Abstract
The objective of this study is to prepare three dimensional (3D) of mouse mammary epithelial EpH4 and mouse preadipocyte 3T3L1 cells in the presence of gelatin hydrogel microspheres (GM) and evaluate the effect of GM presence on the survival and functions of cells in the 3D cell aggregates. Gelatin was dehydrothermally crosslinked at 140 °C for 48 h in a water-in-oil emulsion state to obtain the GM with average diameters of 50 and 200 μm, followed by treatment with fibronectin (FN). EpH4 and/or 3T3L1 cells were cultured with or without the FN-treated GM in round U-bottom wells of 96-multiwell culture plates which had been coated with poly (vinyl alcohol) (PVA) to allow the cells to form their aggregates. On the other hand, EpH4 cells were precultured with the FN-treated GM, and then continued to culture with 3T3L1 cells in the same condition described above. The EpH4 cells attached onto the GM in the cell number dependent manner, irrespective of their size. When 3T3L1 cells were incubated with the original and GM-preincubated EpH4 cells in the presence of both the FN-treated GM, the number of alive cells in the aggregates was significantly high compared with that for the absence of FN-treated GM. In addition, higher β-casein expression level of EpH4 cells in EpH4/3T3L1 cells aggregates in the presence of FN-treated GM was observed than that of cells in the absence of FN-treated GM. Laminin secretion was also promoted for the cells aggregates cultured with FN-treated GM. It is concluded that the presence of FN-treated GM in the EpH4/3T3L1 cells aggregates gave a better condition to cells, resulting in an enhanced generation of β-casein from EpH4 cells in the aggregates.
Keywords: Cells aggregates, Epithelial–mesenchymal cells interactions, Three-dimensional cells culture, Gelatin hydrogel microspheres, Cells-gelatin hydrogel microspheres aggregates
1. Introduction
Epithelial–mesenchymal interactions are important and indispensable in the development of most organs, such as tooth, mammary gland, lung, kidney, and hair follicle [1], [2], [3], [4], [5]. The organ fundamentally develops to form from epithelium and mesenchyme by the epithelial–mesenchymal interactions during the early stage of morphogenesis. It is well recognized that the interactions are mediated by soluble paracrine factors, direct cell–cell contact, and cell-extracellular matrix (ECM) interactions [6].
Recently, epithelial and mesenchymal cells are co-cultured to investigate the mechanism of organs development [7], [8], wound healing and fibrosis [9], cancer progression and metastasis [10], and cell-based tissue and organization [11]. However, most of the researches have been performed in two dimensional (2D) systems, which is quite different from the three dimensional (3D) cell environment of living tissues. In addition, epithelial cells are not proliferated, during the culture, their polarity and functions are lost [12]. On the other hand, several 3D cell culture technologies have been reported [13], [14], [15], [16]. Considering at the structure of body tissues, such as liver and bone, cell aggregates, physiologically work as the minimum unit of cellular function [17]. For example, embryonic stem cells generally aggregate to form an embryoid body, and consequently initiate the cell differentiation into different lineages [18]. In addition, cell aggregates produce extracellular matrix proteins more efficiently than single cells [19]. It is possible that cell aggregation physiologically induces the cell–cell interactions, resulting in enhanced biological functions of cells. However, some technological problems still remain unsolved for the cell aggregates culture. One of the largest problems is that when as the size of cell aggregates become large, cells in the center of aggregates weaken and die. This is mainly due to the lack of oxygen and nutrients inside the aggregates [20], [21]. Another problem is difficulty to control the cells distribution for their better interaction in co-culture of epithelial and mesenchymal cells [7].
