and compared the changes of colony profiles from the aspect of morphological categories. By comparing the Manhattan chart of morphological clusters, the differences between human skills, which can disturb the final quality of the same iPSC culture protocol, could be visualized. For this investigation, we have set three types of experiments to evaluate the influential factors in the iPSC culture skill that may disturb the undifferentiated quality of iPSC colonies: (Exp. 1) technical differences in passage skills, (Exp. 2) technical differences in feeder-cell preparation, and (Exp. 3) technical differences in maintenance skills (medium exchange frequency with the combination of manual removal of abnormal colonies) listed in Table 1.
Fig. 1.
Schematic illustration of colony morphology analysis in this work to compare culture operations. The colony morphology analysis in this study consist of 4 steps; experiment, image processing and measurement, data analysis, and visualization. In this study, cell culture operation operations, which are not literarily described although, have deep impact on the resultant colony quality. Phase contrast microscopic images were acquired from the experimental samples, and processed to measure all the colonies with 9 morphological parameters. Using the collected database of colony morphologies, hierarchical clustering followed by its segmentation by a threshold of correlation coefficient produces clusters of morphological sub-categories. Manhattan-bar-chart is used for visualization of colony number transition with morphological cluster differences for quantitative comparison of operation conditions.
Table 1.
List of experimental conditions.
| Exp. 1 | Condition name | Operation 1 | Operation 2 | ||
| Passages of iPSC | P0 | P4 | |||
| Exp. 2 | Conditions | Operation 1 | Operation 2 | ||
| Passages of iPSC | P0 | P0 | |||
| Passages of feeder cells (SNL) | P3 | P11 | |||
| Exp. 3 | Conditions | Operation 1 | Operation 2 | Operation 3 | Operation 4 |
| Medium change volume | Half volume | Half volume | All volume | None | |
| Medium change frequency | Everyday | Everyday | Everyday | None | |
| Physical colony removal maintenance | + | − | − | − | |
In Exp. 1, the influence of passage skill was evaluated. The skill of stressless passage is known to be important for maintaining undifferentiated iPSC colonies; however its definition had been ambiguous. We compared the morphologically categorized colony profiles of iPSCs between “before” and “after” 4-repeated times of intentional stressful passages. Practically, in the stressful passage condition, the daily removal of morphologically irregular colonies was neglected during the four passages. By such passage operation, we mimicked to perform the negative influence caused by a careless operator.
In Exp. 2, the influence of feeder-cell preparation skill was evaluated. Feeder cells are known to influence the quality of PSCs [18], [19], therefore early passage of cells are commonly recommended. However, the definition of “early” had been ambiguous. The suggested definition of cellular usage by “passage numbers” may result differently, even with immortal cells, because their detailed maintenance conditions including culture skills can be different between facilities. To evaluate such ambiguous facility-specific influential factor, the morphologically categorized colony profiles of the same iPSCs on different feeder-cell conditions (“before” and “after” 8-repeated times of intentional over-passages) were compared.
In Exp. 3, the influence of culture maintenance skill was evaluated. As a maintenance skill, it is known that the control of frequencies/volume of medium exchange can change the cellular condition. Moreover, the removal skill of morphologically irregular colonies is also an important skill. However, its individual effect or their combinational effect on iPSCs' undifferentiated state had been ambiguous. By visualizing the morphologically categorized colony profiles, four types of manners which change the rate of medium change and colony removal were quantitatively compared.
In this work, the image-based quantitation of morphologies of the cultured iPSCs was found to be effective for visualization and understanding of the heterogenic changes in culturing iPSCs. We propose that our method of visualization can provide quantitative approach to evaluate the delicate skill-derived effects in the culture process which were not objectively analyzed before. We consider our method can provide real-time culture record of iPSCs to evaluate, compare, and optimize the ambiguous cell culture operation skills.
