Abstract
Objective
To examine the natural history of acute alcoholic hepatitis (AH) and identify predictors of mortality for AH using data from a prospective multicenter observational study.
Participants and Methods
We analyzed data from 164 patients with AH and 131 heavy-drinking controls with no liver disease. Participants underwent clinical/laboratory assessment at baseline and 6 and 12 months after enrollment. Multivariable analyses were conducted to identify variables associated with mortality and examine the association between coffee drinking and risk of AH.
Results
Thirty-six patients with AH died during follow-up, with estimated 30-day, 90-day, 180-day, and 1-year survival of 0.91 (95% CI, 0.87-0.96), 0.85 (95% CI, 0.80-0.91), 0.80 (95% CI, 0.74-0.87), and 0.75 (95% CI, 0.68-0.83), respectively. In the multivariable analysis, higher serum bilirubin level (hazard ratio [HR]=1.059; 95% CI, 1.022-1.089), lower hemoglobin level (HR=1.263; 95% CI, 1.012-1.575), and lower platelet count (HR=1.006; 95% CI, 1.001-1.012) were independently associated with mortality in AH. Compared with controls, fewer patients with AH regularly consumed coffee (20% vs 44%; P<.001), and this association between regular coffee drinking and lower risk of AH persisted after controlling for relevant covariates (odds ratio=0.26; 95% CI, 0.15-0.46). Time-dependent receiver operating characteristic curve analysis revealed that Model for End-Stage Liver Disease; Maddrey Discriminant Function; age, serum bilirubin, international normalized ratio, and serum creatinine; and Child-Pugh scores all provided similar discrimination performance at 30 days (area under the curve=0.73-0.77).
Conclusion
Alcoholic hepatitis remains highly fatal, with 1-year mortality of 25%. Regular coffee consumption was associated with lower risk of AH in heavy drinkers.
Abbreviations and Acronyms: ABIC, age, serum bilirubin, international normalized ratio, and serum creatinine; AH, alcoholic hepatitis; AIC, Akaike Information Criterion; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under the curve; BMI, body mass index; CP, Child-Pugh; HR, hazard ratio; INR, international normalized ratio; IQR, interquartile range; mDF, Maddrey Discriminant Function; MELD, Model for End-Stage Liver Disease; NA, not applicable; OR, odds ratio; ROC, receiver operating characteristic; STOPAH, Steroids or Pentoxifylline for Alcoholic Hepatitis; TREAT, Translational Research and Evolving Alcoholic Hepatitis Treatment; WBC, white blood cell
Excessive alcohol consumption is a growing trend in the United States, with approximately 87% of the general population older than 18 years reporting some history of alcohol consumption and approximately 25% meeting the criteria for heavy drinking.1, 2, 3 Alcoholic hepatitis (AH) is a unique syndrome in patients with chronic and active harmful alcohol use. It is associated with poor prognosis and reported short-term mortality of 16% to 50% within 1 month of presentation, depending on initial disease severity.4, 5, 6, 7, 8 Based on the National Inpatient Sample analysis, AH accounts for 0.7% of all hospital admissions in the United States, which is higher than that for myocardial infarction, acute cerebrovascular disease, or acute pancreatitis.9, 10 No recent studies have prospectively examined the natural history of AH in the United States. In addition, the lack of prospective clinical studies makes it challenging to validate existing risk stratification models, such as the Model for End-Stage Liver Disease (MELD) score; Lille score; age, serum bilirubin, international normalized ratio, serum creatinine (ABIC) score; Child-Pugh (CP) score; or Maddrey Discriminant Function (mDF) score, to classify disease severity and predict survival in patients with AH.11, 12, 13
The risk of alcoholic liver disease is related to the amount and duration of alcohol use.14, 15 However, only a small proportion of heavy drinkers develop AH, suggesting the role of host and environmental factors on the development of AH.2, 4, 5, 16 Recent genomic studies reported higher variants of hepatic antiapoptotic genes such as K8/K18 (keratinocytes) in white individuals or the PNPLA3 gene in the Hispanic population, which predisposes them to liver injury.17, 18, 19 Furthermore, several studies have suggested the benefit of regular coffee consumption in preventing liver disease, albeit limited to epidemiologic or retrospective studies involving nonalcoholic fatty liver disease and liver cancer.20, 21, 22, 23 There are limited data, either prospective or retrospective, that evaluate the relationship between coffee consumption and AH in heavy drinkers.
The main objectives of this study were to describe (1) the outcomes of AH in a cohort using the new diagnostic definition,24 (2) variables associated with mortality in AH, (3) the performance of commonly used risk stratification models, and (4) the relationship between coffee consumption and the risk of AH in heavy drinkers using a multicenter prospective cohort. The data presented herein provide additional complementary analyses beyond a recent and initial publication describing this cohort.25
Participants and Methods
This article is concerned with an ongoing prospective, multicenter, observational study of patients with well-characterized AH (cases) and heavy drinkers without evidence of liver disease (controls) conducted by the Translational Research and Evolving Alcoholic Hepatitis Treatment (TREAT) Consortium. The TREAT Consortium, consisting of Indiana University, Virginia Commonwealth University, and Mayo Clinic, is funded by the National Institute on Alcohol Abuse and Alcoholism and its objectives are to conduct clinical research in AH and develop novel treatments.