The previous study demonstrated that the incorporation of gelatin hydrogel microspheres prevented the mouse preosteoblast MC3T3-E1 cells in the aggregates suffering from a lack of oxygen and nutrient necessary for their survival because oxygen and nutrients can be permeated through the hydrogel matrix [21], resulting in a promoted their proliferation and osteogenic differentiation [22]. Gelatin is a biodegradable biomaterial which has been extensively used for medical, pharmaceutical, and cosmetic applications. Its biosafety has been proven through the long-term practical usage [23]. Gelatin hydrogels of different shapes can be formulated, while their feasibility as cell culture substrates [24], [25], [26] and cell scaffolds for tissue regeneration [27], [28], [29], [30], [31] or as carriers of growth factors and drugs release [32], [33], [34], [35], [36], has been experimentally demonstrated. Gelatin hydrogels can release growth factors to induce tissue regeneration [37], [38], [39]. In addition, some researches have been reported on the cell aggregates incorporating the microspheres of gelatin, poly(lactic-co-glycolic acid) (PLGA), and alginate [22], [40], [41], [42]. Gelatin has an inherent ability of cell adhesion ability superior to PLGA and alginate microspheres. Based on the availability and nature, in this study, gelatin was selected as the hydrogel material.
The objective of this study is to design a 3D cell culture technology of epithelial and mesenchymal cells aggregates for an improved epithelial cell functions and epithelial–mesenchymal interactions. This technology will be useful and available to investigate the mechanism of organs development and cell-based tissue and organization. In this study, 3D of mouse mammary epithelial EpH4 and mouse preadipocyte 3T3L1 cells in the presence of gelatin hydrogel microspheres (GM) were prepared and the effect of GM presence on the survival and functions of cells in the 3D cell aggregates were evaluated. For epithelial cells, mouse mammary epithelial EpH4 cells were used because the mammary gland provides an excellent system to investigate the hormonal regulation of specific gene expression and differentiation [43], [44], [45], [46], [47]. For mesenchymal cells, mouse preadipocyte 3T3L1 cells were selected. Because it is known that the cells express high levels collagen type IV and laminin of two essential basement membrane proteins [48], [49]. GM were prepared by the dehydrothermally crosslinking method, followed by treatment with fibronectin (FN) to give a cell adhesion property to the GM surface. Following, EpH4 and/or 3T3L1 cells were incubated with FN-treated GM to form their aggregates. The survival, distribution, l-lactic acid/glucose ratio (an oxygen condition measure), and β-casein expression of the cells aggregates in the presence of GM were evaluated comparing with those of GM-absent aggregates.
2. Materials and methods
2.1. Preparation of gelatin hydrogel microspheres
Gelatin hydrogel microspheres (GM) were prepared by the chemical cross-linking of gelatin in a water-in -oil emulsion state according to the method previously reported [38]. Briefly, an aqueous solution (20 ml) of 10 wt % gelatin (isoelectric point 5.0, weight-averaged molecular weight 1,00,000, Nitta Gelatin Inc., Osaka, Japan) was preheated at 40 °C, and then added dropwise into 600 ml of olive oil (Wako Ltd, Osaka, Japan) at 40 °C, followed by stirring at 150 rpm for 10 min to prepare a water-in-oil emulsion. The emulsion temperature was decreased to 4 °C for the gelation of gelatin solution to obtain non-crosslinked GM. The resulting microspheres were washed three times with cold acetone by centrifugation (5000 rpm, 4 °C, 5 min) to completely exclude the residual oil. Then, they were fractionated by size using sieves with apertures of 20, 32, 75, and 105 μm (Iida Seisakusyo Ltd, Osaka, Japan) and air-dried at 4 °C. The non-crosslinked and dried GM (200 mg) were treated in a vacuum oven at 140 °C and 0.1 Torr for the dehydrothermal crosslinking of gelatin for 48 h according to the method previously reported [21]. The pictures of GM in the water-swollen and dispersed states were taken with a light microscope (CKX41, Olympus Ltd, Tokyo, Japan). For each sample, the size of 100 microspheres was measured with a computer program Image J (NIH Inc., Bethesda, USA), and the average size was calculated. For the fibronectin (FN) treatment of GM, 20 μl of 100 μg/ml FN aqueous solution (Sigma–Aldrich Inc., St. Louis, USA) was dropped onto 2 mg of freeze-dried GM, followed by leaving at 37 °C for 1 h for adsorption of fibronectin onto the microspheres. During this treatment, it is likely that FN molecules interact with gelatin of GM because of the bioaffinity for gelatin.
