Abstract
The objective of this study is to investigate the effect of matrigel microspheres (MM), gelatin hydrogel microspheres (GM), and matrigel-coated GM on the proliferated and biological functions of epithelial cells in cell aggregates incorporating the microspheres. The MM were prepared by a coacelvation method. When mammary epithelial EpH4 cells were cultured with the MM, GM, and matrigel-coated GM in round U-bottom wells of 96-multiwell culture plates which had been coated with poly (vinyl alcohol) (PVA) to suppress the cell adhesion, EpH4 cell aggregates with each microspheres homogeneously incorporated were formed. Higher EpH4 cells proliferation was observed for cell aggregates incorporating MM, GM, and matrigel-coated GM compared with the conventional 3-dimensional (3D) culture method. When examined to evaluate the epithelial differentiation of EpH4 cells, the β-casein expression was significantly higher for the cell aggregates incorporating MM than that of aggregates incorporating GM and matrigel-coated GM or the conventional 3D culture method. It is concluded that the proliferation and differentiation of mammary epithelial EpH4 cells were promoted by the incorporation of MM.
Keywords: Cell aggregates, Three-dimensional culture, Epithelial cells, Gelatin hydrogel microspheres, Matigel microspheres, β-casein
1. Introduction
Recently, cell researches have become more and more popular to clarify the molecular mechanisms of cell proliferation and differentiation. The epithelium is the first emerging tissue during ontogenesis, and epithelial cells play fundamental roles in embryo morphogenesis and organ development [1], [2], [3], [4], [5]. Epithelial cells have segregated apical and basolateral plasma membrane domains with asymmetric compositions of nutrient and fluid transporters which are required to carry out crucial vectorial transport functions and cytoplasmic polarity to generate different cell progenies for tissue morphogenesis [6], [7]. However, there have been some problems by the culture of epithelial cells. In two-dimensional (2D) cell culture systems on a plastic plate, epithelial cells quickly lose their functions, and do not always proliferate as well as other types of cells. Because the local environment of epithelial cells is different from that of mesenchymal cells in living tissues [8]. As one tried to tackle this problems, epithelial cells are cultured with the feeder layer of fibroblasts for their proliferation, but their functions are biologically insufficient because of the lack of basement membrane components [9], [10], [11]. In three-dimensional (3D) cell culture systems, epithelial cells are often cultured with 3D basement membrane component-rich gels [12], [13]. Cell aggregates are formed with a central lumen and polarized structures, but cells are not proliferated well, while cells in center of aggregates die by apoptosis [14], [15], [16], [17], [18]. We demonstrate that mouse preosteoblast MC3T3-E1 cells were cultured with gelatin hydrogel microspheres (GM) to form the MC3T3-E1 cell aggregates homogeneously incorporating GM for an enhanced cell proliferation and osteogenic differentiation [19]. The GM incorporation enabled cells to rescue the lack of oxygen in cell aggregates.
In the physiological condition, most cells are present in a 3D structure in which the cell–cell and cell–extracellular matrix interactions are naturally to allow cells to survive and biologically function [20]. This 3D structure of cells is important and essential to promote their functions. For example, embryonic stem cells generally aggregate to form an embryoid body, and consequently initiate their differentiation into different cell lineages [21]. The aggregation of liver cells to form a spheroid is necessary to enhance their metabolic activity [22]. Cell aggregates produce extracellular matrix proteins more efficiently than single cells [23]. Considering the cell structure of body tissues, such as liver and bone, cell aggregates biologically function as the minimum unit [24].
The objective of this study is to prepare a new 3D aggregates culture system of epithelial cells for an enhanced cell proliferation and differentiation. In this study, matrigel microspheres (MM) and matrigel-coated GM were prepared. Mouse mammary epithelial EpH4 cells were cultured with the microspheres to form cell aggregates homogeneously incorporating microspheres to evaluate the proliferation and differentiation in terms of the expression of differentiation markers. We examine the effect of MM, GM, and matrigel-coated GM on the cell behavior.
2. Materials and methods
2.1. Preparation of matrigel microspheres
Matrigel microspheres (MM) were prepared by a coacelvation method [25]. According to the coacelvation method, nanospheres or microspheres with narrow-size distribution and small size were prepared. Briefly, 1.0 ml of 10 vol% aqueous Becton, Dickinson and Company (BD) Matrigel™ Basement Membrane Matrix (BD Biosciences, Inc., Franklin Lakes, America) solution was prepared at 4 °C. Then, 4 ml of 2-butanol (Nacalai Tesque, Inc., Kyoto, Japan) was added to the matrigel solution at 4 °C. The resulting microspheres were gelationed for 1 h at 37 °C. Then, 2-butanol was removed by evaporation, and followed by centrifuged for 5 min at 14,000 rpm at 4 °C to obtain MM. The MM were stored at −30 °C until to use.
2.2. Preparation of gelatin hydrogel microspheres
Gelatin hydrogel microspheres (GM) were prepared by the chemical crosslinking of gelatin in a water-in-oil emulsion state according to the method previously reported [26]. Briefly, an aqueous solution (20 ml) of 10 wt% gelatin (isoelectric point 5.0, weight-averaged molecular weight 1,00,000, Nitta Gelatin Inc., Osaka, Japan) was preheated at 40 °C, and then added dropwise into 600 ml of olive oil (Wako Ltd, Osaka, Japan) at 40 °C, followed by stirring at 400 rpm for 10 min to prepare a water-in-oil emulsion. The emulsion temperature was decreased to 4 °C for the natural gelation of gelatin solution to obtain non-crosslinked hydrogel microspheres. The resulting microspheres were washed three times with cold acetone in combination with centrifugation (5000 rpm, 4 °C, 5 min) to completely exclude the residual oil. Then, they were fractionated by size using sieves with apertures of 20 μm (Iida Seisakusho Ltd, Osaka, Japan) and air dried at 4 °C. The non-crosslinked and dried gelatin hydrogel microspheres (200 mg) were treated in a vacuum oven at 140 °C and 0.1 Torr for the dehydrothermal crosslinking of gelatin for 24 h according to the method previously reported [19]. The pictures of gelatin hydrogel microspheres in the water swollen state were taken with a microscope (CKX41, Olympus Ltd, Tokyo, Japan). The size of 100 microspheres for each sample was measured using the computer program Image J (NIH Inc., Bethesda, USA) to calculate the average size.
2.3. Preparation of matrigel-coated gelatin hydrogel microspheres
The matrigel solution (20 μl) was dropped onto 2 mg of freeze-dried gelatin hydrogel microspheres (GM), followed by leaving at 4 °C for overnight to allow matrigel to absorb onto the microspheres. The matrigel solution was completely absorbed into the GM because the solution volume was much less than that theoretically required for the equilibrated swelling of microspheres. To evaluate the matrigel existence on the surface of matrigel-coated GM were incubated with an anti-laminin antibody (Abcam Inc., Cambridge, UK) for 60 min at 25 °C and subsequently Alexa Fluor® 488 Donkey anti-rabbit (Thermo Fisher Inc., Massachusetts, America) for 30 min at 25 °C, followed by fluorescent viewing with confocal laser scanning microscope (FV1000D, Olympus Ltd, Tokyo, Japan).