2. Methods
2.1. Cells and cell culture
Human iPS cell line, 201B7, (provided by Dr. Shinya Yamanaka, Center for iPS Cell Research and Application, Kyoto University) was used in this study. For feeder cells, SNL 76/7 feeder cells (European Collection of Authenticated Cell Cultures (ECACC), Salisbury, UK) were used for Exp. 1 and 2, and MEF feeder cells (Merck Millipore, Billerica, MA, USA) were used for Exp. 3 (detailed experimental conditions are listed in Table 1). For both types of feeder cells, cells were only used within passage 3 from the first seeding of purchased cells in their usual maintenance. Only for Exp. 2, we over passaged the SNL cells for 8 repeated times (passage was decided by their sub-confluent status) after passage 3. Feeder cells were seeded at a density of 8.0 × 104 cells/well in 6-well plate. For MEF culture, EmbryoMax® 0.1% Gelatin Solution (Merck Millipore) was coated for 1 h in the incubator (37 °C, 5% CO2 condition). iPSCs were maintained in Knockout DMEM/F-12 (Life Technologies, Carlsbad, CA, USA) containing 0.1 mM Non-aminoessential acid (Life Technologies), 0.1 mM 2-Mercaptoethanol (Life Technologies), 2 mM l-glutamine (Life Technologies), and 20% Knockout serum replacement (Life Technologies). The iPSC colonies were treated by 1 mg/ml Dispase II (Roche Applied Science, Penzberg, Upper Bavaria, Germany) until the colony edge shows slight lift-up (within 3–10 min), After sucking Dispase II, new medium were added 5 ml and colonies were collected with scraper and pipetting. iPSCs aggregates were dispersed by pipetting and split into five portions for each new vessel for passage. The morphologically irregular colonies are removed using cell scraper or sucked by aspirator. In their maintenance culture, medium was changed every day supplemented with 10 ng/ml bFGF (Life Technologies). In usual maintenance culture for cell expansion, above described cell collection protocol was carried out with the cell split ratio (1:4 to 1:10). During the maintenance culture, the irregular colonies are also scraped off in medium change process. Cells were cultured in the 37 °C, 5% CO2 condition. Prior to the start of each experiment designed in this experiment, colonies were stained with SSEA4, Tra-1-60, and OCT 3/4 to confirm their undifferentiation status (Representative staining images provided in Supplementary information Fig. S1). All cell culture and maintenance were conducted by two operators (Exp 1 and 2: 5 years of culture experiences, Exp 3: 2 years of culture experiences). Both operators were trained until morphologically irregular colonies can be eliminated by their own decision in daily maintenance.
2.2. Image acquisition
Phase contrast microscopic images of iPSCs were obtained by IX81 (Olympus, Tokyo, Japan) with camera C11440-10C (Hamamatsu Photonics, Hamamatsu, Japan). In Exp. 1 and 2, five view fields (center position and four positions 2.2 mm from the center in the well of 6-well plate) were acquired from each well. In Exp. 3, the five positions in the view fields in each well were manually selected and memorized as x–y coordinates before the image acquisition. The time-course image acquisition was done semi-automatically according to the x–y coordinates by the x–y stage (Olympus) regulated by MetaMorph (Molecular Devices, Sunnyvale, CA, USA). Image acquisition intervals were; 6 h (Exp. 1), 4 h (Exp. 2), and 12 h (Exp. 3). Fluorescent images for confirming the staring status of iPSCs were obtained by IX81 with camera C11440-10C (Exp 1, and 2), and with BioStation CT (Nikon, Tokyo, Japan) (Exp. 3).
2.3. Image processing
In Exp. 1 and Exp. 2, images were processed by MetaMorph. In Exp. 1 and Exp. 2, images were preprocessed that were binarized successive segment of the 20 pixel under 89 brightness value by the original program written in C language. After this process, images were processed through “Close filter”, “binarization filter”, and “Close-Open filter” (MetaMorph) for all field of images. In Exp. 3, imaged were binarized the colony area (MetaMorph) which was segmented manually by regions tool (MetaMorph). All colonies recognized in each image were measured with 9 morphology parameters (Supplementary information Fig. S2). The 9 parameters were selected from 44 morphology parameters in MetaMorph to escape from multicollinearity. Moreover, 9 parameters were chosen because most of them were related to the morphological characteristic features which related to the conventionally described irregular colony morphology. For quantification of immunohistochemical staining result (SSEA4 and TRA-1-60), fluorescent images were quantified by CL-Quant software (Nikon Corp.). Stained colonies in the images were recognized by soft-matching algorithm following the manufactures' protocol, and the intensity of each colony was measured, and calculated as “total intensity/total pixel area in a colony”.
2.4. Immunohistochemistry staining
Cells were fixed with 4% paraformaldehyde in PBS, washed, and permeablized with PBS containing 0.1% Triton X-100 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 15 min at room temperature. After the incubation with blocking solution (4% goat serum (16210-064, Life Technologies) in phosphate buffered saline (PBS)) for 15 min, primary antibodies were hybridized overnight, followed by secondary antibodies for 1 h at room temperature. The following antibodies were used: anti-SSEA4 mouse IgG1 (V6630, Life Technologies), anti-TRA-1-60 mouse IgG (sc-21705, Santa Cruz Biotechnology), Alexa Fluor® 488 Goat Anti-Rabbit IgG (Life Technologies), and Alexa Fluor® 488 Goat Anti-Mouse IgG (Life Technologies).