2.2. Cell culture experiments
EpH4 cells of a mouse mammary epithelial cell line were transfected and clones selected as previously described [50]. EpH4 were cultured in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) (Thermo Inc., Waltham, USA) supplemented with 2 vol % fetal calf serum (FCS) (Thermo Inc., Waltham, USA), gentamicin, 3 μg/ml prolactin (Sigma–Aldrich Inc., St. Louis, USA), 1 μg/ml hydrocortisone (Sigma–Aldrich Inc., St. Louis, USA), and 5 μg/ml insulin (Sigma–Aldrich, St. Louis, USA) (standard medium), and cultured at 37 °C in a 95% air-5% carbon dioxide atmosphere. The culture medium was changed every 2 days and confluent cells were subcultured through trypsinization. In the experiment of phosphotidylinositol 3-kinase (PI3K) inhibition of EpH4 cells, the medium containing 50 μM of PI3K inhibitor LY294002 (Abcam Inc., Cambridge, UK) was used to culture for 72 h 3T3L1 preadipocytes were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen Inc., Carlsbad, USA) supplemented with 10 vol% FCS, penicillin (50 U/ml), and streptomycin (50 U/ml) (standard medium) and cultured at 37 °C in a 95% air-5% carbon dioxide atmosphere. The culture medium was changed every 2 days and confluent cells were subcultured through trypsinization. For the adipogenic differentiation of 3T3L1 cells, the medium supplemented using AdipoInducer Reagent (Takara-bio Inc., Shiga, Japan) including insulin, dexamethasone, and 3-isobutyl-1-methylxanthine was used according to the manufacturer's instructions.
2.3. Attachment of EpH4 cells to gelatin hydrogel microspheres with or without fibronectin-treatment
Agarose (Sigma–Aldrich Inc., St. Louis, USA) was dissolved in phosphate buffered saline (PBS) (1 wt %). This solution (600 μl/well) was added to each flat-bottomed well of 12-multiwell culture plate with flat bottom and incubated at 25 °C for 30 min. On the other hand, FN-treated GM and EpH4 cells were separately suspended in the standard medium. The suspensions of GM (4 mg/ml, 500 μl/well) was added into the agarose-coated wells, and then the EpH4 cells suspension (1 × 106, 2 × 106, 4 × 106, 8 × 106 cells/ml, 500 μl/well) was added to culture at 37 °C for 6 h. Next, the mixture of GM and EpH4 cells was passed through a cell strainers (40 μm; Becton, Dickinson and Company Inc., Franklin Lakes, USA) to remove single EpH4 cells to collect EpH4 cells attached GM. The number of EpH4 cells attached on GM was determined by the fluorometric quantification of cellular DNA according to the method reported by Rao et al. [51]. Briefly, the cells-attached GM were lysed in 30 mM sodium citrate-buffered saline solution (SSC) (pH 7.4) containing 0.2 mg/ml sodium dodecylsulfate (SDS) at 37 °C for 12 h with occasional mixing. The cell lysate (30 μl) was mixed with a dye solution (70 μl; 30 mM SSC, 10 μg/ml Hoechst 33258 dye) and the fluorescent intensity of mixed solution was measured in a fluorescence spectrometer (F-2000, HITACHI, Japan) at the excitation and emission wavelengths of 355 and 460 nm, respectively. The calibration curve between the DNA and cell number was prepared by use of cell suspensions at different cell densities. The DNA assay was done three times independently for every experimental sample unless otherwise mentioned.