2.4. EpH4 cell culture
EpH4 cells of a mouse mammary epithelial cell line were transfected and clones selected as previously described [27]. EpH4 were cultured in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) (Thermo Inc., Waltham, USA) supplemented with 2 vol% fetal calf serum (FCS) (Thermo Inc., Waltham, USA), gentamicin, 3 μg/ml prolactin (Sigma–Aldrich Inc., St. Louis, America), 1 μg/ml hydrocortisone (Sigma–Aldrich Inc., St. Louis, America) and 5 μg/ml insulin (Sigma–Aldrich Inc., St. Louis, America) (standard medium) and cultured at 37 °C in a 95% air–5% CO2 atmosphere. The culture medium was changed every 2 days and confluent cells were subcultured through trypsinisation. In the experiment of phosphotidylinositol 3-kinase (PI3K, Sigma–Aldrich Inc., St. Louis, America) inhibition experiment of EpH4 cells, the standard medium containing a PI3K inhibitor LY294002 (50 μM, Abcam Inc., Cambridge, British) was used and the cells were cultured for 72 h.
2.5. Preparation of cell aggregates incorporating MM, GM, and matrigel-coated GM
Poly(vinyl alcohol) (PVA, 1800 degree of polymerization and 88 mol% saponification) kindly supplied from Unichika (Tokyo, Japan) was dissolved in 1× Dulbecco's phosphate buffered saline (PBS, Nissui Ltd, Tokyo, Japan) to give a concentration of 1 wt%. The PVA solution (100 μl/well) was added to each well of 96-multiwell culture plate with either flat- or round-bottomed (U-bottomed) wells and incubated at 37 °C for 15 min. Then, the solution was removed by aspiration and the wells were washed twice with PBS (100 μl/well). MM, GM or matrigel-coated GM and EpH4 cells were separately suspended in the standard medium. The microspheres-free standard medium or the suspensions of MM, GM, and matrigel-coated GM (0, 2 × 103, 2 × 104, 2 × 105 microspheres/ml) (50 μl/well) were added to the PVA-coated wells, followed by the addition of EpH4 cells suspension (2 × 105 cells/ml, 50 μl/well). For the conventional 3D epithelial cells culture, 10 μl of matrigel solution was placed into the flat-bottomed wells of 96-well flat-bottomed culture plates at 4 °C, and then the culture plates were incubated at 37 °C for 15 min for matrigel polymerization. Next, EpH4 cells suspension (1 × 105 cells/ml, 100 μl/well) containing 2 vol% matrigel was added in the matrigel-treated wells. The pictures of cells 3D cultured with were taken with a microscope (CKX41, Olympus Ltd, Tokyo, Japan).
2.6. Evaluation of cells viability in cell aggregates incorporating MM, GM, and matrigel-coated GM
Live/dead assays were conducted using Live/Dead@ Viability/Cytotoxicity assay (Invitrogen Inc., Carlsbad, UK) according to the manufacture's protocol. After 7 days culture, cell aggregates were rinsed with PBS, and then incubated with a solution containing 2 μM calcein AM and 4 μM EthD-1 in PBS for 30 min at 37 °C in the dark, followed by fluorescent viewing with the confocal laser scanning microscope.
2.7. Measurement of live cells number in cell aggregates incorporating MM, GM, and matrigel-coated GM
The number of live cells in EpH4 cell aggregates with MM, GM or matrigel-coated GM incorporation was determined by counting the number of cell nuclei after the crystal violet staining [28]. Briefly, a mixed solution of 0.2 M citric acid and 0.2 wt% crystal violet was added (100 μl/well) to each well of well plate 7 days after EpH4 cell culture with MM, GM, and matrigel-coated GM. After crystal violet staining the cells were lysed in 0.1 wt% Triton X-100 in PBS at 37 °C overnight to extract the nuclei from the cells. After pipetting, the nuclei collected were viewed under a microscope (CKX31-11PHP, Olympus Ltd, Tokyo, Japan) and counted in a hemocytometer (OneCell Inc., Hiroshima, Japan). The nuclei of live cells are generally round, while dead cell nuclei are irregularly shaped. Base on the nucleus shape, live cells can be distinguished from dead cells to assess the number of live cells.
2.8. Measurement of l-lactic acid/glucose ratio of cell aggregates incorporating MM, GM, and matrigel-coated GM
EpH4 cells were similarly cultured with MM, GM, or matrigel-coated GM to form the cell aggregates. The amount of glucose consumed by EpH4 cell aggregates was determined by measuring the change in glucose concentration in the culture medium using a Glutest Neo Super test kit (Sanwa kagaku kenkyusyo Ltd, Kyoto, Japan) 7 days after incubation. Similarly, the amount of l-lactic acid produced by EpH4 cell aggregates was determined with an E-kit (R-Biopharm AG Ltd, Germany) 7 days after incubation. The number of live cells was determined by the crystal violet staining as described above and used to normalize the amount of glucose consumption and l-lactic acid produced by the number of live cells. The l-lactic acid/glucose ratio was calculated as a measure of the aerobic metabolism of cells [29].
2.9. Immunostaining of cell aggregates incorporating MM, GM, and matrigel-coated GM
EpH4 cells were similarly cultured with MM, GM, or matrigel-coated GM to form the cell aggregates. After incubation for 7 days, the cell aggregates were fixed with 4 vol% paraformaldehyde at 4 °C for 1 h and embedded in optimal cutting temperature compound (Sakura Finetek Japan Ltd, Tokyo, Japan) and frozen in liquid nitrogen. The frozen samples were sectioned using a cryotome (CM3050S, Leica Microsystems, Wetzlar, Germany) and incubated at 4 °C overnight with the following primary antibodies: β-casein (Santacruz Inc., America, 1:50) or Laminin (Abcam Inc., Cambridge, UK, 1:100). Then, secondary antibodies coupled to Alexa 488 (Molecular Probes, Invitrogen Inc., Carlsbad, America, 1:700) were incubated at 25 °C for 30 min for fluorescent viewing. Next, TO-PRO-3 (Molecular Probes, Eu-gene, USA) was added to incubate at 25 °C for 10 min for the nuclear staining of cells. The sections of 10 μm thickness were viewed in a confocal laser scanning microscope (FV1000D, Olympus Ltd, Tokyo, Japan).
2.10. Measurement of β-casein and E-cadherin expression in cell aggregates incorporating MM, GM, and matrigel-coated GM
The messenger ribonucleic acid (mRNA) expression of β-casein and E-cadherin was evaluated by real-time polymerase chain reaction (PCR). Briefly, EpH4 cells were cultured with MM, GM, or matrigel-coated GM to form the cell aggregates. After incubation for 7 days, RNA was extracted and cleaned up using the Qiagen RNeasy® Plus Mini kit (Qiagen Ltd, Hilden, Germany) according to the manufacturer's instructions. The total RNA sample was reverse-transcribed to cDNA using SuperScript VILO™ (Invitrogen, Carlsbad, CA). The resulting cDNA was then subjected to real-time PCR by using Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems Inc., Carlsbad, CA) to determine the gene expression. The PCR reaction was carried out with specific primers in the presence of Power SYBR Green (Applied Biosystems Inc., Carlsbad, CA) as described in the manufacturer's instruction. The level of gene expression was normalized by that of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression, and calculated as the ratio to that of EpH4 cell culture without microspheres of a control group. Experiments were performed for 4 specimens independently for each sample. The primer sequences for GAPDH and β-casein were as follows, GAPDH: 5-TCCACAAGGACAGAGTCAGATTAC-3, 5-TGGCTCAGATAGGAGGGGTA-3; β-casein: 5-GGTGAATCTCATGGGACAGC-3, 5-TGACTGGATGGTGGAGTGAA-3; E-cadherin: 5-CAGAATGACAACAGGCCAGA, 5-TTCATCACGGAGGTTGGTG.
