Abstract
Introduction
Adipose tissue stromal cells contain a substantial number of mesenchymal stem cells. As such, their application to regeneration of miscellaneous impaired organs has attracted much attention.
Methods
We designed a clinical study to investigate freshly isolated autologous adipose tissue-derived stromal/stem (regenerative) cell (ADRC) therapy for liver cirrhosis and conducted treatment in four cirrhotic patients. ADRCs were isolated from autologous subcutaneous adipose tissue obtained by the liposuction method, followed with use of the Celution system adipose tissue dissociation device. The primary endpoint is assessment of safety one month after treatment. We also characterized the obtained ADRCs.
Results
Two patients had type C cirrhosis, one had nonalcoholic steatohepatitis-cirrhosis, and one had type B cirrhosis. No serious adverse events were observed during the 1-month study period after freshly isolated ADRC infusion. Serum albumin concentrations were maintained or improved during this period as well as during the succeeding follow-up of approximately 1 year in two patients and 6 months in another patient. Liver regeneration-related factors, namely hepatocyte growth factor and interleukin-6, were elevated 1 day after ADRC treatment in all patients. The obtained freshly isolated ADRCs were expanded in culture and found to express mesenchymal stem cell markers. Gene expression profile analysis of ADRCs was shown to involve inflammatory features, suggesting that characteristics of the obtained ADRCs were related to immunomodulatory biological effects.
Conclusion
This clinical study treatment for liver cirrhosis using ADRCs was proven to be safely conductible, and can be further investigated in future for regeneration/repair of liver cirrhosis.
Keywords: Adipose tissue-derived regenerative cells, Stromal cells, Stem cells, Liver cirrhosis
Abbreviations: MSC, mesenchymal stromal cells; ADRC, adipose tissue-derived stromal/stem (regenerative) cells; HGF, hepatocyte growth factor; IL, interleukin; cADSC, cultured adipose tissue-derived stromal cells
Highlights
-
•
Clinical study of liver cirrhosis therapy using adipose tissue-derived stromal cells.
-
•
Autologous adipose tissue-derived stromal/stem (regenerative) cells were administered via intrahepatic arterial transfusion into cirrhotic patients.
-
•
The obtained adipose tissue-derived stromal cells were shown to contain mesenchymal stem cells.
1. Introduction
Liver cirrhosis is the end-stage condition of a variety of chronic liver diseases, including viral hepatitis, primary biliary cirrhosis, alcoholic hepatitis, and nonalcoholic steatohepatitis [1]. Liver transplantation is the ultimate treatment for hepatic failure, which is the end-stage of cirrhosis. Cirrhosis occurs when the fundamental cause of chronic liver disease is not radically eliminated or controlled, resulting in the persistence of hepatic inflammation [2]. However, liver transplantation is associated with many problems because it is an invasive procedure. Moreover, there exists a lack of donors, especially in Japan, where living-donor liver transplantation is still mainly conducted [3]. Therefore, an alternative novel therapy for cirrhosis should be developed to prevent further disease progression, avoid hepatic failure requiring liver transplantation, and decrease the risk of hepatocellular carcinoma, which frequently occurs as a result of the advanced fibrosis of the liver and worsens the prognosis and quality of life of patients with cirrhosis.
Adipose tissue contains a substantial number of stem cells in its stromal fraction [4]. Mesenchymal stromal cells (MSCs) are somatic stem cells capable of differentiating into various types of cells [5], including hepatocytes [6], [7]. In addition, they have strong immunomodulatory effects, which are principally beneficial for inflammation-related diseases in any organs [8], [9]. As such, much attention has been given to their application in regeneration therapy of various impaired organs. In a nonclinical study, we confirmed that adipose tissue-derived stromal cells are therapeutically beneficial in murine cirrhosis models because they improved hepatocyte function and ameliorated hepatic fibrosis and persistent inflammation [10].
We designed and conducted a regenerative therapy clinical study using autologous freshly isolated adipose tissue-derived stromal/stem (regenerative) cells (ADRCs) for liver cirrhosis (UMIN000009122, NCT01062750). The freshly obtained cells were infused through a catheter, the tip of which was placed in the common hepatic artery, and the maximal number of cells administered was 6.6 × 105 cells/kg. Four cirrhotic patients were treated. During the 1-month study period, no serious adverse effects were observed. Serum hepatocyte growth factor (HGF) and interleukin (IL)-6 levels were elevated 1 day after ADRC treatment in all patients. Serum concentrations of albumin in three of the four treated cirrhotic patients were improved after treatment during the study period as well as during follow-up. The obtained ADRCs did not merely contain lineagepotent cells, but were characterized to have immune-related biological functions by gene expression analysis. This clinical study investigating autologous freshly isolated ADRC treatment for liver cirrhosis was successful in demonstrating safety and its potential therapeutic efficacy.
2. Methods
2.1. Clinical study objectives
The primary aim of this clinical study was to confirm the safety of autologous ADRC therapy in cirrhotic patients (clinical research registration: ClinicalTrials.gov, registered number: NCT01062750, UMIN, registered number: UMIN000009122). Eligibility criteria included clinically diagnosed liver cirrhosis with relatively maintained hepatic function (Table 1). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the Japanese Ministry of Health, Labour and Welfare and the institutional review board. The written informed consent was obtained from each patient, respectively. The study was conducted under monitoring by Innovative Clinical Research Center, Kanazawa University.
Table 1.
Eligibility of enrollment.
|
2.2. Autologous ADRC treatment and clinical assessment
Adipose tissue was obtained from the subcutaneum of the patients' abdomen or buttocks using the Tumescent liposuction method under general anesthesia [11], [12]. The obtained adipose tissue was put into the Celution adipose tissue dissociation system (Cytori Therapeutics Inc., San Diego, CA), which is investigational device digesting adipose tissue automatically under sterile conditions to isolate ADRCs [13]. Cell viability was confirmed using the NucleoCounter® (Chemometec A/S, Allerod, Denmark) as well as with microscopic examination using the Trypan-blue dye exclusion test (Sigma–Aldrich, St. Louis, MO). We prepared freshly isolated ADRCs aliquot which contained 1 × 106 cells/ml suspended in lactate ringer liquid.
We administered 3.3 × 105 cells/kg and 6.6 × 105 cells/kg of the freshly isolated ADRCs in two patients, respectively, using an intravascular catheter, which was subcutaneously inserted through the femoral artery and guided to the common hepatic artery. After confirmation of intrahepatic arterial flow into the liver with contrast reagent, the freshly isolated ADRCs were infused directly into the cirrhotic liver. Since ADRCs were freshly isolated without culture, infusion of ADRCs was conducted on the same day of liposuction.
The patients were studied for 1 month following ADRC treatment, and the primary endpoints measured at the end of the study period included confirmation of clinical safety, a physical examination, and enhanced abdominal computed tomography. Additionally, blood samples for generally established clinical laboratory parameters, including relevant examination of liver function, and for RNA, using PAXgene® RNA tubes (PreAnalytiX GmbH, Germany), were collected during the study and follow-up periods.
2.3. Serum concentration of cytokines and chemokines
Peripheral blood was collected prior to and after ADRC infusion. The serum was separated by centrifugation, and the concentrations of various serum cytokines and chemokines were measured using Bio-Plex Pro Human Cytokine Group I and Ⅱ® (Bio-Rad, Tokyo, Japan). The following cytokines and chemokines were measured: interleukin(IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, IL-17, granulocyte-colony stimulating factor, granulocyte macrophage-colony stimulating factor, interferon-γ, monocyte chemoattractant protein-1, macrophage inflammatory protein-1β, tumor necrosis factor-α, IL-1α, IL-2Rα, IL-3, IL-12, IL-16, IL-18, cutaneous T-cell attracting chemokine, HGF, interferon-α2, leukemia inhibitory factor, monocyte chemoattractant protein-3, macrophage-colony stimulating factor, macrophage migration inhibitory factor, monokine induced by gamma interferon, nerve growth factor-β, stem cell factor, stem cell growth factor-β, stromal cell-derived factor-1α, tumor necrosis factor -β, and tumor necrosis factor-related apoptosis-inducing ligand.
2.4. Culture and expansion of ADRCs
The fraction of the obtained freshly isolated ADRCs were cultured in dishes with Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12, supplemented with 10% heat-inactivated fetal bovine serum (Life Technologies, Carlsbad, CA) and 10 ng/ml human basic fibroblast growth factor (PeproTech, Inc., Rocky Hill, NJ) to obtain cultured adipose tissue-derived stromal cells (cADSCs). The size of the ADRCs and the expanded cADSCs were measured and calculated using ImageJ software (http://imagej.nih.gov/ij/).
2.5. FACS analysis of ADRCs and cADSCs
A fraction of the obtained freshly isolated ADRCs and expanded cADSCs were subjected to analysis for surface antigen expression using human MSC antibodies. They were analyzed by flow cytometry using BD Stemflow™ (Human MSC Analysis Kit; Becton, Dickinson and Company, Franklin Lakes, NJ), as well as with FITC-labeled anti-CD31, PE-labeled anti-CD44 (Becton, Dickinson and Company), and APC-labeled anti-CD105 (BioLegend, San Diego, CA) antibodies.