2.4. Preparation of EpH4/3T3L1 cells aggregates without or with fibronectin-treated gelatin hydrogel microspheres incorporation
Poly(vinyl alcohol) (PVA, degree of polymerization = 1800 and 88 mol % saponification) kindly supplied from Unichika Ltd Tokyo, Japan, was dissolved in PBS (1 wt %). This solution (100 μl/well) was added to each round-bottomed (U-bottomed) well of a 96-multiwell culture plate and incubated at 37 °C for 15 min. Then, the solution was removed by aspiration and the wells were washed twice with PBS (100 μl/well). For preparation of EpH4 or 3T3L1 cell aggregates without FN-treated GM, the EpH4 suspension (2 × 105 cells/ml, 50 μl/well) or 3T3L1 suspension (2 × 105 cells/ml, 50 μl/well) was added to the coated wells. For preparation of EpH4/3T3L1 cells aggregates without or with FN-treated GM, EpH4-cells attached and FN-treated GM and 3T3L1 cells were separately suspended in the standard medium. The suspension of FN-treated GM attached with EpH4 cells (1 × 103 and 2 × 104 microspheres/ml, 50 μl/well) were added to the coated wells, followed by the addition of 3T3L1 cells suspension (1 × 105 cells/ml, 50 μl/well). On the other hand, FN-treated GM was dehydrothermally crosslinked for 48 h, EpH4 cells, and 3T3L1 cells were separately suspended in the standard medium. The suspension of FN-treated GM (0, 1 × 103 and 2 × 104 microspheres/ml, 50 μl/well) was added to the coated wells, followed by the EpH4 suspension (1 × 105 cells/ml, 50 μl/well) and 3T3L1 suspension (1 × 105 cells/ml, 50 μl/well). Experiments were performed on three wells for each sample unless mentioned otherwise. Photographs of cell aggregates with or without gelatin microspheres were taken with a microscope (CKX41, Olympus, Tokyo, Japan).
2.5. Measurement of cell number of live EpH4 and/or 3T3L1 cells aggregates without or with fibronectin-treated gelatin hydrogel microspheres incorporation
The number of live cells in EpH4/3T3L1 cells aggregates without or with FN-treated GM was determined by counting the number of cells nuclei after their crystal violet staining [41]. Briefly, a mixed solution of 0.2 M citric acid and 0.2 wt % crystal violet was added (100 μl/well) to each well of 96-multiwell culture plate 7 days after EpH4/3T3L1 cells were cultured without or with FN-treated GM dehydrothermally crosslinked for 48 at 140 °C. After the crystal violet staining, the cells were lysed in 0.1 wt % Triton X-100 in PBS at 37 °C overnight to separate the nuclei from the cell debris. After pipetting, the cell nuclei collected were viewed on a light microscope (CKX31-11PHP, Olympus, Tokyo, Japan) and counted in a hemocytometer (OneCell Inc., Hiroshima, Japan). The nuclei of live cells are generally round, while that of dead cells are irregularly shaped. Live cells were distinguished from dead cells by their nucleus shape.
2.6. Measurement of l-lactic acid/glucose ratio of EpH4 and/or 3T3L1 cells aggregates without or with fibronectin-treated gelatin hydrogel microspheres incorporation
EpH4 and 3T3L1 cells were cultured with FN-treated GM in the similar way previously described to form the cell aggregates. The amount of glucose consumed by EpH4/3T3L1 cells aggregates was determined by measuring the change in glucose concentration in the culture medium using a Glutest Neo Super test kit (Sanwa kagaku kenkyusyo Ltd, Kyoto, Japan) 7 days after incubation. The amount of l-lactic acid produced by EpH4/3T3L1 cells aggregates was determined with an E-kit (R-Biopharm AG Co. Ltd, Germany) after 7 days incubation. The number of live cells was determined by the crystal violet staining as described above and used to normalize the amount of glucose consumption and l-lactic acid produced to the number of live cells. The l-lactic acid/glucose ratio was calculated as a measure of aerobic condition [52].
2.7. Evaluation of EpH4 and 3T3L1 cells distribution in EpH4/3T3L1 cells aggregates without or with fibronectin-treated gelatin hydrogel microspheres incorporation
To evaluate the cells distribution in cell aggregates, EpH4 cells were stained with PKH67 Green Fluorescent Cell Linker Kit (Sigma–Aldrich Inc., St Louis., USA), while 3T3L1 cells were stained with PKH26 Red Fluorescent Cell Linker Kit (Sigma–Aldrich Inc., St Louis., USA) according to the manufacturer's instructions. PKH67-labeled EpH4 cells and PKH26-labeled 3T3L1 cells were cultured with or without FN-treated GM in the similar way previously described to form the cell aggregates. After incubation for 7 days, the aggregates were fixed with 4 wt % paraformaldehyde at 4 °C for 1 h and embedded in an optimal cutting temperature compound (Sakura Finetek Japan Co. Ltd, Tokyo, Japan) and frozen using liquid nitrogen. The frozen samples were sectioned using a cryotome (CM3050S, Leica Microsystems, Wetzlar Inc., Germany). The sections of 10 μm thickness were viewed in a confocal laser scanning microscope (FV1000D, Olympus Ltd, Tokyo, Japan) to evaluate the cells distribution in aggregates.