2.11. Statistical analysis
All the statistical data are expressed as the mean ± standard error of the mean (SEM). The data were analyzed by t-test to determine the statistical significance of differences between two mean values, which was accepted at <0.05.
3. Results
3.1. Characterization of MM, GM, and matrigel-coated GM
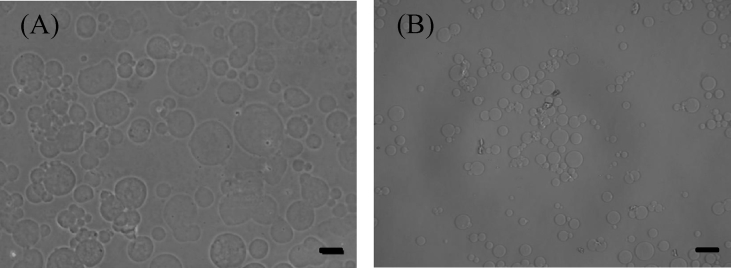
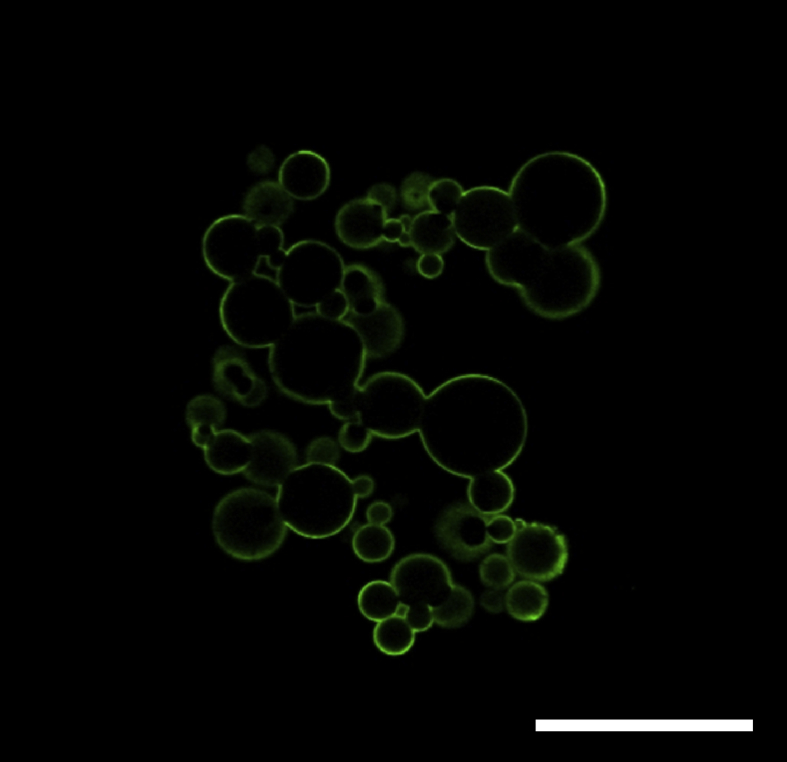
Fig. 1 shows the microscopic pictures of MM and GM dispersed in water. The microspheres were of spherical shape and had a smooth surface. The size of the matrigel and gelatin microspheres in the swollen condition was 19.0 ± 6.5 and 16.0 ± 5.6 μm, respectively. Fig. 2 shows the microscopic picture of matrigel-coated GM dispersed in water. Matrigel was localized on the surface of GM. The size of matrigel-coated GM was 17.0 ± 5.8 μm. The size and aggregate formation of GM were not changed by the matrigel coating.
Fig. 1.
Light microscopic pictures of MM and GM dispersed in water. The GM were dehydrothermally crosslinked for 18 h at 140 °C. Scale bar. 20 μm.
Fig. 2.
A confocal microscopic picture of matrigel-coated GM dispersed in water. The GM were dehydrothermally crosslinked for 18 h at 140 °C. The laminin of matrigel was immunostained. In green. Scale bar. 100 μm.
3.2. Formation of cell aggregates incorporating MM, GM, and matrigel-coated GM
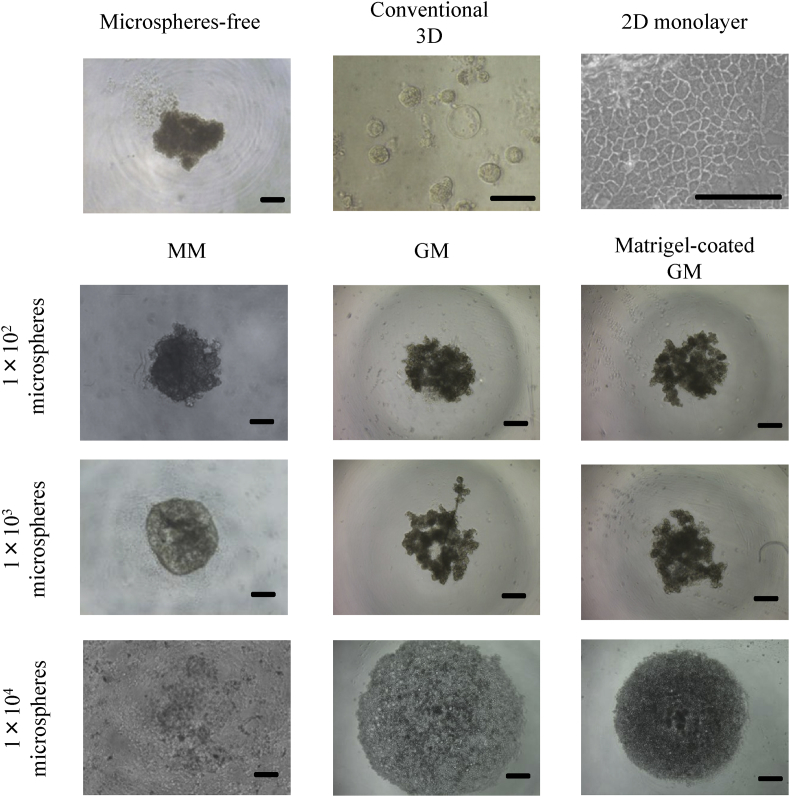
Fig. 3 shows the microscopic pictures of EpH4 cell aggregates 7 days after incubation without or with MM, GM, and matrigel-coated GM in the U-bottomed wells of culture plate, and EpH4 cells cultured by other methods. EpH4 cell aggregates were not formed when the number of microspheres added initially was 1 × 104/well. EpH4 cell aggregates were formed for cultured without or with the microspheres added number of 1 × 102 and 1 × 103/well and the conventional 3D method. The shape of EpH4 cell aggregates was of spherical when the number of MM added initially was 1 × 103/well or for the conventional 3D method. However, the shape of EpH4 cell aggregates was not of spherical, but oval shape for cell aggregates cultured without or with 1 × 102 of MM, GM, and matrigel coated GM.
Fig. 3.
Light microscopic pictures of EpH4 cell aggregates 7 days after incubation of EpH4 cells with MM, GM, and matrigel-coated GM or without microspheres. EpH4 cells were cultured as by the conventional 3D and 2D monolayer culture methods. The number of EpH4 cells added initially was 1 × 104/well while that of microspheres was 1 × 102, 1 × 103 or 1 × 104/well. Scale bar. 100 μm.