2.6. Lineagepotency analysis of cADSCs
cADSCs obtained from the culture of ADRCs were examined for lineagepotency using the Human Mesenchymal Stem Cell Functional Identification Kit® (R&D Systems, Minneapolis, MN). Immunohistochemical staining of cells that had differentiated into adipocytes, osteocytes, and chondrocytes was performed using anti-FABP-4, anti-osteocalcin, and anti-Aggrecan, antibodies, respectively, followed by staining with the NorthernLights™ 557-conjugated secondary antibody and 4′,6-diamidino-2-phenylindole, in accordance with the manufacturer's instructions.
2.7. Gene expression analysis
RNA was isolated from ADRCs and cADSCs which were obtained from all 4 patients using the Micro RNA isolation kit® (Agilent Technologies, Santa Clara, CA) as well as from the all 4 patients' blood obtained in the PAXgene® RNA tubes (Qiagen, Hilden, Germany), in accordance with the manufacturers' instructions. Gene expression analysis using DNA microarray analysis was performed as previously reported [14]. Briefly, the isolated RNA was amplified, followed by labeling with Cy3 using the Quick Amp Labeling Kit® (Agilent Technologies, Palo Alto, CA). The labeled target complementary RNA was mixed and hybridized using the Whole Human Genome 4 × 44 K Microarray Kit® (Agilent Technologies), and the slides were scanned using a microarray scanner (Model G2505B; Agilent Technologies). Gene expression analysis was performed using Biometric Research Branch array tools (National Cancer Institute, http://linus.nci.nih.gov/BRB-ArrayTools.html). Hierarchical clustering and class comparison analysis of gene expression were performed to identify differentially expressed genes. Biological processes and networks were analyzed using the MetaCore™ software suite (GeneGo, Carlsbad, CA).
3. Results
3.1. Freshly isolated autologous ADRC treatment in four cirrhotic patients
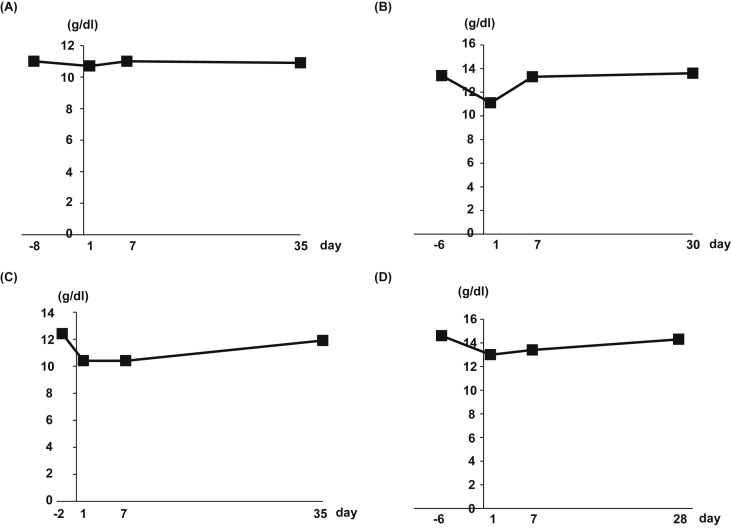
Following the designed clinical study protocol, we registered five cirrhotic patients, eventually excluding one patient who was proven to be non-cirrhotic (Table 2). The etiologies of the four cirrhotic patients were as follows: two patients had type C cirrhosis, one had nonalcoholic steatohepatitis-cirrhosis, and one had type B cirrhosis. The viability of the obtained freshly isolated ADRCs in all four patients was >93.5%. Sterilization of the obtained cells was confirmed by the procedure in the Japanese Pharmacopoeia. The former and latter two patients received 3.3 × 105 and 6.6 × 105 cells/kg, respectively. We have not observed serious exacerbation of serum AST (Supplemental Fig. 1A), ALT (Supplemental Fig. 1B), LDH (Supplemental Fig. 1C) and CK (Supplemental Fig. 1D) activity and clinical symptoms such as dyspnea or chest pain indicating lung embolisms. We did not observe occurrence of severe anemia requiring blood transfusion (Fig. 1) or physical hematoma. The subcutaneous color change to be purple as a consequence of liposuction completely disappeared in all patients at the end of observation period, 1 month. No serious harmful adverse events were noted during the 1-month period after ADRC treatment (Table 2).
Table 2.
Characteristics of the treated 4 patients.
| Registration number | HI-01 | HI-03 | HI-04 | HI-05 |
|---|---|---|---|---|
| Etiology of cirrhosis | Type C | Type C | NASHa | Type B |
| Age (years) | 50's | 30's | 60's | 60's |
| Height (cm) | 162.0 | 163.1 | 163.8 | 155.5 |
| Body weight (Kg) | 66.0 | 67.9 | 87.0 | 63.0 |
| Body mass index | 25.1 | 25.5 | 32.4 | 26.1 |
| Alcohol drinking habit | No | No | No | No |
| The obtained fat volume (ml) | 320 | 150 | 425 | 210 |
| Viability of the obtained cells (%) | 94.5 | 100 | 93.5 | 94.7 |
| Total number of the obtained viable cells | 5.2 × 107 | 2.5 × 109 | 4.5 × 107 | 6.6 × 107 |
| Number of the administered cells | 2.2 × 107 | 2.2 × 107 | 4.4 × 107 | 4.4 × 107 |
| Outcome for the primary endpoint | No serious adverse event | No serious adverse event | No serious adverse event | No serious adverse event |
NASH:non-alcoholic steatohepatitis.
Fig. 1.
Hemoglobin concentration in peripheral blood of each patient before and after ADRCs administration. Four cirrhotic patients were enrolled in this study. Two patients had type C cirrhosis [(A) HI-01, (B) HI-03], one patient had nonalcoholic steatohepatitis cirrhosis [(C) HI-04], and one patient had type B cirrhosis [(D) HI-05].
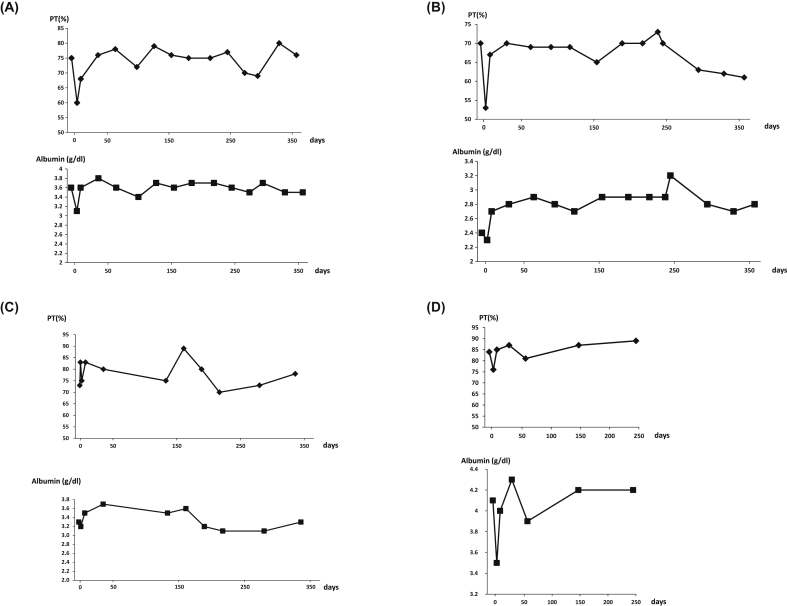
Serum albumin and prothrombin activities and laboratory examinations reflecting hepatic function, especially for cirrhosis, were assessed during the study period as well as during the follow-up period. The type C HI-01 patient maintained prothrombin activity and serum albumin concentrations for approximately 1 year (Fig. 2A), while the type C HI-03 patient maintained a prothrombin time and improved serum albumin concentrations for 1 year (Fig. 2B). The patient with nonalcoholic steatohepatitis-cirrhosis (HI-04) maintained prothrombin activity and albumin levels for approximately 6 months (Fig. 2C), and the patient with type B cirrhosis (HI-05) had improved serum albumin concentrations during the follow-up observation (Fig. 2D).
Fig. 2.
Clinical course of serum albumin concentrations and prothrombin activity. (A) HI-01, (B) HI-03, (C) HI-04, and (D) HI-05.