2.8. Immunostaining of β-casein and laminin expression of EpH4/3T3L1 cells aggregates without or with fibronectin-treated gelatin hydrogel microspheres incorporation
EpH4/3T3L1 cells aggregates with or without FN-treated GM were prepared. After incubation for 7 days, the aggregates were fixed with 4 wt % paraformaldehyde at 4 °C for 1 h and embedded by using an optimal cutting temperature (O.C.T) compound (Sakura Finetek Japan Ltd, Tokyo, Japan) and frozen using liquid nitrogen. The frozen samples were sectioned using a cryotome (CM3050S, Leica Microsystems, Wetzlar Inc., Germany) and incubated at 4 °C overnight with the following primary antibody against: β-casein (Santacruz Inc., America, USA, 1:50) or laminin (Abcam Inc., Cambridge, UK, 1:100). Then, a secondary antibody coupled to Alexa 488 (Molecular Probes, Invitrogen Inc., USA, 1:700) was incubated at 25 °C for 30 min to immunologically stain the corresponding protein. Next, TO-PRO-3 (Molecular Probes, Eu-gene Inc., USA) was incubated at 25 °C for 10 min for the nuclear detection. The sections of 10 μm thickness were viewed in a confocal laser scanning microscope (FV1000D, Olympus Ltd, Tokyo, Japan).
2.9. Measurement of β-casein expression of EpH4/3T3L1 cells aggregates without or with fibronectin-treated gelatin hydrogel microspheres incorporation
The messenger ribonucleic acid (mRNA) expression of β-casein was evaluated by the conventional real-time polymerase chain reaction (PCR). Briefly, EpH4 and/or 3T3L1 cells aggregates with or without FN-treated GM were prepared similarly. After incubation for 7 days, RNA was extracted and cleaned up using the Qiagen RNeasy® Plus Mini kit (Qiagen Inc., Germany) according to the manufacturer's instructions. The total RNA sample was reverse-transcribed to cDNA using SuperScript VILO™ (Invitrogen Inc., Carlsbad, CA). The resulting cDNA was subjected to the real-time PCR assay by using Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems Inc., Carlsbad, CA) to determine the gene expression of β-casein. The PCR reaction was carried out with specific primers in the presence of Power SYBR Green (Applied Biosystems Inc., Carlsbad, CA) as described in the manufacturer's instruction. The level of gene expression was normalized by that of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression, and calculated as the ratio to EpH4 cell culture without GM as a control group. Experiments were performed for 5 specimens independently for each sample. The primer sequences for GAPDH and β-casein were as follows, GAPDH: 5-TGAAGCAGGCATCTGAGGG-3, 5-CGAAGGTGGAAGAGTGGGAG-3; β-casein: 5-GGTGAATCTCATGGGACAGC-3, 5-TGACTGGATGGTGGAGTGAA-3.
2.10. Statistical analysis
All the statistical data are expressed as the mean ± standard error of the mean (SEM). The data were analyzed by t-test to determine the statistical significance of differences between two mean values, which was accepted at a p value of <0.05.
3. Results
3.1. Size of gelatin hydrogel microspheres
Fig. 1 shows typical microscopic pictures of gelatin hydrogel microspheres (GM). The microspheres were of spherical shape and had a smooth surface. The sizes of GM prepared in the water-swollen condition were 50.1 ± 18.3 and 194.3 ± 33.0 μm.
Fig. 1.
Light microscopic pictures of gelatin microspheres with diameters of 50.1 ± 18.3 μm (GM50) and 194.3 ± 33.0 μm (GM200) dispersed in water. Scale bar: 100 μm.
3.2. Number of EpH4 cells attached onto gelatin hydrogel microspheres without or with treatment of fibronectin
Fig. 2 shows the number of cells attached onto FN-treated GM50 and GM200 dispersed in water. The number of cells attached to FN-treated GM50 was about 5, while that of cells attached to FN-treated GM200 was about 100. On the other hand, the number of cells was less than 1 for GM50 and GM200 without treatment of FN (data not shown). The EpH4 adhesion to GM was improved by the treatment of FN, and a higher number of EpH4 cells attached onto GM was observed for FN-treated GM200.