3.3. Cell viability of cell aggregates incorporating MM, GM, and matrigel-coated GM
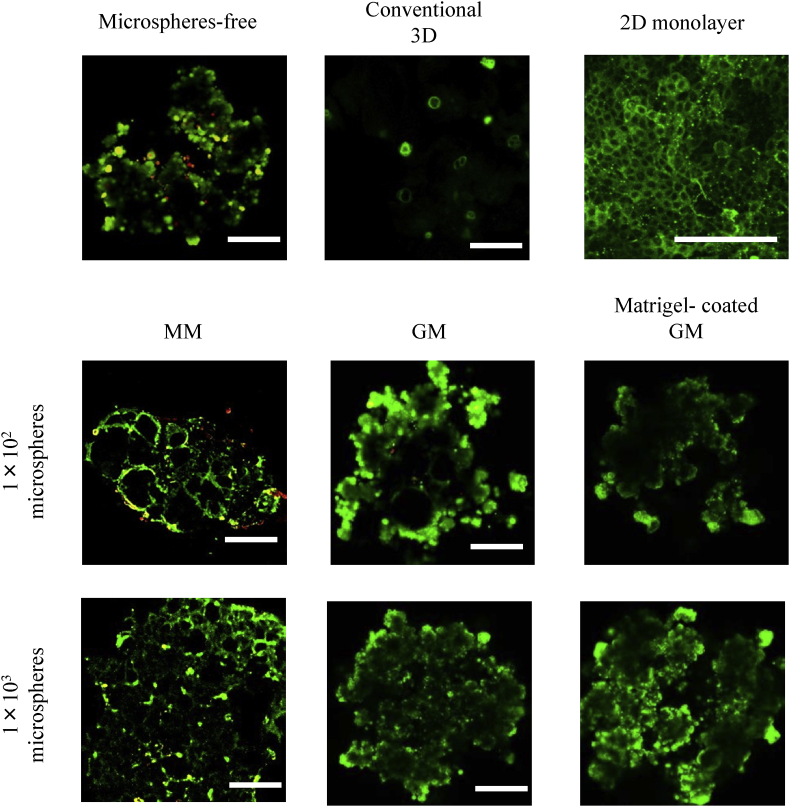
Fig. 4 shows the fluorescent pictures of EpH4 cell aggregates 7 days after incubation without or with MM, GM, and matrigel-coated GM incorporating. Irrespective of the culture method, EpH4 cells in cell aggregates were alive.
Fig. 4.
Live/dead assay of EpH4 cell aggregates 7 days after incubation of EpH4 cells with MM, GM, and matrigel-coated GM or without microspheres. EpH4 cells were cultured by the conventional 3D and 2D monolayer methods. The number of EpH4 cells added initially was 1 × 104/well while that of microspheres was 1 × 102 or 1 × 103/well. Scale bar. 100 μm.
3.4. Live cells number of cell aggregates incorporating MM, GM, and matrigel-coated GM
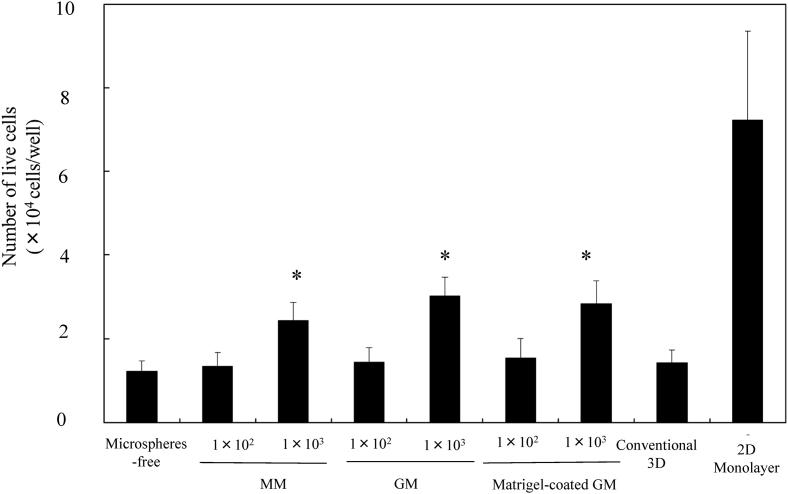
Fig. 5 shows the number of live EpH4 cells 7 days after incubation without or with MM, GM, and matrigel-coated GM incorporation, and cultured by the conventional 3D and 2D monolayer culture methods. The number of live EpH4 cells of cell aggregates incorporating 1 × 103 of MM, GM, and matrigel-coated GM was significantly high compared that of cell aggregates without microspheres incorporation and the conventional 3D method. However, the number was lower than that of 2D culture method.
Fig. 5.
The number of live EpH4 cells 7 days after incubation of EpH4 cells with MM, GM, and matrigel-coated GM or without microspheres. EpH4 cells were cultured by the conventional 3D and 2D monolayer methods. The number of EpH4 cells added initially was 1 × 104/well while that of microspheres was 1 × 102 or 1 × 103/well. *, p < 0.05; significant against the number of live EpH4 cells in EpH4 cell aggregates cultured without microspheres.
3.5. l-lactic acid/glucose ratio of cell aggregates incorporating MM, GM, and matrigel-coated GM
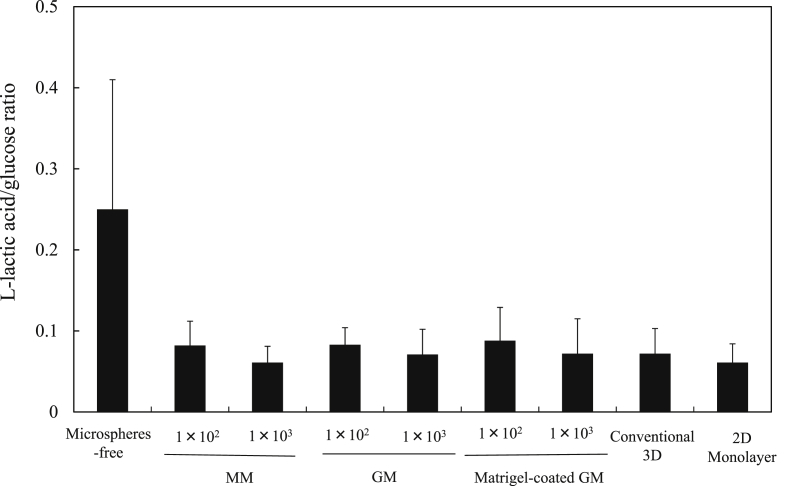
Fig. 6 shows the l-lactic acid/glucose ratio of EpH4 cells in EpH4 cell aggregates 7 days after incubation with or without MM, GM, and matrigel-coated GM. The higher l-lactic acid/glucose ratio was observed for EpH4 cells in cell aggregates without microspheres, although there was no significant difference in the l-lactic acid/glucose ratio among the experiment groups.
Fig. 6.
The l-lactic acid/glucose ratio of live EpH4 cells 7 days after incubation of EpH4 cells with MM, GM, and matrigel-coated GM or without microspheres. EpH4 cells were cultured by the conventional 3D and 2D monolayer methods. The number of EpH4 cells added initially was 1 × 104/well while that of microspheres was 1 × 102 or 1 × 103/well.