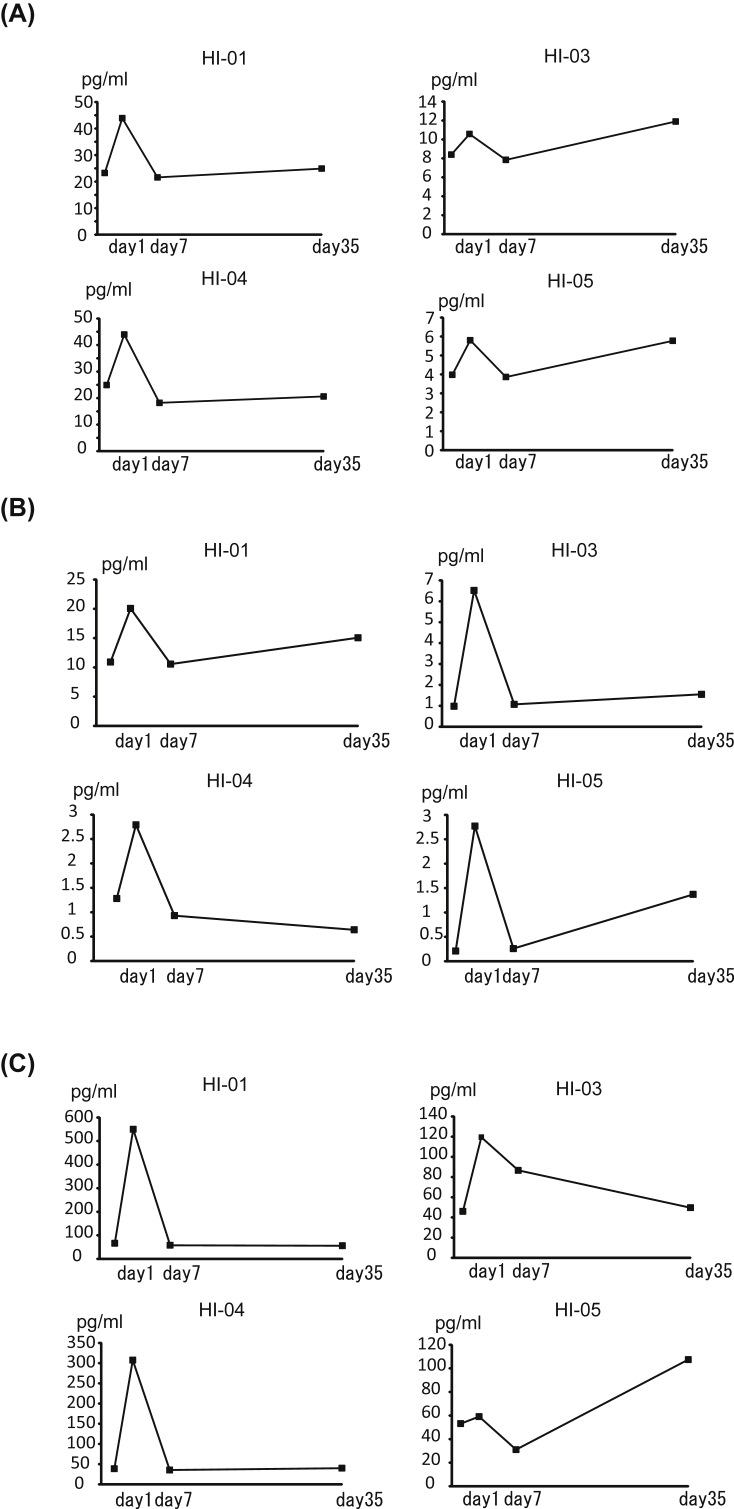
Additionally, we examined how cytokine/chemokine concentrations were affected after ADRC treatment. Among the examined cytokine/chemokine panels, serum HGF and IL-6 concentrations were elevated in all four treated patients 1 day after ADRC treatment compared with the pretreatment levels (Fig. 3). Moreover, serum M-colony stimulating factor, macrophage migration inhibitory factor, and IL-18 levels were also increased (Fig. 4), suggesting that active immune-modulatory humoral factors and liver regeneration-related factors were involved in modulating treatment with ADRCs.
Fig. 3.
Serum HGF and IL-6 concentrations. Serum was collected from each enrolled patient before and after ADRC treatment. Cytokine concentrations were measured using luminescent-conjugated beads. (A) HGF concentrations and (B) IL-6 concentrations.
Fig. 4.
Serum interleukin-18, macrophage colony-stimulating factor, and macrophage migration inhibitory factor concentrations. Serum was collected from each enrolled patient before and after ADRC treatment. Cytokines were measured using luminescent-conjugated beads. (A) interleukin-18, (B) macrophage colony-stimulating factor, (C) macrophage migration inhibitory factor concentration.
3.2. Surface antigen expression and lineagepotency of the obtained cells
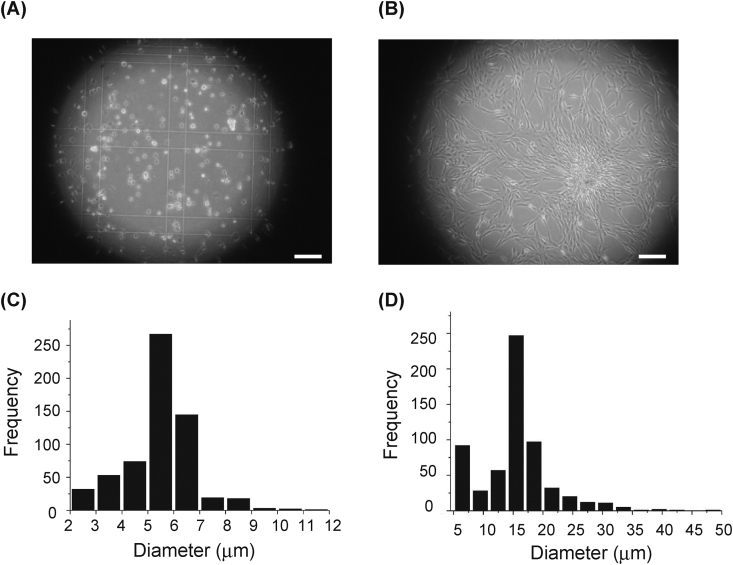
We examined the size and surface marker expression of the freshly isolated ADRCs as well as the cADSCs (Fig. 5, Table 3, Supplemental Fig. 2). The average diameter of the ADRCs was 5.47 μm (Fig. 5A and C), whereas that of cADSCs was 13.21 μm (Fig. 4B and D), indicating a 2.5-fold increase in size during culture. We observed that CD44 mesenchymal antigen expression was prominent in the freshly isolated ADRCs because it was present in approximately 10%–45% of the obtained ADRC population (Table 3, Supplemental Fig. 2A). We successfully cultured ADRCs to expand the typical spindle-shaped MSCs (Fig. 5). CD44, CD105, and CD73, all of which are considered to be mesenchymal stem cell surface markers, were expressed in the cADSCs (Table 4, Supplemental Fig. 2B). These findings suggest that the ADRCs contained the phenotypic populations of MSCs.
Fig. 5.
Microscopic appearance and size of ADRCs and expanded cADSCs. The obtained ADRCs and expanded cADSCs were microscopically observed. Image of them were analyzed for the size by ImageJ software. (A) ADRCs and (B) c ADSCs. (C, D) A histogram of the frequency and diameter of (C) ADRCs and (D) cADSCs. Bars: 100 μm.
Table 3.
Surface antigens expression of the ADRCs.
| Antigen | Registered number |
|||
|---|---|---|---|---|
| HI-01 | HI-03 | HI-04 | HI-05 | |
| CD90 | 12.7% | 1.73% | 5.6% | 0.1% |
| CD105 | 1.76% | 0.01% | 0% | 0.2% |
| CD73 | 11.2% | 0.42% | 4.8% | 0.2% |
| CD45 | 3.7% | 0% | 0.6% | 2.5% |
| CD44 | 10.3% | 32.7% | 28.8% | 45.8% |
| CD34 | 8.4% | 0.9% | 6.2% | 0.5% |
| CD31 | 5.6% | 1.9% | 1.5% | 1.2% |
Table 4.
Surface antigens expression of the cultured cADSCs.
| Antigen | Registered number |
|||
|---|---|---|---|---|
| HI-01 | HI-03 | HI-04 | HI-05 | |
| CD90 | 96.6% | 86.0% | 37.2% | 43.9% |
| CD105 | 95.5% | 82.9% | 98.2% | 95.3% |
| CD73 | 96.7% | 96.4% | 99.2% | 99.1% |
| CD45 | 0% | 0% | 4.4% | 21.1% |
| CD44 | 80.9% | 96.6% | 98.4% | 98.9% |
| CD34 | 0% | 0.6% | 0.3% | 0.1% |
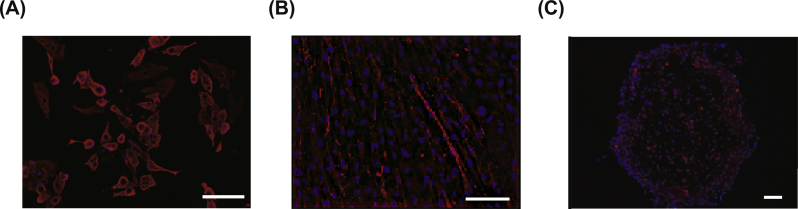
To further confirm that the expanded cADSCs were as lineagepotent as MSCs, we attempted to differentiate the cADSCs into adipocyte, osteocyte, and chondrocyte. Using immunohistochemical staining and morphological appearance, the cADSCs were proven to be lineagepotent in the differentiation-conditioned culture media (Fig. 6).
Fig. 6.
Lineagepotency of the expanded cADSCs. A fraction of the residually obtained ADRCs was expanded in culture media to obtain cADSCs. (a–c) The expanded cADSCs were further cultured in media supplemented with (A) adipocyte, (B) osteocyte, and (C) chondrocyte differentiation-inducing factors. Anti-FABP-4 (A), anti-Osteoalcin (B), and anti-Aggrecan (C) antibodies were used for imunohisotochemical staining for each differentiation, respectively. The stained cells represented the features as followings; (A) cells with droplets in the cytoplasm, (B) flat and oblong cells, and (C) relatively small and round cells, respectively. Bars: (A, B) 100 μm; (C) 200 μm.