Fig. 2.
The number of EpH4 cells attached onto gelatin microspheres dispersed in water. The number of cells was about 5 for GM50 (A) and the number of cells attached was about 100 for GM200 (B).
3.3. EpH4 and/or 3T3L1 cell aggregates formed without or with fibronectin-treated gelatin hydrogel microspheres incorporation
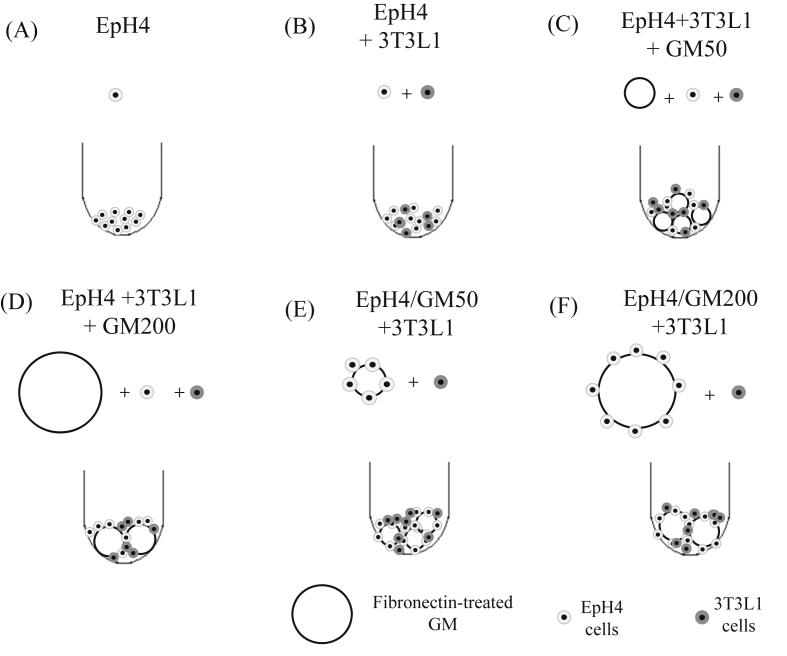
EpH4 and/or 3T3L1 cells aggregates without or with FN-treated GM were formed in different culture conditions (as shown in Fig. 3). Fig. 4 shows microscopic pictures of EpH4 and/or 3T3L1 cell aggregates 1, 3, 5, and 7 days after incubation without FN-treated GM. For 3T3L1 cells only or mixture of EpH4 and 3T3L1 cells, the cells aggregates were rapidly formed and their shape was spherical. On the contrary, aggregates of EpH4 cells only were formed slowly and not of spherical shape. Fig. 5 shows microscopic pictures of EpH4 and 3T3L1 cells aggregates in the presence FN-treated GM with or without EpH4 cells pre-incubation 7 days after incubation. In all cases, the EpH4/3T3L1 cells aggregates with both types of FN-treated GM were formed.
Fig. 3.
Experimental conditions of EpH4 and/or 3T3-L1 cells cultured with or without FN-treated GM; EpH4 cells without GM (A), EpH4 and 3T3L1 cells aggregates without GM (B), EpH4 and 3T3L1 cells and FN-treated GM50 (C), EpH4 and 3T3L1 cells and FN-treated GM200 (D), EpH4 cells precultured with GM50 and 3T3L1 cells (E), and EpH4 cells precultured with GM200 and 3T3L1 cells (F).
Fig. 4.
Light microscopic pictures of EpH4 and/or 3T3L1 cells aggregate 1, 3, 5, and 7 days after incubation without FN-treated GM. Scale bar: 100 μm.
Fig. 5.
Light microscopic pictures of EpH4 cells preincubated with FN-treated GM50 and GM200 and 3T3L1 cells 7 days after incubation. Scale bar: 100 μm.