Participants
For the most part, cases were enrolled from inpatient clinical sites associated with the respective center in the consortium, and controls were enrolled from 1 or more of the local alcohol rehabilitation facilities. However, outpatients from liver clinics were also candidates for inclusion as cases in the study, but these individuals’ laboratory values were typically out of the range for inclusion as cases and, thus, they were unlikely to be enrolled. Heavy drinkers were defined as having average daily alcohol consumption greater than 40 g/d for women or greater than 60 g/d for men for the last 5 years and active drinking within the 6 weeks before study enrollment, amounts that are believed to be reasonable minimal thresholds for the development of AH.24 Frequency-matched (for similar alcohol consumption history) heavy drinkers without liver disease were recruited to the control group. Absence of liver disease in the control group was ascertained based on history, physical examination findings, and normal liver enzyme levels. The diagnosis of AH was made by the admitting clinician based on a combination of appropriate clinical and laboratory data as per the new consensus definition of AH24, 25 criteria in the presence of heavy drinking for a minimum of 6 months and within 6 weeks before enrollment. Subsequent testing, including liver biopsy in some cases, was performed at the discretion of the managing clinician for patients in whom the diagnosis was still in question based on these criteria. Individuals with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus were eligible for enrollment as cases to include the full spectrum of patients seen with AH in practice. Exclusion criteria for either cases or controls included (1) younger than 21 years; (2) evidence of other liver diseases, such as autoimmune or drug induced, hemochromatosis, or Wilson disease; (3) active intravenous drug use; or (4) comorbidities such as chronic obstructive pulmonary disease, congestive heart failure, or multiorgan failure. This study was approved by the institutional review boards at the respective institutions, and all the participants signed an informed consent form before enrollment. These cohorts were described in a recent publication by the TREAT Consortium.24
Detailed data were collected on (1) demographic factors (age, sex, race and ethnicity, marital status, highest educational level), vital signs, anthropometry, medical history, and concomitant medications; (2) quantity of coffee and tea consumed; (3) complications of portal hypertension (ascites, jaundice, varices, hepatic encephalopathy, hepatocellular carcinoma); (4) laboratory values with calculated mDF, MELD, ABIC, and CP scores; and (5) hepatitis B virus, hepatitis C virus, or human immunodeficiency virus status. Both cases and controls had follow-up visits 6 and 12 months after enrollment.
Statistical Analyses
Baseline Differences and Logistic Regression
First, descriptive statistics were used to describe selected baseline characteristics for cases and controls. χ2 and t tests were used to test univariate differences in proportions/means by group for categorical and continuous variables, respectively. Subsequently, logistic regression was performed to examine the relationship between selected baseline characteristics (body mass index [BMI; calculated as the weight in kilograms divided by the height in meters squared]; age; sex; regular coffee consumption [≥4 times a week for 5 years]; regular tea consumption [≥4 times a week for 5 years], measured separately for black and green tea26; educational level measured as low, medium, and high; and marital status) and the presence of AH, with risk estimates reported as odds ratios (ORs). Race was not considered for multivariable analyses due to a very small percentage of nonwhite participants in the study. All the variables were screened for entry into the final model using a cutoff value of P<.20 in univariate models. The Akaike Information Criterion (AIC)27 was subsequently used to examine relative model fit for all possible models built using main effects of variables identified in the first stage. The model with the minimum AIC was selected as the best model.
Variables Associated With Mortality in AH
A Kaplan-Meier estimate of survival in patients with AH was constructed, with CIs as dotted lines. The Kaplan-Meier estimate for controls was not calculated because only 1 death (due to drug overdose) was observed. Next, Cox proportional hazards19 modeling was used to examine the relationship between selected variables (age; sex; BMI; race; marital status; educational level; regular coffee use; regular black tea use; regular green tea use; mean arterial pressure; serum levels of albumin, creatinine, bilirubin, alanine aminotransferase, and aspartate aminotransferase; hemoglobin level; white blood cell count; platelet count; and the risk scores [MELD, ABIC, mDF, and CP]) and mortality for patients with AH, with risk estimates reported as hazard ratios (HRs). All the variables (except for the risk scores) were screened for entry using a cutoff value of P<.20 in univariate models. The risk scores were analyzed separately using time-dependent receiver operating characteristic (ROC) curve analysis (see below). The AIC27 was subsequently used to examine relative model fit for all possible models built using main effects of variables identified in the first stage. The model with minimum AIC was selected as the best model.
Comparing Various Risk Scores for Predicting Mortality in AH
Next, time-dependent ROC curve analysis28 was applied separately for MELD, mDF, ABIC, and CP scores to determine optimal cutoff values for predicting mortality among AH cases at the 30-day, 90-day, 180-day, and 1-year intervals, with area under the curve (AUC) values and 95% CIs presented for all scores and intervals. A bootstrap resampling procedure29 was used to estimate standard errors and compare time-dependent AUCs by risk score, with 1000 samples drawn. The Lille score and Glasgow alcoholic hepatitis score were not assessed because blood urea nitrogen level (included in the Glasgow calculation) was not measured and bilirubin level at day 7 (included in Lille score calculation) was measured only for a small percentage of study participants.
For each risk score, the optimal baseline cutoff score for predicting 90-day mortality was determined by minimizing the distance on the respective ROC curves and perfect performance, ie, 100% sensitivity and 100% specificity. These cutoff points were then used to stratify the sample of AH cases and plot group-specific Kaplan-Meier survival curves during the 1-year study follow-up with associated CIs at the 30-day, 90-day, 180-day, and 1-year intervals.