3.3. Biological characteristics of ADRCs and cADSCs suggested by gene expression profile
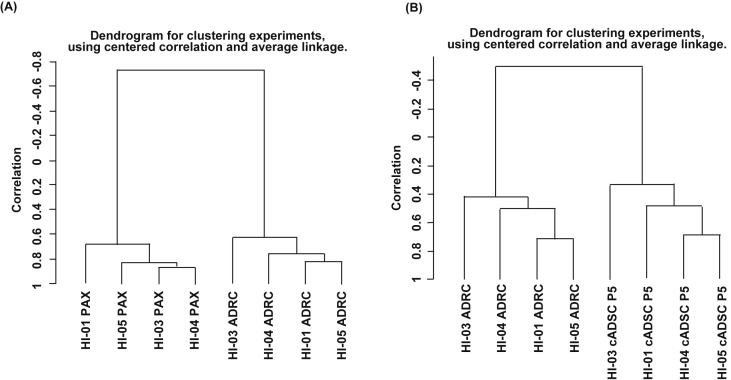
To further characterize the biological characteristics of freshly isolated ADRCs and cADSCs, we performed gene expression analysis using DNA microarray. When we compared the gene expression of ADRCs with that of whole blood obtained using PAXgene® tubes before ADRC treatment by unsupervised clustering analysis, two clusters representing peripheral blood and ADRC were formed using 14,334 filter-passed evaluable genes (Fig. 7A). We identified 769 significantly up-regulated genes, up to 10-fold, in ADRCs compared with peripheral blood (p < 0.001). The biological characteristics of these involved a cytoskeleton development involving WNT signaling and signal transduction involving Notch signaling, suggesting that ADRC gene expression profiles involve a regenerative processes (Table 5).
Fig. 7.
Hierarchical gene expression clustering analysis of peripheral blood, ADRCs, and cADSCs. RNA was extracted from peripheral blood in PAXgene® tubes and from freshly isolated ADRCs and expanded cADSCs. Gene expression data were obtained by DNA microarray and followed by unsupervised hierarchical clustering analysis. (A) Clustering analysis of gene expression data of peripheral blood and ADRCs. (B) Clustering analysis of gene expression data of ADRCs and cADSCs.
Table 5.
Biological processes for up-regulated genes in ADRCs compared to peripheral blood.
| Networks | Total genes | p-Value | FDR | Genes in data | Network objects from active data |
|---|---|---|---|---|---|
| Cytoskeleton_Actin filaments | 176 | 3.61E−11 | 4.65E−09 | 35 | c-Abl, MYH11, Talin, Myosin I, Transgelin, WASF3 (WAVE3), Beta TnTF, ERM proteins, Drebrin, Troponin T, skeletal, MyHC, MYLK1, Gamma-sarcoglycan, Filamin B (TABP), MYH10, Profilin II, Actin, Tropomyosin-1, BPAG1, Calponin-1, Gelsolin, Profilin, MLCK, MRLC, RDX (radixin), Myosin VI, Cofilin, Filamin C, Fascin, Actin muscle, Pacsin, Tropomyosin, Tropomyosin-2, Nephrocystin, FAK1 |
| Development_Skeletal muscle development | 144 | 6.04E−11 | 4.65E−09 | 31 | MYH11, Smooth muscle myosin, Angiotensin II, HB-EGF, Transgelin, Beta TnTF, EGR3, ACTA2, Troponin T, skeletal, MyHC, Gamma-sarcoglycan, VEGF-A, MuRF1, GATA-6, Filamin B (TABP), Actin, Tropomyosin-1, Caveolin-3, MYF6, MRLC, Beta-sarcoglycan, MYRL2, Filamin C, Actin muscle, MKL2(MRTF-B), Tropomyosin, TWIST1, Tropomyosin-2, ITGA11, ACTG2, Collagen IV |
| Development_Ossification and bone remodeling | 157 | 2.63E−09 | 1.35E−07 | 30 | SFRP4, OSF-2, BMP6, TIEG1, HOXA9, Osteonectin, SMAD7, TGF-beta, BMPR1A, Chordin-like 1, TIEG, WNT, SMAD1, Osteopontin, EGFR, Pleiotrophin (OSF1), IGF-2, FOXC1/2, BMP5, BMP7, Osteomodulin, TBX3, FOXC1, IBP, Frizzled, FGFR1, TWIST1, HOXC8, FGFR2, CSF1 |
| Cell adhesion_Integrin-mediated cell-matrix adhesion | 214 | 3.16E−08 | 1.22E−06 | 34 | Tubulin beta, OSF-2, ITGB4, Talin, Lpd, ITGA3, Caveolin-2, Collagen XIV, Fibrillin 1, ERM proteins, LAMA5, ITGB5, Osteonectin, MyHC, Filamin B (TABP), c-Myc, Profilin II, alpha-V/beta-5 integrin, Actin, Osteopontin, Tau (MAPT), Osteomodulin, Profilin, MLCK, MRLC, RDX (radixin), Cofilin, Filamin C, ITGAV, ITGA11, PI3K reg class IA, FAK1, Tetraspanin-8, Collagen IV |
| Development_EMT_Regulation of epithelial-to-mesenchymal transition | 225 | 1.12E−07 | 3.45E−06 | 34 | c-Abl, PDGF receptor, DAB2, HEY1, TGF-beta 2, TGF-beta 3, ACTA2, Jagged1, SMAD7, TGF-beta, WNT, Actin, Tropomyosin-1, SLUG, EGFR, AP-1, JNK(MAPK8-10), PAI1, IGF-2, BMP7, PDGF-D, PDGF-B, Frizzled, FGFR1, LOXL2, Cofilin, MKL2(MRTF-B), TWIST1, PDGF-R-beta, FGFR2, PI3K reg class IA, c-Fos, G-protein alpha-q/11, FAK1 |
| Signal transduction_WNT signaling | 177 | 1.72E−07 | 4.42E−06 | 29 | AKAP12, SFRP4, HB-EGF, Fra-1, LRP5, TGF-beta 2, TGF-beta 3, Matrilysin (MMP-7), HES1, FZD10, PP2A regulatory, SFRP2, VEGF-A, TGF-beta, GATA-6, c-Myc, WNT, SLUG, EGFR, JNK(MAPK8-10), WNT5A, FZD5, Adenylate cyclase, FOXC1, Frizzled, SFRP1, c-Fos, G-protein alpha-q/11, PLC-beta |
| Development_Regulation of angiogenesis | 223 | 2.87E−07 | 6.32E−06 | 33 | Ephrin-A, p21, Angiopoietin 1, Angiotensin II, Leptin receptor, HB-EGF, IL-6, Endoglin, Angiotensin III, DDAH1, Biglycan, TGF-beta 2, Ephrin-A5, Galpha(i)-specific peptide GPCRs, Osteonectin, VEGFR-1, VEGF-A, c-Myc, Ephrin-B, EGFR, Ephrin-A receptors, VEGFR-3, PAI1, Galpha(q)-specific peptide GPCRs, HOXA3, Smoothened, MKL2(MRTF-B), PKC, TRPC1, PI3K reg class IA, G-protein alpha-q/11, Syndecan-3, PLC-beta |
| Development_Neurogenesis_Axonal guidance | 230 | 5.96E−07 | 1.15E−05 | 33 | Ephrin-A, c-Abl, Ephrin-B1, Semaphorin 3C, UNC5B, FEZ1, Netrin-1, ROBO3, Neogenin, Ephrin-A5, ERM proteins, NGFR (ICD), Drebrin, MyHC, NGFR(TNFRSF16), MYH10, Ephrin-B, Actin, Ephrin-A receptors, Ephrin-A receptor 2, PCDHA6, Oligophrenin 1, Syndecan-2, Semaphorin 3B, Semaphorin 3D, Cofilin, Semaphorin 6D, RHO6, PI3K reg class IA, G-protein alpha-q/11, FAK1, PLC-beta, NGFR (CTF) |
| Cell adhesion_Amyloid proteins | 195 | 1.40E−06 | 2.20E−05 | 29 | c-Abl, SFRP4, FZD8, DAB2, alphaAPPs, Amyloid beta, TGF-beta 2, BACE1, SHC3, Jagged1, Nidogen, NGFR (ICD), NGFR(TNFRSF16), Filamin B (TABP), WNT, Actin, WNT5A, APP-C59 (AICD), FZD5, Tau (MAPT), Frizzled, SAA1, Cofilin, ADAM9, PKC, FAK1, APP, Collagen IV, NGFR (CTF) |