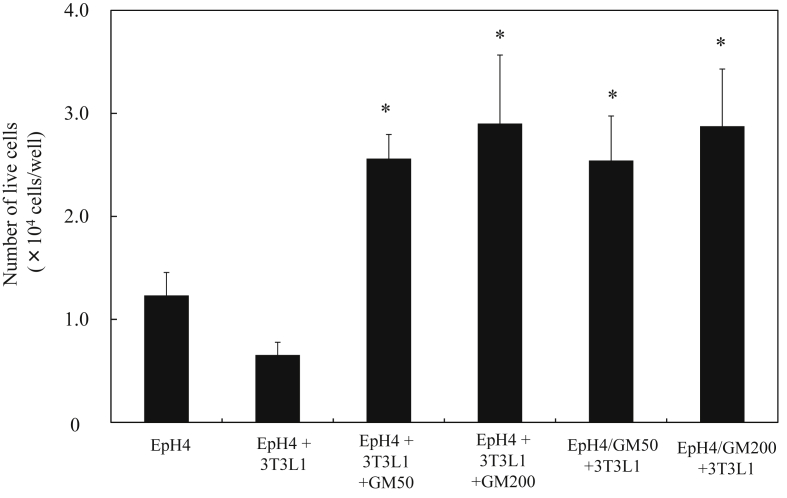
3.4. Number of live cells in EpH4 and/or 3T3L1 cells aggregates without or with fibronectin-treated gelatin hydrogel microspheres incorporation
Fig. 6 shows the number of live EpH4 and 3T3L1 cells in EpH4/3T3L1 cells aggregates 7 days after incubation with or without FN-treated GM. The larger number of live EpH4 and 3T3L1 cells was observed for EpH4/3T3L1 cell aggregates with FN-treated GM, irrespective of their size and EpH4-preincubation. The number of live EpH4 and 3T3L1 cells in EpH4/3T3L1 cells aggregates was influenced by the incorporation of FN-treated GM to aggregates.
Fig. 6.
Number of live EpH4 and 3T3L1 cells in EpH4 and/or3T3L1 cells aggregates with or without FN-treated GM50 7 days after incubation. * p < 0.05, significant against the number of live EpH4 and 3T3L1 cells in EpH4/3T3L1 aggregates cultured without GM.
3.5. l-lactic acid/glucose ratio of EpH4 and/or 3T3L1 cells aggregates without or with fibronectin-treated gelatin hydrogel microspheres incorporation
Fig. 7 shows the l-lactic acid/glucose ratio of EpH4 and 3T3L1 cells in EpH4/3T3L1 cells aggregates 7 days after incubation with or without FN-treated GM. The higher l-lactic acid/glucose ratio of EpH4 and 3T3L1 cells was observed for EpH4/3T3L1 cells aggregates without FN-treated GM. The l-lactic acid/glucose ratio of EpH4 and 3T3L1 cells aggregates decreased to a significantly great extent by the incorporation of GM50 and GM200. The l-lactic acid/glucose ratio of EpH4 and 3T3L1 cells in EpH4/3T3L1 cells aggregates were greatly influenced by the incorporation of FN-treated GM to aggregates.
Fig. 7.
l-lactic acid/glucose ratio of EpH4 and 3T3-L1 cells in EpH4 and/or 3T3-L1 cell aggregates with or without FN-treated GM 7 days after incubation * p < 0.05, significant against the l-lactic acid/glucose ratio of EpH4 cells in EpH4/3T3L1 aggregates cultured without GM.
3.6. Distribution of EpH4 and 3T3L1 cells in EpH4/3T3L1 cells aggregates without or with fibronectin-treated gelatin hydrogel microspheres incorporation
Fig. 8 shows the confocal laser microscopic pictures of EpH4 and 3T3L1 cells in EpH4/3T3L1 cells aggregates 7 days after incubation with or without FN-treated GM. For the EpH4/3T3L1 cells aggregates without FN-treated GM, EpH4/3T3L1 cells aggregates showed a core–shell structure where the 3T3L1 cells and EpH4 cells were localized inside and outside of EpH4/3T3L1 cell aggregates respectively. On the contrary, for the EpH4/3T3L1 cells aggregates with FN-treated GM, the EpH4 and 3T3-L1 cells homogeneously distributed in the EpH4/3T3L1 cells aggregates. However, 3T3L1 cells tended to localize inside of EpH4/3T3L1 cells aggregates incorporating FN-treated GM.