Results
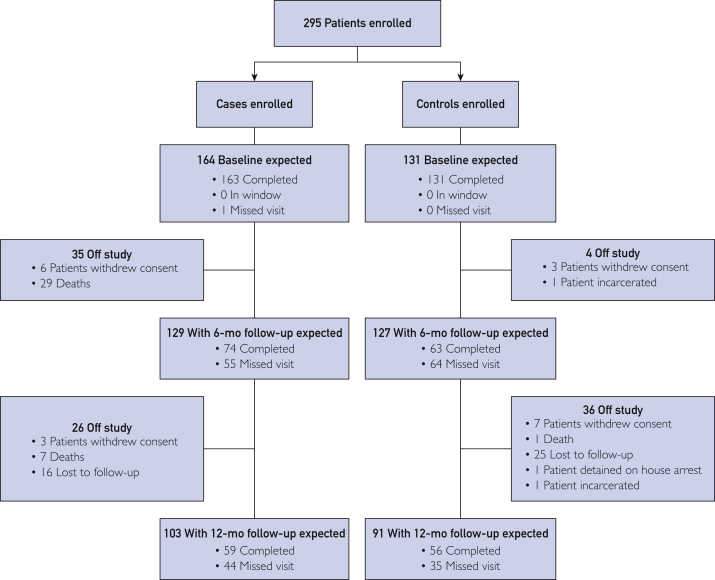
From June 18, 2013, through December 1, 2015, 164 participants with AH and 131 heavy-drinking controls were recruited (Figure 1). Liver biopsy was performed on 41 patients to exclude concurrent liver disease, such as autoimmune, hemochromatosis, or Wilson disease. Thirty-four patients completed liver biopsy during initial enrollment, and the remaining 7 patients completed liver biopsy during follow-up. The median duration of follow-up for all the participants was 324 days (range, 1-365 days). The median duration of follow-up was 281 days (interquartile range [IQR]=93-365 days) in patients with AH and 352 days (IQR=290-365 days) in the control group. Selected clinical characteristics and laboratory values of cases and controls are shown in Table 1. There were no differences in age, sex, BMI, educational level, marital status, black tea use, or green tea use between cases and controls. However, regular coffee use was significantly less frequent in patients with AH than in heavy-drinking controls (20% vs 44%; P<.001) (Table 1). Sixty-six patients received prednisone and 27 received pentoxifylline in the case group.
Figure 1.
Consort diagram describing the cohort experience through 12-month follow-up. Completed indicates completed the visit (regardless of whether completed inside or outside the 3-month window); in window, individual still in window but not completed (3-9 months for the 6-month visit, 9-15 months for the 12-month visit); not yet in window, follow-up time less than 3 months for the 6-month visit and less than 9 months for the 12-month visit; missing visit, past the window/missed the visit.
Table 1.
Selected Clinical and Laboratory Characteristics of Patients With Alcoholic Hepatitis and Heavy-Drinking Controlsa,b,c
| Characteristic | Cases (n=164) | Controls (n=131) | Univariable P value |
|---|---|---|---|
| Age (y) | 46.8±10.9 | 44.4±12.5 | .08 |
| Male sex (No. [%]) | 99 (60.4) | 84 (64.1) | .50 |
| White race (No. [%]) | 144 (87.8) | 108 (82.4) | .20 |
| BMI | 29.1±7.4 | 28.7±7.4 | .60 |
| Prednisone use (No. [%]) | 66 (40.2) | 3 (2.3) | NA |
| Pentoxifylline use (No. [%]) | 27 (16.5) | 1 (0.7) | NA |
| Regular coffee use (No. [%]) | 33 (20.1) | 58 (44.3) | <.001 |
| Black tea use (No. [%]) | 21 (12.8) | 18 (13.7) | .80 |
| Green tea use (No. [%]) | 7 (4.3) | 5 (3.8) | .80 |
| Educational level (No. [%]) | n=160 | n=130 | .08 |
| Low | 26 (16.3) | 10 (7.7) | |
| Medium | 93 (58.1) | 84 (64.6) | |
| High | 41 (25.6 ) | 36 (27.7) | |
| Married (%) | 31.1 | 31.3 | >.99 |
| WBC count (x103/mm3) | 11,400±8300 | 7200±2700 | <.001 |
| MCV (fl) | 101±10 | 92.5±5.7 | <.001 |
| Hemoglobin (f/dL) | 0.10±0.019 | 0.13±0.02 | <.001 |
| Platelets (×103/mm3) | 14,500±8850 | 24,400±7250 | <.001 |
| AST (U/L) | 141.5±90.8 | 27±9.0 | <.001 |
| ALT (U/L) | 64.3±66.9 | 25.6±10.4 | <.001 |
| Alkaline phosphatase (U/L) | 195.0±141.8 | 75.1±32.5 | <.001 |
| Total bilirubin (mg/dL) | 13.5±11.7 | 0.5±0.3 | <.001 |
| Albumin (g/dL) | 2.0±0.7 | 3.9±0.6 | <.001 |
| INR | 1.8±0.5 | 1.0±0.3 | <.001 |
| Creatinine (mg/dL) | 1.0±0.8 | 0.8±0.3 | .07 |
| MELD score | 22.1±7.1 | 7.2±2.2 | <.001 |
| ABIC score | 7.5±1.5 | 5.6±1.3 | <.001 |
| Child-Pugh score | 9.5±1.7 | 5.4±0.6 | <.001 |
| mDF score | 41.6±29.1 | −6.4±11.0 | <.001 |
ABIC = age, bilirubin, international normalized ratio, and creatinine; ALT = alanine aminotransferase; AST = aspartate aminotransferase; BMI = body mass index; INR = international normalized ratio; MCV = mean corpuscular volume; mDF = Maddrey Discriminant Function; MELD = Model for End-Stage Liver Disease; NA = not applicable; WBC = white blood cell.