2.5. Categorization of colonies by morphological parameters
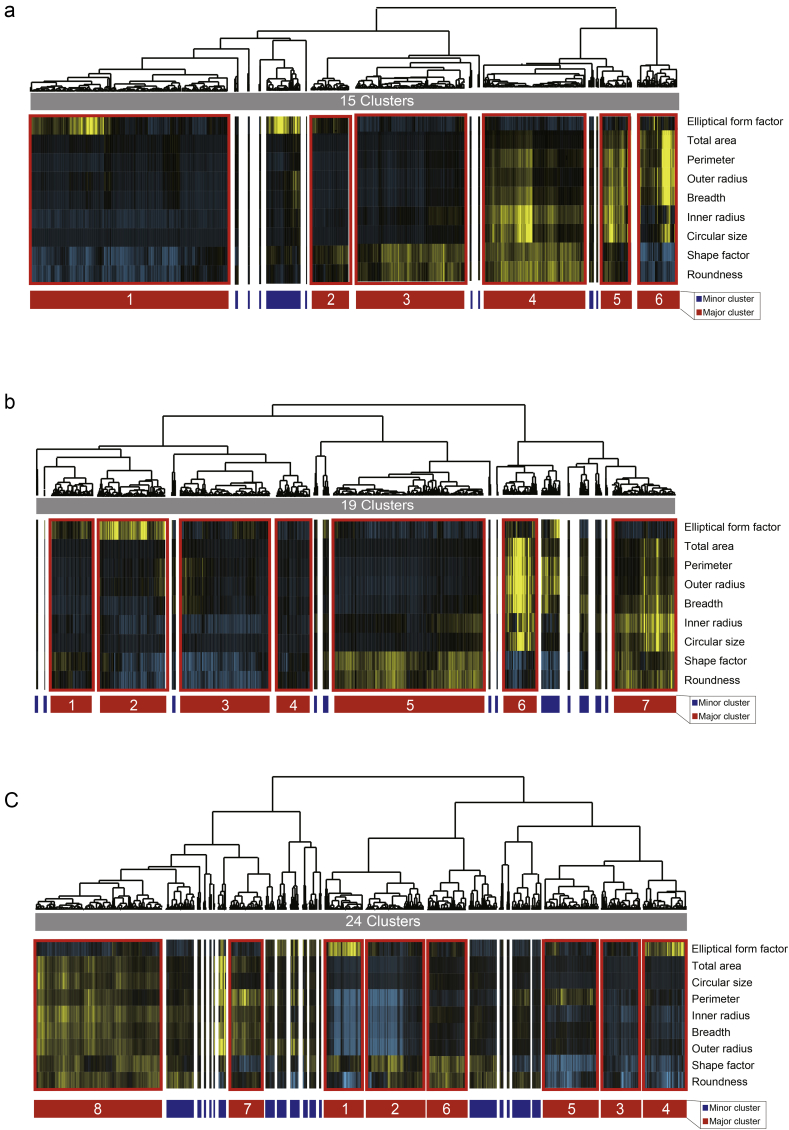
For the morphological categorization of colonies, our classification algorithm consists of two steps. First step, colonies with 9 morphology parameters were categorized with the average linkage hierarchical clustering (uncentred correlation) by the open source clustering software, Cluster 3.0 (University Tokyo, Human Genome Center, Tokyo, Japan). The clustering result was visualized with Java tree view (http://jtreeview.sourceforge.net/). Second step, the hierarchical clustering tree was pruned by the threshold of Pearson's correlation coefficient r > 0.595. This is based on our idea that ‘similar colonies’ should clear the ‘test of no correlation’ by satisfying t > 0.05. Third step, the clusters were evaluated as ‘major cluster’ or ‘minor cluster’ by their size. We defined clusters that consisted of a colony number which exceed more than 5% of total colonies as a ‘major cluster’, and the others were a ‘minor cluster’. This setting was designed to mimic the human recognition of colony morphology by memory. We hypothesized that colonies which only belongs to small cluster are not the colonies that human can recognize as “frequent morphology” (For example, if there is a morphologically characteristic colony that belongs to cluster of 100 colony members, such morphological type is more frequently observed; however if there is a colony that belongs to a morphological cluster of only 2 colony cluster members, such morphology is rarely observed, and difficult to be used in experience-based decision). Practically, the colony number threshold for defining the major clusters were; >287 colonies in Exp. 1 (total 5741 colonies observed), >220 colonies in Exp. 2 (total 4403 colonies observed), and >53 colonies in Exp. 3 (total 1072 colonies observed). We should note that “minor clusters” are not equal to “differentiated colonies”, and “major clusters” are not equal to “undifferentiated colonies”. Major clusters are focused because they are frequent type of morphology, and we hypothesized that they can be the representative morphologies in human memory as representative morphology. If the total colonies clustered consist of both differentiated/undifferentiated colonies, some major/minor cluster can represent either differentiated/undifferentiated status. In Exp. 1 and Exp. 2, since we removed morphologically irregular colonies during their maintenance, there were no “morphologically irregular colonies that can be differentiated”. On the other hand, in Exp. 3, we intentionally did not remove morphologically irregular colonies for the evaluation of skills. Cluster 7 was the major and the morphologically irregular cluster (9% of total colonies).