3.6. Laminin and β-casein expression of cell aggregates incorporating MM, GM, and matrigel-coated GM
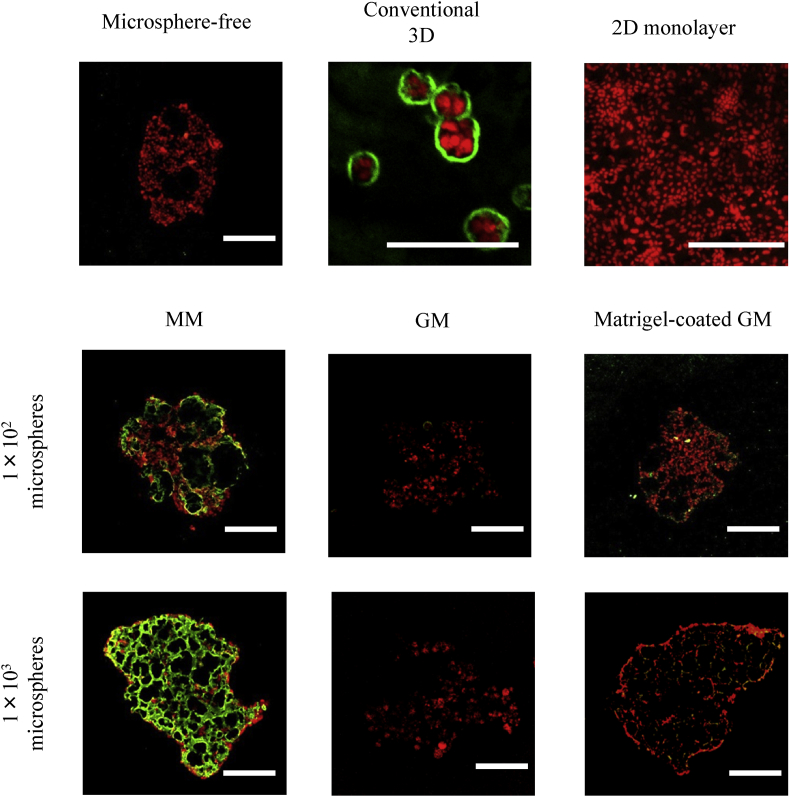
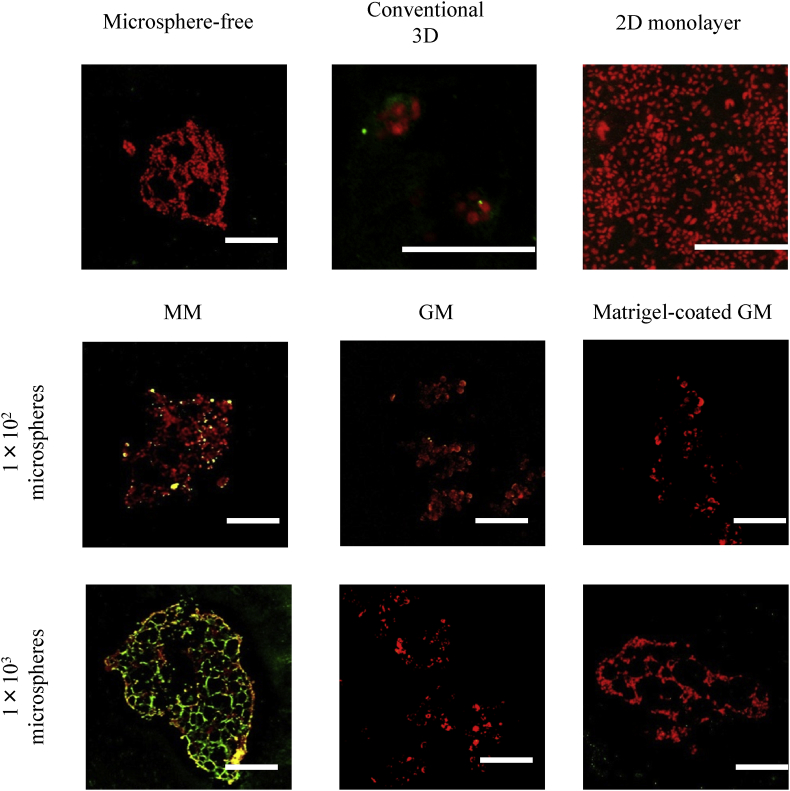
Fig. 7, Fig. 8 show the fluorescent microscopic pictures of laminin and β-casein of cell aggregates 7 days after incubation without or with MM, GM, and matrigel-coated GM incorporation, and cells cultured by the conventional 3D and 2D monolayer culture methods. The higher expression of β-casein was observed for EpH4 cell aggregates incorporating 1 × 103 of MM compared with other groups. For the conventional 3D culture method, the expression of β-casein was observed, but the extent of β-casein expression was lower than that of EpH4 cell aggregates incorporating 1 × 103 of MM. The poor expression of β-casein was observed for EpH4 cell aggregates without or with GM and matrigel-coated GM incorporation. The higher expression of laminin was observed for EpH4 cell aggregates incorporating 1 × 103 of MM, and the conventional 3D culture method. The expression of laminin was higher for EpH4 cell aggregates incorporating 1 × 103 of MM than those of 1 × 102 of MM. The expression of laminin was poor for EpH4 cell aggregates incorporating matrigel-coated GM.
Fig. 7.
Confocal microscopic pictures of laminin expression of EpH4 cells 7 days after incubation of EpH4 cells with MM, GM, and matrigel-coated GM or without microspheres. EpH4 cells were cultured by the conventional 3D and 2D monolayer methods. The number of EpH4 cells added initially was 1 × 104/well while that of microspheres was 1 × 102 or 1 × 103/well. Scale bar. 100 μm.
Fig. 8.
Confocal microscopic pictures of β-casein expression of EpH4 cells 7 days after incubation of EpH4 cells with MM, GM, and matrigel-coated GM or without microspheres. EpH4 cells were cultured by the conventional 3D and 2D monolayer methods. The number of EpH4 cells added initially was 1 × 104/well while that of microspheres was 1 × 102 or 1 × 103/well. Scale bar. 100 μm.