SI conversion factors: To convert WBC count values to ×109/L, multiply by 0.001; to convert hemoglobin values to g/L, multiply by 10.0; to convert platelet count values to ×109/L, multiply by 1.0; to convert AST values to μkat/L, multiply by 0.0167; to convert ALT values to μkat/L, multiply by 0.0167; to convert alkaline phosphatase values to μkat/L, multiply by 0.0167; to convert total bilirubin values to μmol/L, multiply by 17.104; to convert albumin values to g/L, multiply by 10.0; to convert creatinine values to μmol/L, multiply by 88.4.
Values are mean ± SD unless presented otherwise.
Factors Independently Associated with AH
Based on the model selection algorithm described in the “Participants and Methods” section, the best-fitting logistic regression model for baseline AH status included coffee consumption, age, and educational level (low, medium, and high). In summary, regular coffee consumption was found to be independently associated with a significantly lower risk of AH (OR=0.26; 95% CI, 0.15-0.46) (Table 2). Compared with individuals with low educational level, individuals with medium educational level (OR=0.38; 95% CI, 0.16-0.89) were less likely to present with AH at baseline. Increasing age was also positively associated with AH (OR=1.03; 95% CI, 1.01-1.06) (Table 2).
Table 2.
Results of Multivariable Logistic Regression Analysis of Variables Associated With Alcoholic Hepatitisa
| Variable | Estimate | Standard error | χ2 | P value | Odds ratio (95% CI) |
|---|---|---|---|---|---|
| Age | 0.0327 | 0.0114 | 8.2837 | .004 | 1.03 (1.01-1.06) |
| Educational level | |||||
| Medium vs low | −0.9637 | 0.4342 | 4.9257 | .03 | 0.38 (0.16-0.89) |
| High vs low | −0.7445 | 0.4760 | 2.4460 | .12 | 0.48 (0.19-1.21) |
| Regular coffee consumption | −1.3424 | 0.2933 | 20.9498 | <.001 | 0.26 (0.15-0.46) |
The following variables were eligible for inclusion in the final model selected by the Akaike Information Criterion: age, educational level, and coffee consumption.
Predictors of Mortality in AH
During follow-up, 36 patients with AH died, with most deaths occurring within the first 6 months after enrollment (Figure 2). The median time to death was 45 days (IQR=20-120 days). The estimated 30-day, 90-day, 180-day, and 1-year survival in patients with AH were 0.91 (95% CI, 0.87-0.96), 0.85 (95% CI, 0.80-0.91), 0.80 (95% CI, 0.74-0.87), and 0.75 (95% CI, 0.68-0.83), respectively. The most common cause of death in the case group was organ failure (24 of 36), followed by severe infection (5 of 36). Seven patients died of an unknown cause. Among controls, mortality was observed in only 1 individual (heroin overdose).
Figure 2.
Kaplan-Meier curve for survival of 164 patients with alcoholic hepatitis. Solid line represents mean survival; dotted lines, 95% CI.
The results of univariable and multivariable analyses of variables associated with mortality in patients with AH are shown in Table 3. Although pentoxifylline use at baseline was a significant univariate predictor of mortality among AH cases, the association was in the opposite direction as expected. The reason for this contradictory finding is that those given pentoxifylline at baseline had more severe AH than those not given pentoxifylline, as indicated by an average baseline MELD score of 27.89 in the pentoxifylline group vs 20.89 in the nonpentoxifylline group at baseline. For this reason, we chose to focus on liver function and other factors besides treatment to select the final survival model. The final model selected via the algorithm described in the Participants and Methods contained effects for bilirubin, hemoglobin, platelet count, and albumin. Higher serum bilirubin level (HR=1.059; 95% CI, 1.022-1.089), lower hemoglobin level (HR=1.263; 95% CI, 1.012-1.575), and lower platelet count (HR=1.006; 95% CI, 1.001-1.012) were found to be independently associated with increased mortality rates in AH.
Table 3.
Univariable and Multivariable Analysis of Variables Associated With Mortality in Alcoholic Hepatitisa,b
| Parameter | Univariable P value | Multivariable analysis |
|
|---|---|---|---|
| P value | Hazard ratio (95% CI) | ||
| Age | .7 | ||
| Male sex | .6 | ||
| BMI | .1 | ||
| Race | .9 | ||
| Marital status: yes | .3 | ||
| Educational level | |||
| Medium vs low | .6 | ||
| High vs low | .46 | ||
| Heavy drinking | .8 | ||
| Regular coffee consumption | .99 | ||
| Regular black tea consumption | .7 | ||
| Regular green tea consumption | .6 | ||
| Mean blood pressure | .7 | ||
| Albumin (1-U decrease) | .1 | .14 | 1.587 (0.859-2.933) |
| Creatinine | .4 | ||
| Bilirubin | .049 | .001 | 1.059 (1.022-1.089) |
| ALT | .1 | ||
| AST | .8 | ||
| Hemoglobin (1-U decrease) | .1 | .04 | 1.263 (1.012-1.575) |
| WBC count | .5 | ||
| Platelet count (1-U decrease) | .08 | .02 | 1.006 (1.001-1.012) |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; BMI = body mass index; WBC = white blood cell.
The following variables were eligible for inclusion in the final model selected by the Akaike Information Criterion: albumin, bilirubin, ALT, hemoglobin, platelet count, and BMI.