3. Results
3.1. Evaluation of technical differences in passage skills
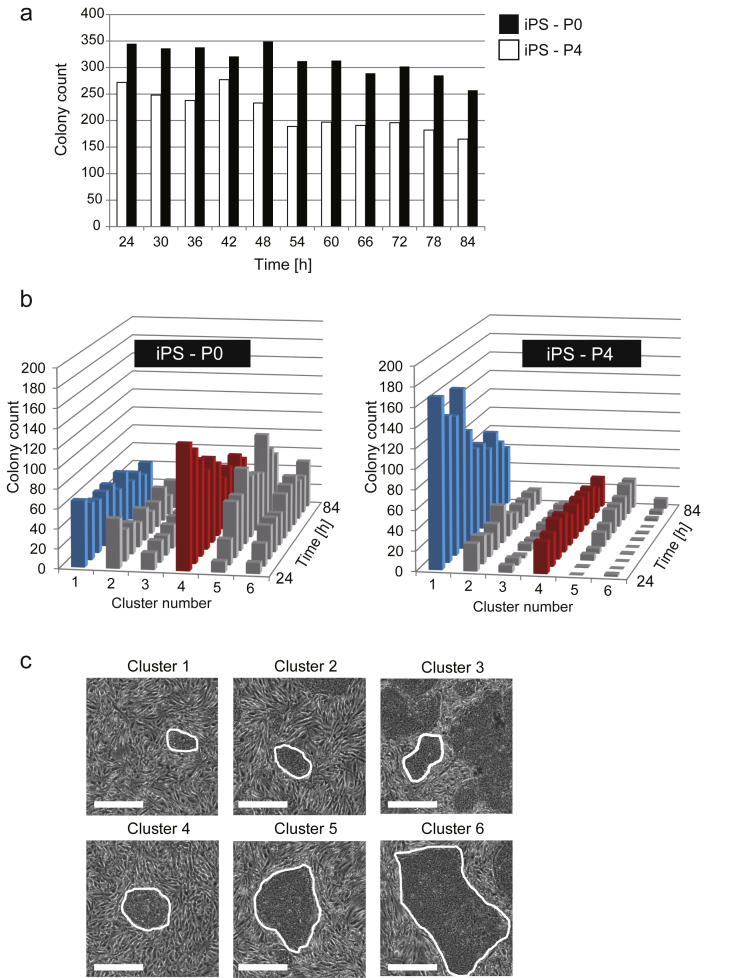
In this analysis, image-based colony morphology analysis was applied to quantitatively compare the difference of cell passage skills. As a model, two types of operations were designed to mimic the difference of passage skills: the operation iPS-P0 (iPS cells which started shortly after the thawing of stock cells), and the operation iPS-P4 (iPS cells passaged 4-repeated times with intentional stressful passage) were compared. During 4 passages, removal of colonies with irregular morphology was not conducted, and dissociation of colonies with pipetting was conducted roughly.
From 5 days of time-lapse image acquisition period, morphologies of 5741 colonies (iPS-P0: 3349, iPS-P4: 2392) were measured by 9 parameters to construct colony morphology database. Using this database, 6 representative clusters (major clusters) were defined by our clustering analysis (Fig. 2a). Compared to the simple colony counts (Fig. 3a), when total colonies were described by Manhattan bar chart using 6 representative morphological clusters (Fig. 3c), the growth profiles of sub-populations (6 morphological clusters) could be quantitatively visualized (Fig. 3b). Such visualization revealed that the most of the number of colonies in iPS-P4 consist of cluster 1-colonies (round but small colonies, with area <6860 μm2). In contrast, the number of cluster 1-colonies was 2-fold smaller in iPS-P0. By such visualization of categories of colony morphologies, the iPS-P0, which total colony number seemed to be less than iPS-P4, were found to consist of grown colonies in cluster 4, 5 and 6 (area >21,360 μm2) (Fig. 3b). In these three clusters, cluster-4-colonies were round (shape factor > 0.67) and showed typical morphology of regular iPSC colonies (Fig. 3c).
Fig. 2.