| Signal Transduction_TGF-beta, GDF and Activin signaling | 154 | 1.49E−06 | 2.20E−05 | 25 | p21, RPTPkappa, IL-6, DAB2, Endoglin, TGF-beta 2, TGF-beta 3, c-Kit, IRS-1, SARA, PP2A regulatory, SMAD7, c-Myc, EGFR, AP-1, JNK(MAPK8-10), PAI1, MYF6, TGF-beta receptor type III (betaglycan), PKC, TSC-22, c-Fos, FAK1, ALK-1, PLC-beta |
| Cell adhesion_Attractive and repulsive receptors | 175 | 1.57E−06 | 2.20E−05 | 27 | Ephrin-A, c-Abl, Ephrin-B1, Semaphorin 3C, UNC5B, ICAM1, Netrin-1, CHL1, ROBO3, Kalirin, Collagen XIV, Neogenin, Ephrin-A5, AF-6, Ephrin-B, Actin, Ephrin-A receptors, Ephrin-A receptor 2, Tau (MAPT), Collagen XII, Cofilin, Semaphorin 6D, PI3K reg class IA (p55-gamma), RHO6, PI3K reg class IA, FAK1, Collagen IV |
| Cell adhesion_Platelet-endothelium-leucocyte interactions | 174 | 4.54E−06 | 5.82E−05 | 26 | Ephrin-B1, PDGF receptor, ICAM1, IL-6, Endoglin, MAGI-1(BAIAP1), TGF-beta 2, TGF-beta 3, KAL1, ESAM, Protein S, VEGF-A, MSR1, TGF-beta, LDLR, Cathepsin G, JAM3, EGFR, PAI1, PDGF-D, PDGF-B, A2M receptor, CD36, PDGF-R-beta, FAK1, Collagen IV |
| Cell adhesion_Cadherins | 180 | 8.54E−06 | 9.56E−05 | 26 | c-Abl, YES, FHL2, SFRP4, LRP5, ITGA3, DAB2, PTPRF (LAR), Nectin-2, AF-6, DKK2, WNT, Actin, PCDA7, EGFR, BPAG1, WNT5A, PCDHA6, Frizzled, DLG5(P-dlg), PEZ, PKC, K-cadherin (CDH6), Nectin-3, FAK1, Protocadherin 18 |
| Signal transduction_NOTCH signaling | 236 | 8.69E−06 | 9.56E−05 | 31 | p21, PDGF receptor, SFRP4, HB-EGF, FZD8, DAB2, HEY1, TGF-beta 2, TGF-beta 3, HES1, FZD10, Jagged1, SFRP2, VEGF-A, TGF-beta, c-Myc, WNT, EGFR, JNK(MAPK8-10), WNT5A, SFRP3 (FRZB1), FZD5, PDGF-D, PDGF-B, JNK3(MAPK10), Frizzled, MPDZ, SFRP1, PDGF-R-beta, PI3K reg class IA, c-Fos |
| Cytoskeleton_Regulation of cytoskeleton rearrangement | 183 | 1.16E−05 | 1.19E−04 | 26 | Tubulin beta, Talin, RhoGAP6, ERM proteins, Drebrin, MyHC, Gamma-sarcoglycan, Filamin B (TABP), PARD6, Profilin II, Actin, Galpha(i)-specific amine GPCRs, BPAG1, Calponin-1, Gelsolin, Profilin, MLCK, MRLC, RDX (radixin), Beta-sarcoglycan, Cofilin, Filamin C, Actin muscle, PKC, RHO6, FAK1 |
| Muscle contraction | 173 | 1.27E−05 | 1.22E−04 | 25 | K(+) channel, subfamily J, MYH11, Junctin, Smooth muscle myosin, Beta TnTF, ACTA2, Galpha(i)-specific peptide GPCRs, Troponin T, skeletal, MyHC, MuRF1, Actin, Tropomyosin-1, Galpha(q)-specific amine GPCRs, Calponin-1, Kir2.2, Galanin, Galpha(q)-specific prostanoid GPCRs, Galpha(q)-specific peptide GPCRs, MLCK, MRLC, MYRL2, Actin muscle, PKC, Tropomyosin, G-protein alpha-q/11 |
| Reproduction_Feeding and Neurohormone signaling | 211 | 1.38E−04 | 1.25E−03 | 26 | PAM, ITGA3, IL-6, HSP70, Amyloid beta, TGF-beta 2, c-Kit, Galpha(i)-specific peptide GPCRs, PP2A regulatory, TGF-beta, c-Myc, CPEB1, CRF-BP, PAI1, APP-C59 (AICD), Galpha(q)-specific peptide GPCRs, ADAM9, BTEB1, IBP3, PI3K reg class IA, c-Fos, G-protein alpha-q/11, FAK1, Amyloid beta 42, APP, PLC-beta |
| Cardiac development_BMP_TGF_beta_signaling | 117 | 2.92E−04 | 2.49E−03 | 17 | MYH11, BMP6, FGF12, Endoglin, TGF-beta 2, TGF-beta 3, MyHC, SMAD7, BMPR1A, MYH10, SMAD1, SLUG, BMP7, FOXC1, TGF-beta receptor type III (betaglycan), MKL2(MRTF-B), PDLIM3 |
| Development_Blood vessel morphogenesis | 228 | 4.78E−04 | 3.88E−03 | 26 | Angiopoietin 1, G-protein alpha-11, HB-EGF, ICAM1, HEY1, PDE, Jagged1, PDE9A, Galpha(i)-specific peptide GPCRs, VEGFR-1, VEGF-A, c-Myc, EGFR, Galpha(q)-specific amine GPCRs, Galpha(i)-specific amine GPCRs, VEGFR-3, FOXC1/2, Galpha(q)-specific peptide GPCRs, FOXC1, Angiopoietin 2, FGFR1, TRPC1, PI3K reg class IA, c-Fos, G-protein alpha-q/11, Transferrin |
| Reproduction_FSH-beta signaling pathway | 160 | 6.71E−04 | 5.17E−03 | 20 | TGF-beta 2, PBX1, IRS-1, PP2A regulatory, VEGF-A, c-Myc, SMAD1, EGFR, AP-1, Adenylate cyclase, IBP, TGF-beta receptor type III (betaglycan), PBX, CREM (activators), CREM (repressors), PBX1/PREP2, IBP3, PI3K reg class IA, c-Fos, PBX1a/HOXA10 |
Next, we compared the gene expression profile of ADRCs and cADSCs. Using 14,099 filter-passed evaluable genes, unsupervised clustering analysis for gene expression of ADRCs and cADSCs showed that two clusters were formed: a classifying ADRC cluster and a cADSC cluster. Thus, the ADRC expression profiles were similar irrespective of the cirrhosis etiology (Fig. 7B). We identified 668 genes with up-regulated expression in ADRCs compared with cADSCs (p < 0.001, 10-fold expression difference). The biological processes of these genes mostly implicated a variety of immune response-related processes, such as antigen presenting cell-related inflammation, natural killer cells, T cells, and cytokine signaling (Table 6). We also identified 141 genes with up-regulated expression in cADSCs compared with ADRCs. The biological processes involving these included development, extracellular matrix remodeling, and WNT signaling, suggesting the involvement of tissue repair-related and regeneration-related processes (Table 7). Taken together, these results demonstrate that freshly isolated ADRCs contain MSCs whose biological effects are related to tissue repair and remodeling as well as development.
Table 6.
Biological processes for up-regulated genes in ADRCs compared to cADSCs.