Risk Scores and Mortality in AH
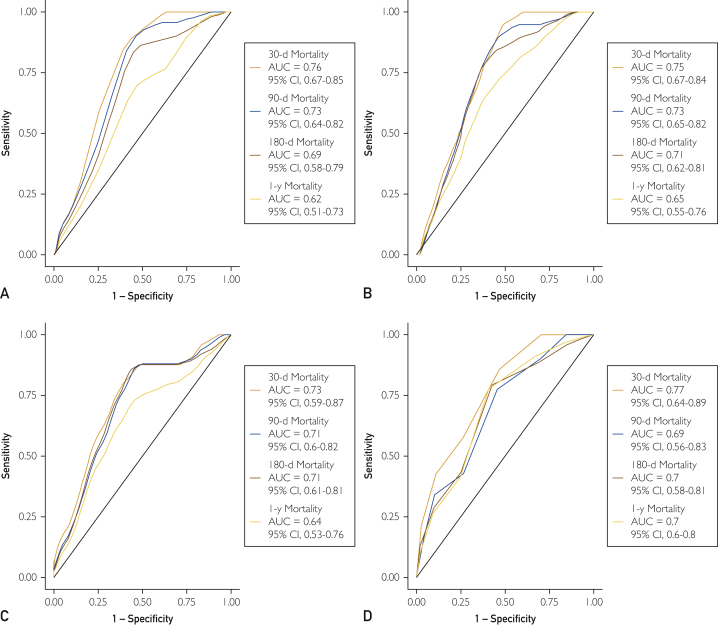
Time-dependent ROC curve analysis (Figure 3) applied separately to each risk score revealed that CP score had the highest estimated discrimination for predicting mortality at the 30-day (AUC=0.77; 95% CI, 0.64-0.89) and 1-year (AUC=0.70; 95% CI, 0.6-0.8) intervals. At the 90-day interval, MELD (AUC=0.73; 95% CI, 0.64-0.82) and mDF (AUC=0.73; 95% CI, 0.65-0.82) scores were tied for highest estimated discrimination for predicting mortality. At the 180-day interval, mDF (0.71; 95% CI, 0.62-0.81) and ABIC (AUC=0.71; 95% CI, 0.61-0.81) scores were tied for highest estimated discrimination for predicting mortality. All 30-day AUCs were greater than 0.70, reflecting fair to good discriminative power over this interval for all risk scores. The AUCs for all risk scores were greater than or very near 0.70 for predicting 90- and 180-day mortality, reflecting fair performance over those intervals. The only risk score with an AUC near 0.7 at 1 year was CP score; however, for all risk scores, 30- and 90-day discrimination performance was at the fair level or higher (AUC≥0.7). Bootstrap CIs (not presented) were calculated for each pairwise AUC difference and showed that there was insufficient statistical evidence to conclude that the markers differed in their discrimination performance.
Figure 3.
Receiver operating characteristic (ROC) curves for Model for End-Stage Liver Disease (MELD); Maddrey Discriminant Function (mDF); age, serum bilirubin, international normalized ratio, and serum creatinine (ABIC); and Child-Pugh (CP) scores for predicting 30-day, 90-day, 180-day, and 1-year mortality rates. A, The ROC curve for MELD scores. The area under the curve (AUC) for 30-day mortality is 0.76 (95% CI, 0.67-0.85); 90-day mortality, 0.73 (95% CI, 0.64-0.82); 180-day mortality, 0.69 (95% CI, 0.58-0.79); and 1-year mortality, 0.62 (95% CI, 0.51-0.73). B, The ROC curve for mDF scores. The AUC for 30-day mortality is 0.75 (95% CI, 0.67-0.84); 90-day mortality, 0.73 (95% CI, 0.65-0.82); 180-day mortality, 0.71 (95% CI, 0.62-0.81); and 1-year mortality, 0.65 (95% CI, 0.55-0.76). C, The ROC curve for ABIC scores. The AUC for 30-day mortality is 0.73 (95% CI, 0.59-0.87); 90-day mortality, 0.71 (95% CI, 0.6-0.82); 180-day mortality, 0.71 (95% CI, 0.61-0.81); and 1-year mortality, 0.64 (95% CI, 0.53-0.76). D, The ROC curve for CP scores. The AUC for 30-day mortality is 0.77 (95% CI, 0.64-0.89), 90-day mortality, 0.69 (95% CI, 0.56-0.83); 180-day mortality, 0.7 (95% CI, 0.58-0.81); and 1-year mortality, 0.7 (95% CI, 0.6-0.8).
The 90-day ROC curve for each risk score was used to calculate optimal cutoff values for predicting survival in this cohort (Table 4). The 90-day AUCs for MELD, mDF, ABIC, and CP scores were 0.76, 0.75, 0.73, and 0.77, respectively. The time-dependent ROC curves at the 90-day interval led to selecting a cutoff score of 22 or greater for MELD, 44.62 or greater for mDF, 7.603 or greater for ABIC, and 9 or greater for CP scores. The Kaplan-Meier survival curves for these classifications are presented in Figure 4, along with tick marks on the y-axis that denote survival probabilities at the 30-day, 90-day, 180-day, and 1-year intervals as well as associated CIs.
Table 4.