Results of hierarchical clustering of morphology parameters obtained from iPSC colonies from the image. (a) Morphological clustering result of colonies in Exp. 1. Fifteen clusters were found from the total colony clustering. (b) Morphological clustering results of colonies in Exp. 2. Nineteen clusters were found from the total colony clustering. (c) Morphological clustering result of colonies in Exp. 3. Twenty-four clusters were found from the total colony clustering. For all clustering, 9 morphological parameters were used (listed in Supplementary information Fig. S2). Major clusters were indicated as red region, and minor clusters were indicated as blue region.
Fig. 3.
Morphological comparison of iPS colonies for the evaluation of technical differences in passage skills. (a) Comparison of total colony count by each time. Black bar is iPS-P0 (iPS colonies without stressful passage) and white bar is iPS-P4 (iPS colonies with 4-repeated times of additional stressful passages). (b) Morphological sub-categories and their transition are depicted by Manhattan-bar-chart. iPS-P0 and iPS-P4 were compared. Clusters are numbered in the order of size (Cluster 1 < size < Cluster 6). (c) Representative images of colony in the corresponding cluster number are indicated in the inlet. Scale bars = 500 μm. Magnification: ×4.
3.2. Evaluation of technical differences in feeder cell preparation
In this analysis, image-based colony morphology analysis was applied to evaluate the influence of stressful passage on feeder cells. The condition of feeder cells is considered to be an influential factor that can change the quality of iPSCs even under the use of same protocol [18], [19], [20]. As a model, the same conditioned iPSCs on two types of feeder cell conditions were designed to mimic the differences of feeder cell preparation skills: (i) Feeder-P3, which only experienced 3 passages after thawing of purchased cryo-vial, and (ii) Feeder-P11, which intentionally continued extra 8-times of passages of Feeder-P3. Although SNL is an established cell line that is commonly more stable to be used in the undifferentiated iPSCs, the examination of their exact usage limitation can be an important factor in the case of their culture automation is large scale.
By analyzing 4403 colonies with 9 morphological parameters, 7 representative clusters were found (Fig. 2b). From our colony morphology analysis, 7 representative morphological clusters could be visualized compared to the simple total colony count results (Fig. 4a and b). It revealed that colony growth profiles in all clusters had nearly no difference (correlation coefficient of all charts = 0.85) in this model study (Fig. 4b). In other words, even if Feeder-P11 can be considered to be over-passaged, this result indicates an example that some feeder cells can be used for long term. Although this is only a limited example, we here show that the influence of feeder cell conditions can be quantitatively compared by the morphologically categorized colony profiles.
Fig. 4.
Morphological comparison of iPS colonies for the evaluation of technical differences in feeder cell preparation. (a) Comparison of total colony count by each time. Black bar is feeder-P3 Feeder-P3 (iPS colonies seeded on feeder cells with passage 3) and white bar is feeder-P11 (iPS colonies seeded on feeder cells with passage 11, which experienced additional stressful passages). (b) Morphological sub-categories and their transition are depicted by Manhattan-bar-chart. Clusters are numbered in the order of size (Cluster 1 < size < Cluster 7). (c) Representative images of colony in the corresponding cluster number are indicated in the inlet. Scale bars = 500 μm. Magnification: ×4.
3.3. Evaluation of technical differences in culture maintenance skills
As the third analysis, our colony morphology analysis was applied to evaluate the effect of more delicate differences that may occur among technical know-hows behind the same culture protocol. As a model of such delicate know-how, (i) the effect of medium exchange manner, and (ii) the effect of colony maintenance skill, which manually scrape-off the morphologically irregular colonies in the culture vessel with microscopic observation, was evaluated. As illustrated in Table 1, four types of operations were designed: (Operation 1) half volume medium change with colony maintenance, (Operation 2) half volume medium change without colony maintenance, (Operation 3) total volume medium change without colony maintenance, and (Operation 4) no medium change without colony maintenance. From Operation 1 to 4, the operations were set to mimic the immatureness of iPSC culture maintenance skills.