| Networks | Total genes | p-Value | FDR | Genes in data | Network objects from active data |
|---|---|---|---|---|---|
| Immune response_Antigen presentation | 197 | 2.10128E−13 | 3.04686E−11 | 39 | CD8 alpha, CIITA, STAT3, ITGAM, CD8, ICAM1, MHC class II beta chain, CD28, HSP70, ICOS, HLA-DQA1, KLRK1 (NKG2D), alpha-L/beta-2 integrin, HA2Z, HLA-DM, HLA-DRB1, ITGAL, ICAM2, NKG2A, Cathepsin S, CD3 gamma, MHC class II, KLRC4 (NKG2F), HLA-DQB1, CD3 zeta, HLA-DRA1, CD2, HLA-DPB1, CD3 delta, HLA-DRB4, CD86, CEACAM1, IFN-gamma, CD74, CD8 beta, CD209, RING6, alpha-M/beta-2 integrin, TNF-alpha |
| Cell adhesion_Leucocyte chemotaxis | 205 | 8.02086E−13 | 5.81512E−11 | 39 | CCL5, ITGB2, ITGAM, ICAM1, GRO-2, CD28, CCL2, ZAP70, CCL14, alpha-L/beta-2 integrin, MIP-1-beta, CCL23, Galpha(i)-specific peptide GPCRs, MIG, ITGAL, ICAM2, IL-8, SKAP55, MHC class II, PLC-gamma 2, ITK, Btk, CXCL16, G-protein alpha-i family, CD2, CX3CR1, Lymphotactin, Lck, Galpha(q)-specific peptide GPCRs, CD86, VAV-1, CXCR4, PLC-gamma, CALDAG-GEFI, SLAP-130(ADAP), CCR7, IP10, CCL13, alpha-M/beta-2 integrin |
| Chemotaxis | 137 | 1.75407E−12 | 8.47803E−11 | 31 | CCL5, ITGB2, Syk, XCL2, C5aR, GRO-2, Prokineticin 2, CCL2, KAL1, alpha-L/beta-2 integrin, MIP-1-beta, alpha-X/beta-2 integrin, CCL23, Galpha(i)-specific peptide GPCRs, MIG, Integrin, IL-8, CCL8, Osteopontin, CXCL16, G-protein alpha-i family, CX3CR1, Lymphotactin, Lck, Galpha(q)-specific peptide GPCRs, IL-16, FPR, CCR7, IP10, CCL13, alpha-M/beta-2 integrin |
| Cell adhesion_Platelet-endothelium-leucocyte interactions | 174 | 1.12476E−09 | 4.07727E−08 | 31 | ITGAX, CCL5, LEKTI, ITGB2, CD84, IL-18, ITGAM, ICAM1, CCL2, JAM1, KAL1, alpha-L/beta-2 integrin, alpha-X/beta-2 integrin, ITGAL, CD34, ESAM, SREC-I, MSR1, IL-8, Cathepsin G, PZP, PECAM1, P-selectin, von Willebrand factor, VEGFR-2, SHP-1, PDGF-B, CD36, alpha-M/beta-2 integrin, Thrombospondin 4, TNF-alpha |
| Immune response_Phagocytosis | 222 | 2.7911E−09 | 8.09419E−08 | 35 | ITGB2, Syk, Myosin I, MELC, Fc gamma RII beta, alpha-X/beta-2 integrin, MyHC, C3, MSR1, CORO1A(CLABP, TACO), Lyn, PLC-gamma 2, Btk, C3b, Hck, Gelsolin, MARCO, SHP-1, VAV-1, iC3b, Fc epsilon RI beta, PLC-gamma, gp91-phox, C3dg, p47-phox, p40-phox, SLAP-130(ADAP), PI3K reg class IA (p55-gamma), p67-phox, CD14, FGR, ELMO1, alpha-M/beta-2 integrin, PI3K reg class IA, Fc gamma RII alpha |
| Inflammation_Complement system | 74 | 4.19219E−09 | 1.01311E−07 | 19 | ITGAX, ITGB2, ITGAM, C5aR, Ficolin, C7, Factor H, alpha-X/beta-2 integrin, C6, Properdin, C3a, C3, L-Ficolin, C3b, iC3b, C1qRp, C3dg, alpha-M/beta-2 integrin, H-Ficolin |
| Inflammation_TREM1 signaling | 145 | 9.53854E−08 | 1.97584E−06 | 25 | ITGAX, ITGB2, Syk, ITGAM, ICAM1, IRF8, CCL2, ZAP70, Matrilysin (MMP-7), IL-8, Nod2 (CARD15), PLC-gamma 2, CD83, DAP12, CD86, IFN-gamma, PLC-gamma, TLR2, WBSCR5(NTAL), PI3K reg class IA (p55-gamma), CD14, MEF2C, PI3K reg class IA, TREM2, TNF-alpha |
| Immune response_Phagosome in antigen presentation | 243 | 9.93211E−07 | 1.8002E−05 | 32 | Syk, HSP70, HLA-DQA1, A2M, C3, Cathepsin S, Lyn, MHC class II, PLC-gamma 2, HLA-DQB1, Btk, HLA-DRA1, Hck, Gelsolin, HLA-DPB1, SHP-1, HLA-DRB4, VAV-1, iC3b, Fc epsilon RI beta, PLC-gamma, CD74, FPR, C3dg, SLAP-130(ADAP), PI3K reg class IA (p55-gamma), CD14, FGR, ELMO1, alpha-M/beta-2 integrin, PI3K reg class IA, Fc gamma RII alpha |
| Inflammation_Histamine signaling | 213 | 1.64374E−06 | 2.64826E−05 | 29 | CCL5, IL-18, PLA2, ICAM1, IRF8, BETA-PIX, CCL2, Guanylate cyclase, Guanylate cyclase alpha, MIP-1-beta, Guanylate cyclase A (NPR1), IL-8, PLC-gamma 2, P-selectin, HDC, GUCY1A3, G-protein alpha-i family, IGHG1, CD86, IFN-gamma, PLC-gamma, LTC4S, gp91-phox, p47-phox, p40-phox, p67-phox, CD14, IP10, TNF-alpha |
| Inflammation_NK cell cytotoxicity | 164 | 3.7358E−06 | 5.41691E−05 | 24 | Syk, ICAM1, EAT-2, ZAP70, KLRK1 (NKG2D), alpha-L/beta-2 integrin, NKp30, ICAM2, NKG2A, KLRC4 (NKG2F), PLC-gamma 2, CD3 zeta, DAP12, CD2, Granzyme B, SHP-1, Lck, Perforin, IFN-gamma, PLC-gamma, Apo-2L(TNFSF10), Serglycin, DAP10, TNF-alpha |
| Inflammation_Neutrophil activation | 215 | 6.02147E−06 | 7.90026E−05 | 28 | ITGB2, STAT3, ITGAM, C5aR, PLA2, ICAM1, GRO-2, CCL2, TNF-R2, Galpha(i)-specific peptide GPCRs, ICAM2, IL-8, P-selectin, Btk, G-protein alpha-15, G-protein alpha-i family, CX3CR1, VAV-1, IFN-gamma, FPR, gp91-phox, p40-phox, PI3K reg class IA (p55-gamma), p67-phox, alpha-M/beta-2 integrin, PI3K reg class IA, Ryanodine receptor 1, TNF-alpha |
| Cell adhesion_Platelet aggregation | 158 | 6.53814E−06 | 7.90026E−05 | 23 | ITGB2, Syk, PLA2, MELC, PAR4, ZAP70, MyHC, THAS, Lyn, Cathepsin G, PECAM1, PLC-gamma 2, P-selectin, G-protein alpha-z, von Willebrand factor, G-protein alpha-i family, VAV-1, PLA2G7, PLC-gamma, CD36, CALDAG-GEFI, PI3K reg class IA (p55-gamma), PI3K reg class IA |
| Immune response_TCR signaling | 174 | 1.0605E−05 | 0.000118287 | 24 | CD8 alpha, ITGB2, CD8, ICAM1, CD28, ICOS, ZAP70, alpha-L/beta-2 integrin, ICAM2, SKAP55, CD3 gamma, MHC class II, CD3 zeta, ITK, SHP-1, CD3 delta, Lck, CD86, VAV-1, IFN-gamma, CD8 beta, SLAP-130(ADAP), PI3K reg class IA, TNF-alpha |
| Inflammation_IL-4 signaling | 115 | 2.46593E−05 | 0.0002554 | 18 | CCL2, HLA-DQA1, IL-8, MHC class II, HLA-DQB1, HLA-DRA1, HLA-DPB1, SHP-1, HLA-DRB4, IGHG1, CD86, Fc epsilon RI beta, CD74, DOK2, SHIP, CCL13, PI3K reg class IA, Fc gamma RII alpha |
| Inflammation_Interferon signaling | 110 | 4.8939E−05 | 0.00045454 | 17 | CIITA, CCL5, STAT3, IRF1, ICAM1, IRF8, CCL2, MIP-1-beta, MIG, CCL8, SHP-1, IL18RAP, CD86, SOCS3, IFN-gamma, Apo-2L(TNFSF10), SERPINB9 |
| Development_Blood vessel morphogenesis | 228 | 5.01561E−05 | 0.00045454 | 27 | LEKTI, STAT3, HB-EGF, ICAM1, Prokineticin 2, Alpha-1B adrenergic receptor, PDE, PDE9A, Galpha(i)-specific peptide GPCRs, Lactoferrin, Galpha(q)-specific nucleotide-like GPCRs, VEGFR-1, Guanylate cyclase A (NPR1), Epiregulin, Endomucin, TIE2, Galpha(q)-specific amine GPCRs, Ceruloplasmin, VEGFR-3, VEGFR-2, G-protein alpha-i family, Galpha(q)-specific peptide GPCRs, CEACAM1, CXCR4, ROBO4, DOK2, PI3K reg class IA |
| Inflammation_Innate inflammatory response | 180 | 5.61052E−05 | 0.000478544 | 23 | STAT3, IL-18, C5aR, PLA2, Beta-defensin 1, C7, TLR1, C6, TLR5, C3a, C3, IL-8, PLC-gamma 2, Btk, C3b, G-protein alpha-15, G-protein alpha-i family, sCD14, PLC-gamma, TLR2, CD14, IP10, TNF-alpha |
| Muscle contraction | 173 | 8.74525E−05 | 0.000704479 | 22 | MYH11, Smooth muscle myosin, KCNAB1, MELC, Phospholamban, Beta TnTF, Galpha(i)-specific peptide GPCRs, HRC, Troponin T, skeletal, IPP-1, MyHC, Guanylate cyclase A (NPR1), Titin, KCNQ1, MYL4, Galpha(q)-specific amine GPCRs, G-protein alpha-i family, Galpha(q)-specific peptide GPCRs, Kv2.1, PKC, Troponin I, fast skeletal muscle, Ryanodine receptor 1 |
| Inflammation_Amphoterin signaling | 118 | 0.000386616 | 0.00295049 | 16 | ITGB2, ITGAM, Calgranulin A, ICAM1, MELC, CCL2, MyHC, Calgranulin B, IL-8, Calgranulin C, IL1RN, TLR2, PI3K reg class IA (p55-gamma), alpha-M/beta-2 integrin, PI3K reg class IA, TNF-alpha |
| Proliferation_Lymphocyte proliferation | 209 | 0.000530476 | 0.003684745 | 23 | CCL5, STAT3, Syk, IL-18, IL10RA, BST2, CD28, ICOS, ZAP70, MIP-1-beta, TNF-R2, CD33, CD30(TNFRSF8), OX40(TNFRSF4), SHP-1, CD86, VAV-1, CD70(TNFSF7), CXCR4, DOK2, EBI3, PI3K reg class IA, TNF-alpha |
Table 7.