Estimated Survival and 95% CIs for MELD, mDF, ABIC, and CP Scores Based on Optimal Cutoff Scores
| Cutoff score | Survival (95% CI) |
|||
|---|---|---|---|---|
| 30 d | 90 d | 180 d | 1 y | |
| MELD | ||||
| ≥22 | 0.84 (0.77-0.93) | 0.74 (0.65-0.84) | 0.67 (0.58-0.78) | 0.66 (0.56-0.77) |
| <22 | 0.99 (0.96-1) | 0.97 (0.94-1) | 0.94 (0.89-0.99) | 0.86 (0.77-0.96) |
| mDF | ||||
| ≥44.62 | 0.84 (0.76-0.93) | 0.73 (0.63-0.85) | 0.65 (0.54-0.78) | 0.63 (0.52-0.76) |
| <44.62 | 0.97 (0.93-1) | 0.94 (0.90-0.99) | 0.92 (0.86-0.98) | 0.84 (0.76-0.94) |
| ABIC | ||||
| ≥7.603 | 0.83 (0.74-0.92) | 0.74 (0.64-0.85) | 0.65 (0.55-0.78) | 0.64 (0.53-0.76) |
| <7.603 | 0.98 (0.95-1) | 0.94 (0.89-0.99) | 0.91 (0.85-0.98) | 0.84 (0.76-0.94) |
| CP | ||||
| ≥9 | 0.88 (0.82-0.94) | 0.82 (0.75-0.89) | 0.75 (0.68-0.84) | 0.69 (0.61-0.79) |
| <9 | 1 (1-1) | 0.95 (0.88-1) | 0.92 (0.83-1) | 0.92 (0.83-1) |
ABIC = age, serum bilirubin, international normalized ratio, and serum creatinine; CP = Child-Pugh; mDF = Maddrey Discriminant Function; MELD = Model for End-Stage Liver Disease.
Figure 4.
Kaplan-Meier survival curves for 4 risk stratification models stratified by optimal cutoff points (90-day interval). A, Model for End-Stage Liver Disease (MELD) score (<22 vs ≥22). B, Maddrey Discriminant Function (mDF) score (<44.62 vs ≥44.62). C, Age, serum bilirubin, international normalized ratio, and serum creatinine (ABIC) score (<7.603 vs ≥7.603). D, Child-Pugh (CP) score (<9 vs ≥9).
Discussion
Alcoholic hepatitis is a form of alcoholic liver disease associated with high morbidity and mortality rates in severe cases. Little is known about the natural history of AH, risk factors, and predictors of mortality and recidivism, especially when patients are defined using the new consensus definition of AH.24 To better characterize the progression of disease, we prospectively observed heavy drinkers with and without a clinical diagnosis of AH over a 1-year period. Through this study we were able to prospectively validate the utility of MELD and other prognostic scores in predicting short- and long-term mortality associated with AH. Furthermore, we were able to elucidate the relationship between coffee consumption and AH.
There are several population-based and retrospective studies reporting the association between coffee consumption and a lower incidence of liver disease.20, 21, 22, 23 We found an association between regular coffee consumption and the presence of AH at baseline, an effect that persisted after controlling for other factors. This effect was denoted by a statistically significant reduction in the odds of AH for participants who regularly consumed coffee in the logistic regression model. In fact, heavy drinkers with regular coffee consumption were 4 times less likely to present with AH. The mechanism behind this protection is incompletely understood, although findings from rodent models suggest favorable alterations in the liver biochemical signaling pathways associated with coffee consumption.30 However, note that this finding does not indicate evidence for causation, only an association. Because we found coffee to be associated with a decreased prevalence of AH in heavy drinkers, we hypothesized that coffee consumption would also provide mortality benefit in this population. However, we did not find an association between coffee consumption and mortality. These data suggest that regular coffee consumption might provide some benefit in reducing the likelihood of AH in heavy drinkers but does not confer survival benefit once AH has developed. Further studies might elucidate whether coffee consumption in heavy drinkers is hepatoprotective, as well as the mechanisms involved.
There are several different prognostic scores used to drive management decisions in AH, such as CP, mDF, ABIC, and MELD scores. For each of these prognostic scores, mortality was extremely unlikely for individuals whose baseline score fell below the corresponding cutoff point (survival >97% and >94% at 30 and 90 days, respectively). However, the scores were less precise in determining which patients above these cutoff scores were at risk for mortality in terms of the mortality rate being lower than expected for individuals presenting scores higher than the cutoff scores at baseline, reflected by the 12% to 17% range of 1-month mortality rates. This inaccuracy may reflect the importance of recidivism in increasing mortality risk. Mortality prediction may improve if a validated recidivism risk score is added to the current models. A drawback of the CP and mDF scores, which are used in patients with alcoholic cirrhosis and AH, is the lack of standardization of the prothrombin time from laboratory to laboratory. Thus, both CP and mDF scores may vary depending on the sensitivity of the reagent used to measure the prothrombin time. This variability has been rectified by expressing the prothrombin time as international normalized ratio taking into consideration the sensitivity of the thromboplastin reagent used for the test by the individual laboratory. Moreover, the CP score includes ascites and hepatic encephalopathy, which are subjective parameters and can depend on the experience of the observer assessing the variable. Also, ABIC score has been criticized because the group characterized as low risk (ABIC score <6.71) exhibited approximately 17% mortality at 84 days.25 Both MELD and CP are generic scores that have been validated across a variety of liver diseases. Moreover, the MELD score is used to prioritize organ allocation for liver transplant in the United States and in other countries. Alcoholic hepatitis–specific scores such as the mDF and the ABIC would be attractive only if they were clearly superior to the generic scores. This was not the case in the present study. Finally, the CP score has not been extensively validated in AH. Therefore, we chose to focus on the MELD score further as described later herein.
Despite retrospective studies supporting its utility, prospective validation and optimization of the ideal cutoff point for pharmacologic therapy are not available.13 The MELD score was previously validated using a retrospective study, with a score 21 or greater reported to have 75% sensitivity and 75% specificity in predicting 90-day mortality.11 In a prospective study, we confirmed the MELD score to be a strong independent factor that predicts mortality in AH. More specifically, we found a baseline MELD score of 22 or greater to be the optimal score that predicts the largest difference in mortality over the year-long period of follow-up. Although the optimal cutoff score in this study was slightly different from that previously observed (≥22 vs ≥21), this study provides substantial evidence for the MELD score as a reliable predictor of mortality in AH. The previously accepted mDF cutoff point of greater than 32 could not be validated. Instead, we identified 44.62 as the optimal mDF cutoff score, a value discordant from the previously reported limit of 32. As discussed later herein, this finding may be attributed to the overall reduced mortality rate observed in the present sample. These findings further validate the utility of the MELD score for predicting the short- and long-term mortality associated with AH. We believe that a MELD score of 22 or greater can be used as a clinical tool to prognosticate patients with AH, to guide treatment decisions in the acute setting, and to stratify patients in investigational protocols.