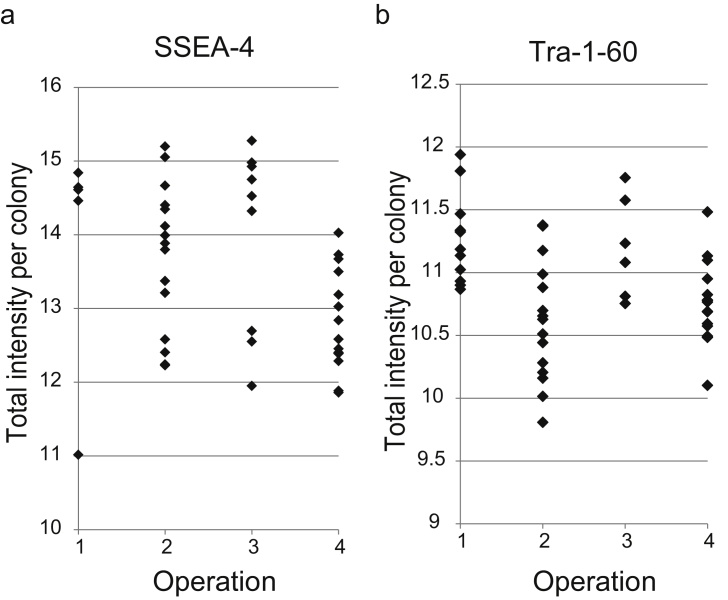
By analyzing 1072 colonies obtained in four operation cultures, 8 representative morphological clusters were found (Fig. 2c). Compare to the simple total colony count results (Fig. 5a), among all operations, cluster 7 and cluster 8 were found to show drastic difference of existence between the compared operations. From their size, these two clusters were grown colonies of all clusters (Supplementary information Fig. S3), and consist of 30% of colonies larger than 1 mm diameter after 5 days of culture (Fig. 5b). The cluster 7, which showed characteristic colony morphology with unclear peripheral outline with fibroblastic cells (Fig. 5c), is one of the typical morphology regarded as irregular colony to be removed. The numbers of cluster 7 colonies were found to be low in Operation 1 and 3, although increased in Operation 2 and 4. Although the low number of cluster 7 colonies in Operation 1 is simply the reflection of colony maintenance, it was found that the cluster 7 colonies were reduced by totally refreshing the medium in Operation 3 (Fig. 5b). The cluster 8, consist of round grown colonies with clear peripheral outline, was a typical morphology for regular iPS colonies (Fig. 5c). The numbers of cluster 8 colonies were found to be drastically low in Operation 4, however were high in other operation operations. The yield of cluster 8 colonies reached the highest level when the half volume of medium was changed (Operation 1 and 2), however the 1.38-fold yield (Operation 1 vs. 3), and 1.27-fold yield (Operation 2 vs. 3) was achieved by simply refreshing the total medium in Operation 3. When a part of morphologically evaluated colonies were stained with SSEA4 or Tra-1-60 for conformation, the staining result per colony was found to be lower in average, and wider in their variance in Operation 2 and 4 which has larger number of cluster-7 colonies (Fig. 6).
Fig. 5.
Morphological comparison of iPS colonies for the evaluation of differences in culture maintenance skills. Four types of operation operations are compared: (Operation 1) half volume medium change with colony maintenance, (Operation 2) half volume medium change without colony maintenance, (Operation 3) total volume medium change without colony maintenance, and (Operation 4) no medium change without colony maintenance. (a) Comparison of total colony count by each time. Black bars, Operation 1; dark gray bars, Operation 2; light gray bars, Operation 3, and white bars, Operation 4. (b) Morphological sub-categories and their transition in each operation are depicted by Manhattan-bar-chart. Clusters are numbered in the order of size (Cluster 1 < size < Cluster 8). Blue bars, Cluster-7 colonies, which indicated irregular colony morphology; Red bars, Cluster-8 colonies, which indicated regular colony morphology. (c) Representative images of colony in the corresponding cluster number are indicated in the inlet. Scale bars = 500 μm. Magnification: ×4.
Fig. 6.
Marker expression differences between four types of operations. Scatter plot indicates the immunostaining intensity per each colony in four types of operations. (a) SSEA-4, (b) Tra-1-60. (Operation 1) half volume medium change with colony maintenance, (Operation 2) half volume medium change without colony maintenance, (Operation 3) total volume medium change without colony maintenance, and (Operation 4) no medium change without colony maintenance. Detailed operation condition is summarized in Table 1.
4. Discussion
For wider distribution of iPSCs for their applications, massive cell manufacturing in industrial level is strongly expected. In such mass production process, automation of iPSC culture is one of the effective and practical concepts. However, although the labor of human can be replaced by robotic automation, the decision of human has been difficult to replace with technology, because there are many unwritten technical skills that had not been quantified as data. Supported by the increasing number of image-based iPSC analysis methods, which indicates the biological meaning of morphological assessment of iPSC colonies, we here tried to apply the morphological analysis for evaluating “culture skills” in the aim of data-driven protocol optimization. We have to clarify that the biological results, such as the correlation between the passage numbers and cellular status, is a case study to examine our analysis methodology, and is not a universal finding, and more detailed study have to be investigated to further describe the underlying biological responses. Our motivation was to indicate the effect and importance of quantitative morphological data, which represent the comprehensive colony profile that could not be recorded in manual microscopic observation. Therefore, as model studies, we here report the applicability of our image-based analysis for examining three types of “technical operation differences” by showing their colony profiles with Manhattan-chart of morphological sub-categories.