3.7. β-casein and E-cadherin expression of cell aggregates incorporating MM, GM, and matrigel-coated GM
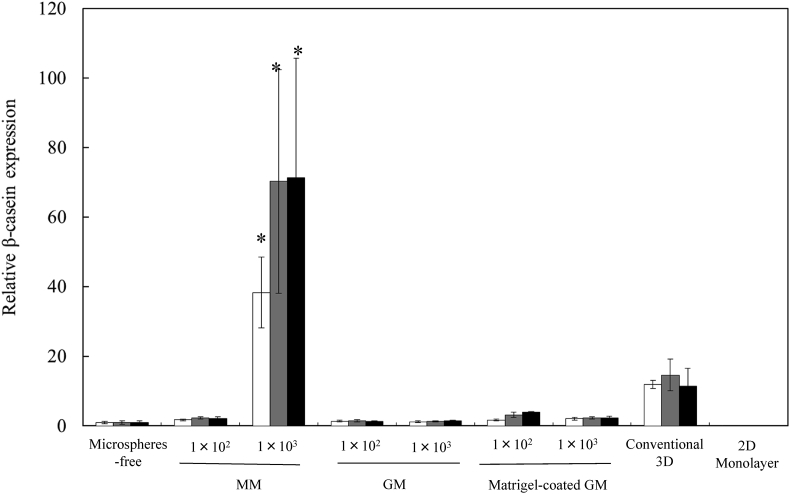
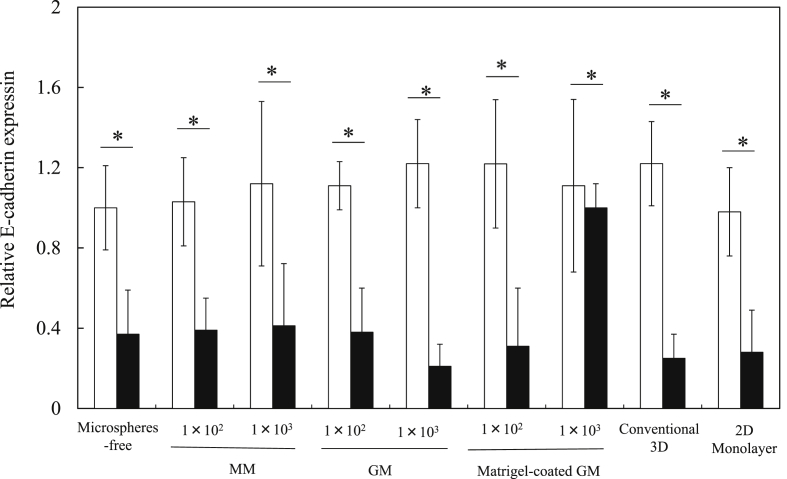
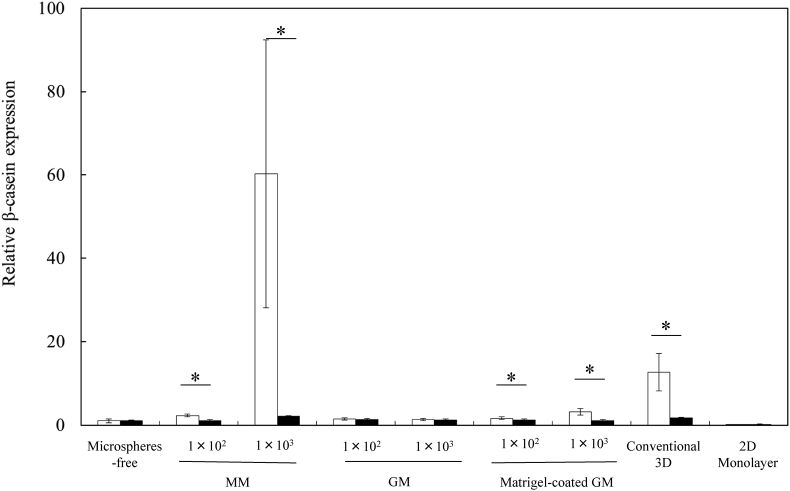
Fig. 9 shows the β-casein expression of EpH4 cell aggregates 4, 7, and 14 days after incubation without or with MM, GM, and matrigel-coated GM and EpH4 cells cultured by the conventional 3D and 2D monolayer culture methods. The β-casein expression of cells cultured by the 3D culture method was higher than that of microspheres-free and 2D monolayer culture methods. The higher expression of β-casein was observed for cell aggregates incorporating 1 × 103 of MM than that of 3D culture methods. Fig. 10, Fig. 11 show the β-casein and E-cadherin expressions of EpH4 cell aggregates 7 days after incubation without or with MM, GM, and matrigel-coated GM incorporation and EpH4 cells cultured by the conventional 3D and 2D monolayer culture methods in the presence or absence of LY294002. For the culture without LY294002 addition, no difference in the expression level of E-cadherin was observed in any experimental conditions. Irrespective of the culture method, the expression of β-casein and E-cadherin significantly was inhibited by the LY294002 addition.
Fig. 9.
The β-casein expression of EpH4 cells 4 (□), 7 ( ), and 14 days (■) after incubation of EpH4 cells with MM, GM, and matrigel-coated GM or without microspheres. EpH4 cells were cultured by the conventional 3D and 2D monolayer methods. The number of EpH4 cells added initially was 1 × 104/well while that of microspheres was 1 × 102 or 1 × 103/well. *, p < 0.05; significant against the other groups at corresponding time points.
), and 14 days (■) after incubation of EpH4 cells with MM, GM, and matrigel-coated GM or without microspheres. EpH4 cells were cultured by the conventional 3D and 2D monolayer methods. The number of EpH4 cells added initially was 1 × 104/well while that of microspheres was 1 × 102 or 1 × 103/well. *, p < 0.05; significant against the other groups at corresponding time points.
Fig. 10.
The E-cadherin expression of EpH4 cells 7 days after incubation of EpH4 cells with MM, GM, and matrigel-coated GM or without microspheres and EpH4 cells were cultured by the conventional 3D and 2D monolayer methods in the absence (□) or presence of PI3K inhibitor LY294002 (■). The number of EpH4 cells added initially was 1 × 104/well while that of microspheres was 1 × 102 or 1 × 103/well. *, p < 0.05; significant against between two groups.
Fig. 11.
The β-casein expression of EpH4 cells 7 days after incubation of EpH4 cells with MM, GM, and matrigel-coated GM or without microspheres and EpH4 cells were cultured by the conventional 3D and 2D monolayer methods in the absence (□) or presence of PI3K inhibitor LY294002 (■). The number of EpH4 cells added initially was 1 × 104/well while that of microspheres was 1 × 102 or 1 × 103/well. *, p < 0.05; significant against between two groups.
4. Discussion
The size of microspheres affects the behavior and functions of cell aggregates. In this study, thus, microspheres with a similar diameter were prepared to exclude the effect of microspheres size (Fig. 1). It is practically difficult to prepare the large size of matrigel microspheres by the coacelvation method. The present method allowed us to prepare only 19.0 ± 6.5 μm of matrigel microspheres with a good reproducibility. In addition, as control, the gelatin microspheres with the same size of matrigel microspheres were readily prepared as well. The matrigel coating did not change the size of GM (Fig. 2). The fluorescent observation indicated that the laminin of a matrigel component was localized on the surface of GM (Fig. 2). Taken together, it is possible that three types of MM, GM, and matrigel-coated GM are good microspheres samples to evaluate the influence of microsphere nature on the biological behavior of cell aggregates.
EpH4 cell aggregates incorporating GM and matrigel-coated GM were formed, but were not of spherical and oval shape. On the other hand, EpH4 cell aggregates incorporating 1 × 103 of MM and prepared by the conventional 3D culture method were of spherical shape (Fig. 3). The reason why the shape of EpH4 cell aggregates was different is unclear at present. It is conceivable that the oval shape of EpH4 cell aggregates may be caused by the lack of matrigels and a strong cell–cell contact. Another study reported that the matrigel/integrin signaling transduction and strong cell–cell contact were important for morphogenetic changes and the formation of spherical structures [30], [31], [32]. On the other hand, the EpH4 cell aggregates incorporating larger number of microspheres were not formed. Therefore, EpH4 cell aggregates incorporating small number of microspheres were used for the following experiments.
The number of live EpH4 cells in cell aggregates incorporating 1 × 103 of MM, GM, and matrigel-coated GM was significantly high compared that of the conventional 3D culture method (Fig. 5). However, the number of live EpH4 cells was much higher for the 2D culture method than other experimental groups. This may be due to the difference of surface area between microspheres and culture plates as the scaffold of cell proliferation. The surface area of 2D culture plates was much higher than that of 1 × 103 of microspheres, resulting in higher proliferation of cells in 2D culture plates. Higher proliferation of EpH4 cells was observed for cell aggregates incorporating microspheres compared with that of 2D culture when the area of microsphere surface was equal to the 2D culture surface (data not shown). In addition, the cell proliferation was observed for EpH4 cell aggregates incorporating larger number of microspheres. This results obtained in this study is similar to the results previously reported [33]. The microspheres would act on the scaffold of cell proliferation. The proliferation was not influences by the type of microspheres incorporated (Fig. 5).