The strengths and weakness of this study merit further discussion. The major strength is the prospective multicenter nature of the study, with close follow-up at tertiary care hepatology centers. The major limitation is the lack of information available on comorbid medical conditions that can affect outcomes, such as infection, gastrointestinal bleeding, or renal failure. This may contribute to the difference in baseline information and outcome compared with the Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial. In addition, information regarding addiction treatment, medication adherence, and relapse information (besides at 6- and 12-month intervals) in the case and control groups is also lacking. This limitation makes it difficult to ascertain the contributions of inadequate treatment, noncompliance, and relapse to risk of mortality. Furthermore, because most (28 of 36) observed deaths in the AH case group occurred before 6 months, relapse information is of limited use in predicting mortality in this study. Future studies should collect more frequent relapse information to aid in assessing the relative contribution of relapse to mortality in AH cases. We recommend the use of telephone interviews, if possible, to reduce study costs and logistical issues. The lack of close follow-up during the first 6 months of the study is a related weakness. The median of all observed death times was 105 days, indicating that a 6-month follow-up visit schedule may be too infrequent to fully capture the relationship between risk scores and risk of mortality in the short-term. Future studies should follow patients more closely during the first 6 months to provide information regarding factors associated with mortality and recidivism. The study inclusion criteria for the AH case group, specifically, total bilirubin level greater than 2 mg/dL (to convert to μmol/L, multiply by 17.104) and aspartate aminotransferase level greater than 50 U/L (to convert to μkat/L, multiply by 0.0167) may also be viewed as limitations because they include mild AH as well as severe AH. However, the authors note that these criteria are similar to guidance provided in a recent commentary article in the journal Gastroenterology and coincide with our colleagues' recently published work from this cohort.24, 25 The mortality rate of the present study is lower and worth discussing. The difference in mortality rates between this study and the STOPAH trial likely represents a difference in patient selection between the 2 studies. The STOPAH trial selected patients with severe AH, which is reflected by the higher mean ± SD mDF score (62.6±27.2 vs 41.6±29.1), and the present study enrolled a wider range of severity, including mild AH. Finally, the relatively small numbers of patients and large proportion of white individuals in the present study make it difficult to explain the relationship between race, educational level, and risk of AH.
Conclusion
We found that patients with AH have high short- and long-term mortality rates, with most deaths (85.7%) occurring within 6 months after enrollment. Regular coffee consumption was associated with lower odds of development of AH in heavy drinkers but did not provide mortality benefit once AH developed. Alcoholic hepatitis with a MELD score of 22 or greater was associated with higher 30-day, 90-day, 180-day, and 1-year mortality rates. This study provides a prospective assessment of a variety of important issues in AH and concurrently uncovers several new issues that warrant future study.
Acknowledgments
Drs Lourens and Sunjaya contributed equally to this work.
Footnotes
Grant Support: The Translational Research and Evolving Alcoholic Hepatitis Treatment (TREAT) Consortium is supported by grants 5U01AA021883-04, 5U01AA021891-04, 5U01AA021788-04, and 5U01AA021840-04 from the National Institute on Alcohol Abuse and Alcoholism.
Potential Competing Interests: Dr Sanyal reports consultancy fees from Intercept, Galectin, BMS, Nitto Denko, Nimbus, Aredlyx, Vivelyx, Teva; pending grants from Gilead, Intercept, Novartis, Merck, BMS, Tobira; receives royalties from UpToDate; and owns stock in Genfit, Akarna, Tiziana, Natural Shield, Durect, Exhalenz. The rest of the authors report no competing interests.
Members of the TREAT Consortium are as follows: Indiana University, Indianapolis: David Crabb, MD, Naga Chalasani, MD, Suthat Liangpunsakul, MD, Barry Katz, PhD, Spencer Lourens, PhD, Andy Borst, BS, Ryan Cook, MPH, Andy Qigui Yu, PhD, David Nelson, PhD, Romil Saxena, MD, Sherrie Cummings, RN, Megan Comerford, BS, and Lakye Edwards, BS; Mayo Clinic, Rochester, MN: Vijay H. Shah, MD, Gregory Gores, MD, Patrick S. Kamath, MD, Vikas Verma, PhD, Sarah Wilder, RN, BSN, Amy Olofson, RN, and Amanda Schimek; Virginia Commonwealth University, Richmond: Arun Sanyal, MD, Puneet Puri, MD, and Susan Walker, RN, MSN; and National Institute on Alcohol Abuse and Alcoholism: Svetlana Radaeva, PhD (project scientist), and Andras Orosz, PhD (program official).
Contributor Information
Vijay H. Shah, Email: shah.vijay@mayo.edu.