Biological processes for up-regulated genes in cADSCs compared to ADRCs.
| Networks | Total gees | p-Value | FDR | Genes in data | Network objects from active data |
|---|---|---|---|---|---|
| Development_Cartilage development | 66 | 7.01E−05 | 0.006166 | 6 | Fibronectin, VDR, ROR2, Activin beta A, RUNX2, Fibrillin |
| Development_Neurogenesis in general | 192 | 0.000202 | 0.008844 | 9 | Frizzled, Galpha(q)-specific amine GPCRs, FZD2, Adenosine A1 receptor, Activin beta A, SERPINE2, WNT, GDNF, ARNT2 |
| Reproduction_FSH-beta signaling pathway | 160 | 0.000301 | 0.008844 | 8 | PSG13, SCCA-1, PSG-5, ROR2, Activin beta A, PSG6, PSG1, PSG11 |
| Cell adhesion_Cell-matrix interactions | 211 | 0.000408 | 0.008969 | 9 | Fibronectin, Tenascin-C, MMP-1, Stromelysin-1, Fibrillin 2, Layilin, Fibrillin, LAMA1, Thrombospondin 1 |
| Development_Ossification and bone remodeling | 157 | 0.001436 | 0.025274 | 7 | Frizzled, Activin, HOXC8, RUNX2, Osteoprotegerin, Cadherin 11, WNT |
| Proteolysis_Connective tissue degradation | 119 | 0.008978 | 0.124094 | 5 | Fibronectin, Tenascin-C, SERPINE2, MMP-1, Stromelysin-1 |
| Development_Neurogenesis_Synaptogenesis | 180 | 0.012735 | 0.124094 | 6 | Frizzled, Nav1.6, Liprin-alpha4, Thrombospondin 1, WNT, Thrombospondin 2 |
| Cell adhesion_Cadherins | 180 | 0.012735 | 0.124094 | 6 | SSX2IP, Frizzled, STMN2, DKK1, Cadherin 11, WNT |
| Proteolysis_ECM remodeling | 85 | 0.013084 | 0.124094 | 4 | Fibronectin, Tenascin-C, MMP-1, Stromelysin-1 |
| Inflammation_IL-13 signaling pathway | 91 | 0.016458 | 0.124094 | 4 | Tenascin-C, IL13RA2, SCCA-1, SCCA-2 |
| Signal Transduction_BMP and GDF signaling | 91 | 0.016458 | 0.124094 | 4 | Gremlin, ROR2, HOXC8, RUNX2 |
| Cell adhesion_Amyloid proteins | 195 | 0.018264 | 0.124094 | 6 | Fibronectin, Frizzled, FZD2, F-spondin, WNT5B, WNT |
| Blood coagulation | 94 | 0.018332 | 0.124094 | 4 | SERPINE2, MMP-1, PAR3, Thrombospondin 1 |
| Cardiac development_Wnt_beta-catenin, Notch, VEGF, IP3 and integrin signaling | 150 | 0.022508 | 0.141479 | 5 | Frizzled, SALL4, DKK1, Thrombospondin 1, WNT |
| Reproduction_Progesterone signaling | 214 | 0.027392 | 0.160698 | 6 | Fibronectin, Frizzled, KIBRA, Galpha(q)-specific peptide GPCRs, WNT, Pitx2 |
| Signal transduction_WNT signaling | 177 | 0.041679 | 0.229236 | 5 | Fibronectin, Frizzled, FZD2, WNT5B, WNT |
| Neurophysiological process_Transmission of nerve impulse | 212 | 0.077966 | 0.393232 | 5 | HTR2A, Glycine receptor beta chain, MaxiK alpha subunit, GluR3, Serotonin receptor |
| Cell adhesion_Integrin-mediated cell-matrix adhesion | 214 | 0.080434 | 0.393232 | 5 | Fibronectin, Tubulin beta 3, Tenascin-C, Tubulin beta, TM4SF9 |
| Muscle contraction | 173 | 0.115956 | 0.518155 | 4 | Galpha(q)-specific amine GPCRs, MaxiK alpha subunit, Galpha(q)-specific peptide GPCRs, Thrombospondin 1 |
| Cell adhesion_Platelet-endothelium-leucocyte interactions | 174 | 0.117762 | 0.518155 | 4 | Fibronectin, SERPINE2, Thrombospondin 1, Thrombospondin 2 |
4. Discussion
We conducted a clinical study investigating freshly isolated autologous ADRC therapy for cirrhotic patients. ADRCs were obtained from autologous subcutaneous adipose tissue. The freshly obtained ADRCs without any processing or manufacturing were infused through a catheter inserted subcutaneously and placed into the hepatic artery. During the 1-month study observation period after ADRC infusion, no serious adverse reactions related to the treatment were observed. The serum albumin level and prothrombin time were maintained or improved during certain periods of the study and follow-up.
In addition, we found elevated serum concentrations of the cytokines HGF and IL-6, as well as other immune-mediating and inflammation-related humoral mediators, in treated patients. HGF enhances the proliferation of liver progenitor cells [15] and has been previously used clinically for hepatic failure [16]. IL-6 is involved in liver regeneration [17]. Therefore, elevation of these two cytokines implies that ADRC treatment may have humoral factor-mediated impacts on regeneration. However, we did not examine the potential mechanisms underlying how infused ADRCs exerted therapeutic effects locally through these liver regeneration-related cytokines in patients with cirrhosis. Importantly, the alteration of these cytokines/chemokines as humoral mediators was transient. The importance of an indirect effect of MSCs on the treatment of liver disease models was previously reported [8]. Liposuction and infusion of ADRCs were conducted on the same day, therefore, it is yet to be fundamentally disclosed whether ADRCs administration rather than liposuction procedure contributed to elevation of HGF, cytokines and chemokines in sera. Furthermore, the significance of these alterations as well as the detailed biological therapeutic mechanism underlying these effects should be further examined in the context of regenerative therapy, if ADRCs administration was beneficial for liver regenerative therapy of cirrhosis.
It is important concern how ADRCs should be delivered to the liver. In non-clinical experiments using murine hepatitis model, we found that majority of the infused adipose-tissue derived stromal cells accumulated in the lung when cells were administered via the peripheral blood with a fraction of cells reached to the liver [8]. In non-clinical experiments using cirrhotic murine models, we observed that adipose-tissue derived stromal cells directly administered into the liver via the splenic-portal vein route, similar route to the direct delivery of cells to the liver via hepatic artery, resided in the cirrhotic murine liver for at least 2 weeks with the enhancement effect of albumin expression [10]. Thus, we administered ADRCs via the hepatic artery to avoid the off-target accumulation in the lung, expecting the administered ADRCs reached and remained in the cirrhotic liver.
Although this clinical study primarily aimed to confirm the safety of freshly isolated ADRC treatment, characterization of the obtained cells was considered to be important. Thus, we assessed the obtained remaining ADRCs. In addition, since adipose tissue-derived stromal cells substantially contain mesenchymal stem cells, we also tried to expand the remaining ADRCs in culture and assessed their biological characteristics of the expanded cells. ADRCs are known to be enriched in mesenchymal stem cells [18], [19]. Flow cytometry analysis of the obtained ADRCs in this study showed that a substantial number of ADRCs expressed CD44 mesenchymal surface antigens, although cells expressing other mesenchymal stem cell antigens, such as CD73, CD90, and CD105, were small and heterogeneous. ADRCs were successfully expanded in vitro, and a considerable proportion of the cADSCs expressed other mesenchymal stem cell surface antigens including CD105, CD90, and CD73 [20], although heterogeneity of surface antigens expression including CD45 in cADSCs was still observed. Thus, versatility of adipose tissue derived stromal cells, both ADRCs and cADSCs, in the context of cell surface antigens, should be further investigated whether they are also functionally heterogeneous or identical. Despite this concern, when cADSCs were cultured in a medium supplemented with growth/differentiation factors for adipocytes, osteocytes, and chondrocytes, they differentiated into these specific cells lineages morphologically and expressed their specific antigens, suggesting that ADRCs definitely contain a substantial fraction of mesenchymal stem cells. It is also important finding that entire features of cADSCs expanded from those of ADRCs were different from the original ADRCs in the context of size (Fig. 4), surface antigen expression (Table 3, Table 4) and gene expression profile (Fig. 7). Therefore, the current clinical study is limited to assessment of safety using freshly isolated ADRCs for liver regenerative therapy.
Using gene expression analysis, the biological process features of ADRCs were shown to be related to development and regeneration when compared with the expression profile of peripheral blood, indicating that ADRCs may be potentially regenerative [13], [18]. Intriguingly, their prominent gene expression features included immunological processes involving a variety of immune-mediating cell types as well as immunological signal transduction when compared with the gene expression profile of cADSCs. Cirrhosis is a condition of chronic hepatitis and persistent intrahepatic inflammation that results in the development of fibrosis, hepatocyte dysfunction, and blood flow alterations [21]. Considering the unique biological characteristics of patient-derived autologous ADRCs, which, as disclosed in this study, contain substantial MSCs with immunomodulating effects, ADRCs may be ideal for the treatment of chronic liver diseases, including cirrhosis. Thus, ADRC therapy could be a potential alternative for the treatment of chronic liver diseases that are not eradicated or uncontrolled, resulting in the destruction of liver function and architecture.