The ratio of l-lactic acid to glucose is a general measure of aerobic metabolism [29]. The lower the ratio is, the higher the aerobic metabolism of cell aggregates is. In the previous study, lower l-lactic acid/glucose ratio was observed for cell aggregates with GM than that of cell aggregates without GM. However, in this study, the l-lactic acid/glucose ratio of cells aggregates incorporating MM, GM, and matrigel-coated GM was similar to that of microspheres-free cell aggregates (Fig. 7). This can be explained by the shape of cell aggregates. Mesenchymal cell aggregates without GM were of spherical shape and the thickness of cell aggregates over 150 μm, which results in lack of the oxygen and nutrients supply into aggregates [19], [33]. However, in this study, the epithelial cell aggregates without GM were of not spherical, but oval shape, and the thickness of cell aggregates was smaller than 100 μm.
Prolactin is an important hormone to stimulate the milk synthesis of mammary epithelial cells. The signals through the Jak2–Stat5 pathway to induce the expression of a milk protein, such as β-casein, which is often used as a marker for mammary differentiation [32], [33], [34], [35], [36]. In addition, cells adhesion to basement membrane proteins is required for the expression of milk protein genes [37], [38], [39]. In the evaluation study of laminin, β-casein, and E-cadherin expression of EpH4 cells in cell aggregates or cultured by other methods, the higher expression of laminin and β-casein was observed for EpH4 aggregates incorporating 1 × 103 of MM than that of aggregates incorporating 1 × 102 of MM and matrigel-coated GM. This can be explained by the amount of laminin in EpH4 cell aggregates incorporating microspheres. Another study reported that the larger amount of laminin enhanced the expression of β-casein of epithelial cells [38], [40]. Laminin is one of the major matrigels components. The amount of laminin for 1 × 103 of MM was larger than that 1 × 102 of MM and matrigel-coated GM (data not shown). After 7 days culture of cell aggregates, no laminin expression of EpH4 cell aggregates was observed for the matrigel-coated GM. It is likely that GM were degraded by enzymes secreted from EpH4 cells, leading to a decrease in the matrigel amount on GM. This may be due to the degradation or detachment of matrigel from GM. In addition, it is conceivable that the stiffness of MM is different from that of matrigel-coated GM, resulting in different cell responses. A small laminin amount and different stiffness of matrigel-coated GM incorporated into EpH4 cell aggregates would lead to poor β-casein expression compared with that of MM. The higher β-casein expression was observed for EpH4 cell incorporated 1 × 103 of MM than that of cultured by the conventional 3D method (Fig. 11). In cell aggregates incorporating MM, the 3D cell–cell interaction is well achieved and also the cells directly interact with matrigel. The better 3D cell–cell interaction of EpH4 cell aggregates than that of the conventional 3D method would enable cells to increase the level of β-casein expression. However, the reason is unclear at present. Detailed investigation on the mechanism of epithelial differentiation is required.
PI3K is a key mediator in the laminin-induced signaling cascade, which controls the activity of transcription factors essential for the gene expression of milk protein [39]. In addition, PI3K maintained the adherence junction for milk protein gene expression [34]. To investigate whether or not the PI3K is involved in the β-casein and E-cadherin expression of EpH4 cells, EpH4 cell aggregates with MM, GM, matrigel-coated GM were cultured with or without LY294002 of a PI3K inhibitor [41]. The β-casein expression was mainly induced by prolactin/STAT5 and laminin/integrin/PI3K signaling cascade. For all the experiment groups, the β-casein expression was inhibited by the LY294002 addition. This indicates that the PI3K signal is required for the β-casein expression of EpH4 cells. Based on this, laminin present in MM would induce to promote the PI3K signaling cascade, resulting in higher β-casein expression. The result of this study was similar to that previously reported. In this study, for the culture without LY294002 addition, no difference in the expression of E-cadherin was observed in any experimental conditions without LY294002 addition (Fig. 7). This can be explained by the aerobic metabolism of EpH4 cell aggregates. It has been demonstrated that epithelial cells often lost the E-cadherin expression by epithelial–mesenchymal transition under a hypoxic condition [42], [43]. In this study, EpH4 cells in cell aggregates without or with microspheres incorporation were not under a hypoxic condition (Fig. 4, Fig. 6). Taken together, we can say certainty that the E-cadherin expression was maintained without epithelial–mesenchymal transition.
5. Conclusions
Upon co-culturing EpH4 cells with MM in PVA-coated U-bottomed wells, cell aggregates were formed with MM homogeneously distributed. The EpH4 cells proliferation was higher for EpH4 cell aggregates containing MM compared with the conventional 3D culture method. The expression of β-casein expression as a measurement of epithelial differentiation was higher for EpH4 cell aggregates containing MM compared with that of the conventional 3D culture method. It is concluded that incorporation into EpH4 cell aggregates is promising to promote the proliferation and differentiation of EpH4 cells in aggregates.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Thesleff I., Vainio S. Epithelial-mesenchymal interactions in tooth morphogenesis: the roles of extracellular matrix, growth factors, and cell surface receptors. J Craniofac Genet Dev Biol. 1991;11(4):229–237. [PubMed] [Google Scholar]
- 2.Cunha G.R., Hom Y.K. Role of mesenchymal-epithelial interactions in mammary gland development. J Mammary Gland Biol Neoplasia. 1996;1(1):21–35. doi: 10.1007/BF02096300. [DOI] [PubMed] [Google Scholar]
- 3.Minoo Parviz, King Richard J. Epithelial-mesenchymal interactions in lung development. Annu Rev Physiol. 1994;56:13–45. doi: 10.1146/annurev.ph.56.030194.000305. [DOI] [PubMed] [Google Scholar]
- 4.Aufderheide E., Chiquet-Ehrismann R., Ekblom P. Epithelial-mesenchymal interactions in the developing kidney lead to expression of tenascin in the mesenchyme. J Cell Biol. 1987;105(1):599–608. doi: 10.1083/jcb.105.1.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botchkarevn Vladimir A., Kishimoto Jiro. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc. 2003;8(1):46–55. doi: 10.1046/j.1523-1747.2003.12171.x. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Boulan Enrique, Macara Ian G. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15(4):225–242. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergstralh D.T., Haack T., St Johnston D. Epithelial polarity and spindle orientation: intersecting pathways. Philos Trans R Soc Lond B Biol Sci. 2013 Sep 23;368(1629) doi: 10.1098/rstb.2013.0291. 20130291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shamir Eliah R., Ewald Andrew J. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Mol Cell Biol. 2014;15:647–664. doi: 10.1038/nrm3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor-Papadimitriou J., Shearer M., Stoker M.G. Growth requirements of human mammary epithelial cells in culture. Int J Cancer. 1977;20:903–908. doi: 10.1002/ijc.2910200613. [DOI] [PubMed] [Google Scholar]