TREAT Consortium:
David Crabb, Naga Chalasani, Suthat Liangpunsakul, Barry Katz, Spencer Lourens, Andy Borst, Ryan Cook, Andy Qigui Yu, David Nelson, Romil Saxena, Sherrie Cummings, Megan Comerford, Lakye Edwards, Vijay H. Shah, Gregory Gores, Patrick S. Kamath, Vikas Verma, Sarah Wilder, Amy Olofson, Amanda Schimek, Arun Sanyal, Puneet Puri, Susan Walker, Svetlana Radaeva, and Andras Orosz
References
- 1.Mandayam S., Jamal M.M., Morgan T.R. Epidemiology of alcoholic liver disease. Semin Liver Dis. 2004;24(3):217–232. doi: 10.1055/s-2004-832936. [DOI] [PubMed] [Google Scholar]
- 2.Singal A.K., Anand B.S. Recent trends in the epidemiology of alcoholic liver disease. Clin Liver Dis. 2013;2:53–56. doi: 10.1002/cld.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute on Alcohol Abuse and Alcoholism Alcohol facts and statistics. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics Published 2014. Accessed April 10, 2017.
- 4.Singal A.K., Kamath P.S., Gores G.J., Shah V.H. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12(4):555–564. doi: 10.1016/j.cgh.2013.06.013. quiz e31-e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucey M.R., Mathurin P., Morgan T.R. Alcoholic hepatitis. N Engl J Med. 2009;360(26):2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . World Health Organization; Geneva, Switzerland: 2014. Global Status Report on Alcohol and Health 2014; p. XIV. [Google Scholar]
- 7.Substance Abuse and Mental Health Services Administration . Substance Abuse and Mental Health Services Administration; Rockville, MD: 2014. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH series H-48, HHS publication No. (SMA) 14-4863. [Google Scholar]
- 8.Thursz M.R., Richardson P., Allison M., et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372(17):1619–1628. doi: 10.1056/NEJMoa1412278. [DOI] [PubMed] [Google Scholar]
- 9.Jinjuvadia R., Liangpunsakul S., Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol. 2015;49(6):506–511. doi: 10.1097/MCG.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liangpunsakul S. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J Clin Gastroenterol. 2011;45(8):714–719. doi: 10.1097/MCG.0b013e3181fdef1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn W., Jamil L.H., Brown L.S., et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41(2):353–358. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 12.Louvet A., Naveau S., Abdelnour M., et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45(6):1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 13.Srikureja W., Kyulo N.L., Runyon B.A., Hu K.Q. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol. 2005;42(5):700–706. doi: 10.1016/j.jhep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Corrao G., Bagnardi V., Zambon A., Torchio P. Meta-analysis of alcohol intake in relation to risk of liver cirrhosis. Alcohol Alcohol. 1998;33(4):381–392. doi: 10.1093/oxfordjournals.alcalc.a008408. [DOI] [PubMed] [Google Scholar]
- 15.Becker U., Deis A., Sorensen T.I., et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23(5):1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- 16.Bellentani S., Saccoccio G., Costa G., et al. The Dionysos Study Group Drinking habits as cofactors of risk for alcohol induced liver damage. Gut. 1997;41(6):845–850. doi: 10.1136/gut.41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strnad P., Zhou Q., Hanada S., et al. Keratin variants predispose to acute liver failure and adverse outcome: race and ethnic associations. Gastroenterology. 2010;139(3):828–835. doi: 10.1053/j.gastro.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian C., Stokowski R.P., Kershenobich D., Ballinger D.G., Hinds D.A. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010;42(1):21–23. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 19.Buch S., Stickel F., Trepo E., et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47(12):1443–1448. doi: 10.1038/ng.3417. [DOI] [PubMed] [Google Scholar]
- 20.Larsson S.C., Wolk A. Coffee consumption and risk of liver cancer: a meta-analysis. Gastroenterology. 2007;132(5):1740–1745. doi: 10.1053/j.gastro.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 21.Ruhl C.E., Everhart J.E. Coffee and tea consumption are associated with a lower incidence of chronic liver disease in the United States. Gastroenterology. 2005;129(6):1928–1936. doi: 10.1053/j.gastro.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 22.Ruhl C.E., Everhart J.E. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128(1):24–32. doi: 10.1053/j.gastro.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 23.Chen S., Teoh N.C., Chitturi S., Farrell G.C. Coffee and non-alcoholic fatty liver disease: brewing evidence for hepatoprotection? J Gastroenterol Hepatol. 2014;29(3):435–441. doi: 10.1111/jgh.12422. [DOI] [PubMed] [Google Scholar]
- 24.Crabb D.W., Bataller R., Chalasani N.P., et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150(4):785–790. doi: 10.1053/j.gastro.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liangpunsakul S., Puri P., Shah V.H., et al. Effects of age, sex, body Weight, and quantity of alcohol consumption on occurrence and severity of alcoholic hepatitis. Clin Gastroenterol Hepatol. 2016;14(12):1831–1838. doi: 10.1016/j.cgh.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arab L., Biggs M.L., O'Meara E.S., Longstreth W.T., Crane P.K., Fitzpatrick A.L. Gender differences in tea, coffee, and cognitive decline in the elderly: the Cardiovascular Health Study. J Alzheimers Dis. 2011;27(3):553–566. doi: 10.3233/JAD-2011-110431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akaike H. In: Second International Symposium on Information Theory. Petrov B.N., Csaki F., editors. Akademiai Kiado; Budapest, Hungary: 1973. Information theory and an extension of the maximum likelihood principle; pp. 267–281. [Google Scholar]
- 28.Heagerty P.J., Lumley T., Pepe M.S. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 29.Efron B. Bootstrap methods: another look at the jackknife. Ann Statist. 1979;7(1):1–26. [Google Scholar]
- 30.Arauz J., Moreno M.G., Cortes-Reynosa P., Salazar E.P., Muriel P. Coffee attenuates fibrosis by decreasing the expression of TGF-β and CTGF in a murine model of liver damage. J Appl Toxicol. 2013;33(9):970–979. doi: 10.1002/jat.2788. [DOI] [PubMed] [Google Scholar]