In the cell culture, there still exist various types of unwritten techniques based on experiences. However, since most of techniques are influenced by many parameters, it requires cost and labor to evaluate such process one by one. Moreover, in some cases, the protocol should be examined to adapt to the status of cell types, which may vary their stiffness, density, or sensitivity to physical contacts. For such flexible adaptation examination based on quantitative data, our morphological colony evaluation can provide important visual clues to compare the conditions as shown in our results.
The volume of medium change is one of delicate factor to control in cell culture. The remaining volume of medium can consist of precious cytokines and factors as conditioned medium [21], [22]. The use of conditioned medium is known as effective culture method to enhance the cellular growth or recovery of cellular activities. However, such conditioned medium is also known to consist of cell-growth inhibitory factors, such as lactic acid [23]. Therefore, it is always a difficult decision in which ratio should we refresh the medium. Since the metabolic profiles are still unclear in the total process of iPSC culture, there is still no golden standard for such technique. Moreover, the colony maintenance (removal of unwanted colonies) by morphological decision has been a critically important technique in iPSC culture; however the skill greatly relied on experts' experiences. The rate and frequency of such colony maintenance greatly differ by the person. There had been no quantitative data to standardize the manner of colony maintenance, or the morphology of objective maintenance target. By our approach (Fig. 5), the detailed effect which reflected in numbers of morphological sub-categories could be determined. From the results, the medium change was found to increase the colony yield of “regular morphology”, which categorized as cluster-8 in our analysis. However, it was found that the remaining of conditioned medium can increase such cluster-8 colony growth, together with the unwanted growth of cluster-7 colonies (Fig. 5). It can be interpreted that the iPSC culture experts can enhance colony yield with their medium change timings together with reduction effort of unwanted colonies by their colony maintenance skills. In contrast, it can be said that it is found that the total refreshment of medium without any colony maintenance (Operation 3) can produce 62% yield of cluster-8 colonies and low production rate of cluster-7 colonies compared to the maximum yield with manual efforts (Operation 1). Such process-less technique with certain production efficiency should be an important clue to design process model for automated iPSC culture machineries. It should be more effective to clarify the actual metabolic molecules combined with our morphological evaluation, which is our next investigation.
Although our proposed profiling of colony morphologies can provide quantitative patterns and changes of colony sub-categories in real-time, there is several functions to be achieved to enhance our method. The most important function should be the time-course change of individual colonies, because our present analysis can grasp the total morphological changes in the culture vessel, however cannot evaluate single colonies. For example, in the morphological clusters that contain large colonies, both the single colonies that reached to the size and the colonies enlarged by several colonies merging are included.
5. Conclusion
Through our investigation, we have evaluated that measurement of colonies with multiple parameters, followed by clustering can profile the heterogenic patterns and their time-lapse changes of iPSC colonies. The manual operation techniques to regulate iPSC culture, which have not been quantitatively described with their direct result of morphological phenotypes, were found to be compared and distinguished by our concept. Since our system can evaluate such morphological profiles by phase-contrast microscopic images, our methodology is complete non-invasive real-time measurement and evaluation method to support the understanding of iPSC colony heterogeneity. Hence, we expect our proposing colony morphological analysis methodology can contribute in the stable and safe processing of iPSCs used for regenerative medicine products.
Conflict of interest
H.K., T.U. and Y.K. are employed by Nikon Corporation. BioStation CT and related software used in the present study are marketed products of Nikon Corporation. R.K. is one of the primary investigators on 7 patents (pending) related to basic algorithm technologies for cellular image processing. The other authors report no conflicts of interest.