Gene expression profile of ADRCs was suggestive of regeneration-related processes compared to that of peripheral blood gene expression, and mesenchymal stem cell marker, CD44, was substantially expressed in ADRCs. However, the number of cells expressing other mesenchymal antigens such as CD73, CD90, and CD105 were small and heterogeneous. Therefore, further studies are to be conducted to find out the specific subpopulation in ADRCs, which is the most contributable to liver regeneration.
Utilization of freshly isolated ADRCs is beneficial because it avoids the need to manufacture cells. Similarly, clinical studies using freshly isolated cells from bone marrow without any manufacturing have been reported for therapy of liver cirrhosis [22]. Adipose tissue and bone marrow are known to contain MSCs [23] and are obtainable from a patient's own tissues; thus, both are attracting much attention in the context of practical implementation. Conducting further clinical trials investigating these cells, which are potentially beneficial for the regeneration of impaired organs, is extremely important to provide potential therapeutic beneficial properties. In addition, we could not assess how many cells were the most appropriate for obtaining sufficient therapeutic effect, since the current study was aimed to assess safety of the ADRC regenerative therapy enrolling and treating 4 patients. The future studies should also elucidate how frequent or many times ADRCs should be administered, leading to enhanced development of novel treatments for liver cirrhosis as well as other diseases [24].
5. Conclusion
We conducted a clinical study investigating regenerative therapy for liver cirrhosis using intrahepatic arterial administration of freshly isolated autologous ADRCs. Further clinical trials are warranted to elucidate the potential therapeutic effects of ADRCs and to shed light on the important properties of mesenchymal stem cells on liver diseases.
Acknowledgements
We thank Ms. Masayo Matsumoto, Ms. Sachie Yamazaki, Ms. Ayano Nomura, and Ms. Keiko Yoshida for their excellent technical assistance. This work is supported by subsidies from the Japanese Ministry of Health, Labour and Welfare (grant no. 25040101) and the Japan Agency for Medical Research and Development (grant no. 15bk0104002h0003) and in part by a subsidy from Kanazawa University Hospital.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.reth.2016.12.001.
Conflicts of interest
There are no conflicts of interest.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Clinical course of serum AST, ALT, LDH and CK activity in the treated patients. The clinical courses of serum enzymatic activity, AST (A), ALT(B), LDH(C) and CK(D), before as well as after ADRCs treatment in each patient (HI-01, HI-03, HI-04, and HI-05), were shown.
The representative FACS histogram of ADRC and cADSC. The histograms of surface antigens expression by FACS analysis for ADRCs (A) and cADSCs (B) of HI-04 were descripted. The each antigen expressed in the freshly isolated ADRCs and cultured cADSCs was analyzed using the fluorescence-conjugated antibody by FACS. The negative regions of isotype control antibodies with fluorescence in the histograms were set for gating, and frequency of cells stained with each antibody was assessed, followed by subtraction of frequency in the control histogram.
References
- 1.Bacon B.R. Cirrhosis and its complications. In: Longo Dan L., Kasper Dennis L., Hauser Stephen L., Larry Jameson J., Loscalzo Joseph., editors. Harrison's Principles of internal medicine. 2012. pp. 2592–2603. [Google Scholar]
- 2.Tsochatzis E.A., Bosch J., Burroughs A.K. New therapeutic paradigm for patients with cirrhosis. Hepatology. 2012;56:1983–1992. doi: 10.1002/hep.25915. [DOI] [PubMed] [Google Scholar]
- 3.Kaido T., Uemoto S. Does living donation have advantages over deceased donation in liver transplantation? J Gastroenterol Hepatol. 2010;25:1598–1603. doi: 10.1111/j.1440-1746.2010.06418.x. [DOI] [PubMed] [Google Scholar]
- 4.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 5.Porada C.D., Zanjani E.D., Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1:365–369. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- 6.Banas A., Teratani T., Yamamoto Y., Tokuhara M., Takeshita F., Osaki M. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24:70–77. doi: 10.1111/j.1440-1746.2008.05496.x. [DOI] [PubMed] [Google Scholar]
- 7.Banas A., Teratani T., Yamamoto Y., Tokuhara M., Takeshita F., Quinn G. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 8.Higashimoto M., Sakai Y., Takamura M., Usui S., Nasti A., Yoshida K. Adipose tissue derived stromal stem cell therapy in murine ConA-derived hepatitis is dependent on myeloid-lineage and CD4+ T-cell suppression. Eur J Immunol. 2013;43:2956–2968. doi: 10.1002/eji.201343531. [DOI] [PubMed] [Google Scholar]
- 9.Uccelli A., Moretta L., Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 10.Seki A., Sakai Y., Komura T., Nasti A., Yoshida K., Higashimoto M. Adipose tissue-derived stem cells as a regenerative therapy for a mouse steatohepatitis-induced cirrhosis model. Hepatology. 2013;58:1133–1142. doi: 10.1002/hep.26470. [DOI] [PubMed] [Google Scholar]
- 11.Habbema L. Safety of liposuction using exclusively tumescent local anesthesia in 3240 consecutive cases. Dermatol Surg. 2009;35:1728–1735. doi: 10.1111/j.1524-4725.2009.01284.x. [DOI] [PubMed] [Google Scholar]
- 12.Mann M.W., Palm M.D., Sengelmann R.D. New advances in liposuction technology. Semin Cutan Med Surg. 2008;27:72–82. doi: 10.1016/j.sder.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Zhu M., Zhou Z., Chen Y., Schreiber R., Ransom J.T., Fraser J.K. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64:222–228. doi: 10.1097/SAP.0b013e31819ae05c. [DOI] [PubMed] [Google Scholar]
- 14.Komura T., Sakai Y., Harada K., Kawaguchi K., Takabatake H., Kitagawa H. Inflammatory features of pancreatic cancer highlighted by monocytes/macrophages and CD4+ T cells with clinical impact. Cancer Sci. 2015;106:672–686. doi: 10.1111/cas.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasuike S., Ido A., Uto H., Moriuchi A., Tahara Y., Numata M. Hepatocyte growth factor accelerates the proliferation of hepatic oval cells and possibly promotes the differentiation in a 2-acetylaminofluorene/partial hepatectomy model in rats. J Gastroenterol Hepatol. 2005;20:1753–1761. doi: 10.1111/j.1440-1746.2005.03922.x. [DOI] [PubMed] [Google Scholar]
- 16.Ido A., Tsubouchi H. Translational research to identify clinical applications of hepatocyte growth factor. Hepatol Res. 2009;39:739–747. doi: 10.1111/j.1872-034X.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- 17.Tiberio G.A., Tiberio L., Benetti A., Cervi E., Montani N., Dreano M. IL-6 Promotes compensatory liver regeneration in cirrhotic rat after partial hepatectomy. Cytokine. 2008;42:372–378. doi: 10.1016/j.cyto.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Fraser J.K., Hicok K.C., Shanahan R., Zhu M., Miller S., Arm D.M. The celution system: automated processing of adipose-derived regenerative cells in a functionally closed system. Adv Wound Care (New Rochelle) 2014;3:38–45. doi: 10.1089/wound.2012.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser J.K., Zhu M., Wulur I., Alfonso Z. Adipose-derived stem cells. Methods Mol Biol. 2008;449:59–67. doi: 10.1007/978-1-60327-169-1_4. [DOI] [PubMed] [Google Scholar]
- 20.Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 21.Ramadori G., Saile B. Inflammation, damage repair, immune cells, and liver fibrosis: specific or nonspecific, this is the question. Gastroenterology. 2004;127:997–1000. doi: 10.1053/j.gastro.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 22.Terai S., Sakaida I. Autologous bone marrow cell infusion therapy for liver cirrhosis patients. J Hepatobiliary Pancreat Sci. 2011;18:23–25. doi: 10.1007/s00534-010-0305-1. [DOI] [PubMed] [Google Scholar]
- 23.English K., French A., Wood K.J. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7:431–442. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Gotoh M., Yamamoto T., Kato M., Majima T., Toriyama K., Kamei Y. Regenerative treatment of male stress urinary incontinence by periurethral injection of autologous adipose-derived regenerative cells: 1-year outcomes in 11 patients. Int J Urol. 2014;21:294–300. doi: 10.1111/iju.12266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical course of serum AST, ALT, LDH and CK activity in the treated patients. The clinical courses of serum enzymatic activity, AST (A), ALT(B), LDH(C) and CK(D), before as well as after ADRCs treatment in each patient (HI-01, HI-03, HI-04, and HI-05), were shown.
The representative FACS histogram of ADRC and cADSC. The histograms of surface antigens expression by FACS analysis for ADRCs (A) and cADSCs (B) of HI-04 were descripted. The each antigen expressed in the freshly isolated ADRCs and cultured cADSCs was analyzed using the fluorescence-conjugated antibody by FACS. The negative regions of isotype control antibodies with fluorescence in the histograms were set for gating, and frequency of cells stained with each antibody was assessed, followed by subtraction of frequency in the control histogram.