- 10.Miller D.R., Hamby K.M., Slaga T.J. Contact-stimulated proliferation of cultured mouse epidermal cells by 3T3 feeder layers: inhibition of proliferation by 12-O-tetradecanoylphorbol-13-acetate (TPA) J Cell Physiol. 1982;112(1):76–82. doi: 10.1002/jcp.1041120112. [DOI] [PubMed] [Google Scholar]
- 11.Levine J.F., Stockdale F.E. 3T3-L1 adipocytes promote the growth of mammary epithelium. Exp Cell Res. 1984;151(1):112–122. doi: 10.1016/0014-4827(84)90361-6. [DOI] [PubMed] [Google Scholar]
- 12.Baatout S., Cheţa N. Matrigel a useful tool to study endothelial differentiation. Rom J Intern Med. 1996;34(3–4):263–269. [PubMed] [Google Scholar]
- 13.Kleinman H.K., Martin G.R. Matrigel basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Weaver V.M., Lelièvre S., Lakins J.N., Chrenek M.A., Werb Z., Bissell M.J. Beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2(3):205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debnath J., Mills K.R., Collins N.L., Reginato M.J., Muthuswamy S.K., Brugge J.S. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002 Oct 4;111(1):29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 16.Niemann C., Brinkmann V., Spitzer E., Hartmann G., Sachs M., Birchmeier W. Reconstitution of mammary gland development in vitro: requirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis. J Cell Biol. 1998 Oct 19;143(2):533–545. doi: 10.1083/jcb.143.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinkmann V., Foroutan H., Sachs M., Weidner K.M., Birchmeier W. Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J Cell Biol. 1995;131:1573–1586. doi: 10.1083/jcb.131.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C.F., Chang Y.J., Hsueh Y.Y., Hughes M., Chuong C.M., Wu C.C. Assembling composite dermal papilla spheres with adipose-derived stem cells to enhance hair follicle induction. Sci Rep. 2016;23 doi: 10.1038/srep26436. 26436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tajima Shuhei, Tabata Yasuhiko. Preparation and functional evaluation of cell aggregates incorporating gelatin microspheres with different degradabilities. J Tissue Eng Regen Med. 2013;10:801–811. doi: 10.1002/term.1469. [DOI] [PubMed] [Google Scholar]
- 20.Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103(5):389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda J., Sakai Y., Nakazawa K. Novel hepatocyte culture system developed using microfabrication and collagen/polyethylene glycol microcontact printing. Biomaterials. 2006;27(7):1061–1070. doi: 10.1016/j.biomaterials.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-Enríquez S., Gallardo-Pérez J.C., Avilés-Salas A., Marín-Hernández A., Maldonado-Lagunas V., Moreno-Sánchez R. Energy metabolism transition in multi-cellular human tumor spheroids. J Cell Physiol. 2008;216(1):189–197. doi: 10.1002/jcp.21392. [DOI] [PubMed] [Google Scholar]
- 23.Lin R.Z., Chang H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3(9–10):1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 24.Nelson L.J., Walker S.W., Hayes P.C., Plevris J.N. Low-shear modelled microgravity environment maintains morphology and differentiated functionality of primary porcine hepatocyte cultures. Cells Tissues Organs. 2010;192(2):125–140. doi: 10.1159/000308893. [DOI] [PubMed] [Google Scholar]
- 25.Coester C.J., Langer K., van Briesen H., Kreuter J. Gelatin nanoparticles by two step desolvation – a new preparation method, surface modifications and cell uptake. J Microencapsul. 2002;17:187–193. doi: 10.1080/026520400288427. [DOI] [PubMed] [Google Scholar]
- 26.Patel Z.S., Ueda H., Yamamoto M., Tabata Y., Mikos A.G. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm Res. 2008;25(10):2370–2378. doi: 10.1007/s11095-008-9685-1. [DOI] [PubMed] [Google Scholar]
- 27.Reichmann E., Ball R., Groner B., Friis R.R. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J Cell Biol. 1988;108:1127–1138. doi: 10.1083/jcb.108.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung H.J., Park T.G. Injectable cellular aggregates prepared from biodegradable porous microspheres for adipose tissue engineering. Tissue Eng Part A. 2009;15(6):1391–1400. doi: 10.1089/ten.tea.2008.0344. [DOI] [PubMed] [Google Scholar]
- 29.Nam J.H., Ermonval M., Sharfstein S.T. Cell attachment to microcarriers affects growth, metabolic activity, and culture productivity in bioreactor culture. Biotechnol Prog. 2007;23(3):652–660. doi: 10.1021/bp070007l. [DOI] [PubMed] [Google Scholar]
- 30.Streuli Charles H., Bailey Nina, Bissell Mina J. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theveneau Eric, Mayor Roberto. Collective cell migration of epithelial and mesenchymal cells. Cell Mol Life Sci. 2013;70:3481–3492. doi: 10.1007/s00018-012-1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fessart Delphine, Delom Frederic. Three-dimensional culture model to distinguish normal from malignant human bronchial epithelial cells. Eur Respir J. 2013;42:1345–1356. doi: 10.1183/09031936.00118812. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi K., Tabata Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011;7(7):2797–2803. doi: 10.1016/j.actbio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Somasiri A., Wu C., Ellchuk T., Turley S., Roskelley C.D. Phosphatidylinositol 3-kinase is required for adherens junction-dependent mammary epithelial cell spheroid formation. Differentiation. 2000;66:116–125. doi: 10.1046/j.1432-0436.2000.660206.x. [DOI] [PubMed] [Google Scholar]
- 35.Zoubiane G.S., Valentijn A., Lowe E.T., Bagley S., Gilmore A.P., Streuli C.H. A role for the cytoskeleton in prolactin-dependent mammary epithelial cell differentiation. J Cell Sci. 2004;117(2):271–280. doi: 10.1242/jcs.00855. [DOI] [PubMed] [Google Scholar]
- 36.Hinshelwood R.A., Clark S.J. Breast cancer epigenetics: normal human mammary epithelial cells as a model system. J Mol Med (Berl) 2008;86(12):1315–1328. doi: 10.1007/s00109-008-0386-3. [DOI] [PubMed] [Google Scholar]
- 37.Li M.L., Aggeler J., Farson D.A., Hatier C., Hassell J., Bissell M.J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci USA. 1987;84(1):136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novaro V., Roskelley C.D., Bissell M.J. Collagen-IV and laminin-1 regulate estrogen receptor alpha expression and function in mouse mammary epithelial cells. J Cell Sci. 2003;116(Pt 14):2975–2986. doi: 10.1242/jcs.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu R., Spencer V.A., Groesser D.L., Bissell M.J. Laminin regulates PI3K basal localization and activation to sustain STAT5 activation. Cell Cycle. 2010;9(21):4315–4322. doi: 10.4161/cc.9.21.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell J.J., Davidenko N., Caffarel M.M., Cameron R.E., Watson C.J. A multifunctional 3D co-culture system for studies of mammary tissue morphogenesis and stem cell biology. PLoS One. 2011;6(9):e25661. doi: 10.1371/journal.pone.0025661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gharbi S.I., Zvelebil M.J., Shuttleworth S.J., Timms J.F., Waterfield M.D. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404(Pt 1):15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Lin, Huang Gang, Li Xiaowu, Zhang Yujun, Dong Jiahong, Qian Cheng. Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor -1α in hepatocellular carcinoma. BMC Cancer. 2013;13:108. doi: 10.1186/1471-2407-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y.J., Na H.J., Suh M.J., Ban M.J., Chang J.W., Koh Y.W. Hypoxia induces epithelial-mesenchymal transition in follicular thyroid cancer: involvement of regulation of twist by hypoxia inducible factor-1α. Yonsei Med J. 2015;56(6):1503–1514. doi: 10.3349/ymj.2015.56.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]