Acknowledgments
This study was supported by grants-in-aid from the New Energy and Industrial Technology Development Organization (NEDO) (09C46036a) and the Japan Agency for Medical Research and Development (AMED) (15652971) to Y.K., R.K. and M.K.F.; Industrial Technology Research (Financial Support to Young Researchers, 09C46036a) to R.K. We thank for Dr. Miho Kusuda-Furue (Laboratory of Stem Cell Cultures, National Institutes of Biomedical Innovation, Health and Nutrition) for the discussion in writing the manuscript.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.reth.2016.12.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Doi D., Samata B., Katsukawa M., Kikuchi T., Morizane A., Ono Y. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2014;2(3):337–350. doi: 10.1016/j.stemcr.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura S., Takayama N., Hirata S., Seo H., Endo H., Ochi K. Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell. 2014;14(4):535–548. doi: 10.1016/j.stem.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Assawachananont J., Mandai M., Okamoto S., Yamada C., Eiraku M., Yonemura S. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Rep. 2014;2(5):662–674. doi: 10.1016/j.stemcr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashita A., Morioka M., Yahara Y., Okada M., Kobayashi T., Kuriyama S. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Rep. 2015;4(3):404–418. doi: 10.1016/j.stemcr.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otsuji T.G., Bin J., Yoshimura A., Tomura M., Tateyama D., Minami I. A 3D sphere culture system containing functional polymers for large-scale human pluripotent stem cell production. Stem Cell Rep. 2014;2(5):734–745. doi: 10.1016/j.stemcr.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haraguchi Y., Matsuura K., Shimizu T., Yamato M., Okano T. Simple suspension culture system of human iPS cells maintaining their pluripotency for cardiac cell sheet engineering. J Tissue Eng Regen Med. 2013;9:1363–1375. doi: 10.1002/term.1761. [DOI] [PubMed] [Google Scholar]
- 8.Kami D., Watakabe K., Yamazaki-Inoue M., Minami K., Kitani T., Itakura Y. Large-scale cell production of stem cells for clinical application using the automated cell processing machine. BMC Biotechnol. 2013;13:102. doi: 10.1186/1472-6750-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Na J., Baker D., Zhang J., Andrews P.W., Barbaric I. Aneuploidy in pluripotent stem cells and implications for cancerous transformation. Protein Cell. 2014;5(8):569–579. doi: 10.1007/s13238-014-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amps K., Andrews P.W., Anyfantis G., Armstrong L., Avery S., Baharvand H. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol. 2011;29(12):1132–1144. doi: 10.1038/nbt.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinton D.A., Daley G.Q. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481(7381):295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakao S., Kitada M., Kuroda Y., Ogura F., Murakami T., Niwa A. Morphologic and gene expression criteria for identifying human induced pluripotent stem cells. Plos One. 2012;7(12):e48677. doi: 10.1371/journal.pone.0048677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The International Stem Cell Banking Initiative Consensus guidance for banking and supply of human embryonic stem cell lines for research purposes. Stem Cell Rev. 2009;5(4):301–314. doi: 10.1007/s12015-009-9085-x. [DOI] [PubMed] [Google Scholar]
- 14.Narva E., Autio R., Rahkonen N., Kong L., Harrison N., Kitsberg D. High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nat Biotechnol. 2010;28(4):371–377. doi: 10.1038/nbt.1615. [DOI] [PubMed] [Google Scholar]
- 15.Tokunaga K., Saitoh N., Goldberg I.G., Sakamoto C., Yasuda Y., Yoshida Y. Computational image analysis of colony and nuclear morphology to evaluate human induced pluripotent stem cells. Sci Rep. 2014;4:6996. doi: 10.1038/srep06996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherf N., Herberg M., Thierbach K., Zerjatke T., Kalkan T., Humphreys P. Imaging, quantification and visualization of spatio-temporal patterning in mESC colonies under different culture conditions. Bioinformatics. 2012;28(18):i556–i561. doi: 10.1093/bioinformatics/bts404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suga M., Kii H., Niikura K., Kiyota Y., Furue M.K. Development of a monitoring method for nonlabeled human pluripotent stem cell growth by time-lapse image analysis. Stem Cells Transl Med. 2015;4(7):720–730. doi: 10.5966/sctm.2014-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan C., Hicks A., Guan X., Chen H., Bishop C.E. SNL fibroblast feeder layers support derivation and maintenance of human induced pluripotent stem cells. J Genet Genomics Yi chuan xue bao. 2010;37(4):241–248. doi: 10.1016/S1673-8527(09)60042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park Y.G., Lee S.E., Kim E.Y., Hyun H., Shin M.Y., Son Y.J. Effects of feeder cell types on culture of mouse embryonic stem cell in vitro. Dev Reprod. 2015;19(3):119–126. doi: 10.12717/DR.2015.19.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon C., Escobedo C., Valbuena D., Genbacev O., Galan A., Krtolica A. First derivation in Spain of human embryonic stem cell lines: use of long-term cryopreserved embryos and animal-free conditions. Fertil Steril. 2005;83(1):246–249. doi: 10.1016/j.fertnstert.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig T.E., Levenstein M.E., Jones J.M., Berggren W.T., Mitchen E.R., Frane J.L. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24(2):185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes T.G., Fernandes-Platzgummer A.M., da Silva C.L., Diogo M.M., Cabral J.M. Kinetic and metabolic analysis of mouse embryonic stem cell expansion under serum-free conditions. Biotechnol Lett. 2010;32(1):171–179. doi: 10.1007/s10529-009-0108-0. [DOI] [PubMed] [Google Scholar]
- 23.Chen X., Chen A., Woo T.L., Choo A.B., Reuveny S., Oh S.K. Investigations into the metabolism of two-dimensional colony and suspended microcarrier cultures of human embryonic stem cells in serum-free media. Stem Cells Dev. 2010;19(11):1781–1792. doi: 10.1089/scd.2010.0077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.