Fig. 8.
Fluorescent microscopic pictures of EpH4 and 3T3-L1 cells in EpH4/3T3L1 cells aggregates 7 days after incubation with or without FN-treated GM; EpH4 cells without gelatin microspheres (A), EpH4 and 3T3L1 cells aggregates without gelatin microspheres (B), EpH4 and 3T3L1 cells and FN-treated GM50 (C), EpH4 and 3T3L1 cells and FN-treated GM200 (D), EpH4 cells precultured with GM50 and 3T3L1 cells (E), and EpH4 cells precultured with GM200 and 3T3L1 cells (F). Scale bar: 100 μm.
3.7. Expression of β-casein and laminin of EpH4/3T3L1 cells aggregates without or with fibronectin-treated gelatin hydrogel microspheres incorporation
Fig. 9, Fig. 10 show the confocal laser microscopic pictures of laminin and β-casein expression of EpH4 cells in EpH4/3T3L1 cells aggregates 7 days after incubation with or without FN-treated GM. The higher laminin and β-casein expressions were observed for EpH4/3T3L1 cells aggregates with FN-treated GM than those of GM-absent EpH4/3T3L1 cells aggregates. Figure 11 shows the β-casein mRNA expression of EpH4 cells in EpH4/3T3L1 cells aggregates 4, 7, and 14 days after incubation with or without FN-treated GM. The higher β-casein expression was observed for EpH4/3T3L1 cells aggregates with FN-treated GM than that of without FN-treated GM even after a long-term culture of 14 days. A higher β-casein expression was observed for EpH4/3T3L1 cells aggregates with FN-treated GM50 than that of aggregates with GM200 added at the same time. When EpH4 cells, 3T3L1 cells, and GM were added at the same time, the β-casein expression was high compared with that of 3T3L1 cells cultured with GM-preincubated EpH4 cells. The laminin and β-casein expressions of EpH4 and 3T3L1 cells in EpH4/3T3L1 cells aggregates were influenced by the incorporation of FN-treated GM to aggregates. In addition, the β-casein expression of EpH4 cells in EpH4/3T3L1 cells aggregates was modified by the size of FN-treated GM.
Fig. 9.
Fluorescent microscopic pictures of laminin expression in EpH4/3T3L1 cells aggregates with or without FN-treated GM50 7 days after incubation. Scale bar: 100 μm.
Fig. 10.
Fluorescent microscopic pictures of β-casein expression in EpH4/3T3L1 cells aggregates with or without FN-treated GM50 7 days after incubation. Scale bar: 100 μm.
Fig. 11.
β-casein mRNA expression of EpH4 cells in EpH4/3T3L1 cells aggregates with or without FN-treated GM50 and GM200 4 (□), 7 (■), and 14 (■) days after incubation. * p < 0.05, significant against the β-casein mRNA expression of EpH4 cells in EpH4/3T3L1 cells aggregates without GM at the corresponding incubation time.
Fig. 12 shows the effect of LY294002 addition on the β-casein mRNA expression of EpH4 cells in EpH4/3T3L1 cells aggregates 7 days after incubation with or without FN-treated GM. Irrespective of the FN-treated GM size and the incubation manner, the β-casein expression was inhibited by the addition of LY294002.
Fig. 12.
β-casein mRNA expression of EpH4 cells in EpH4/3T3L1 cells aggregates with or without FN-treated GM50 and GM200 7 days after incubation in the absence (□) and presence (■) of LY294002. * p < 0.05, significant between two groups.
4. Discussion
Gelatin hydrogel microspheres were fractionated by using size sieves to obtain the different sizes of microspheres in the water dispersed state (Fig. 1). The GM were treated by fibronectin (FN) because it allowed the microspheres to enhance their EpH4 cell attachment. It is known that FN is one of the cell adhesion molecules [53]. Less number of EpH4 cells was attached to GM without treatment of FN (data not shown). For FN-treated GM, as expected, the FN-treated GM were susceptible to the attachment of the larger number of EpH4 cells attached onto GM with the larger size (Fig. 2). It is possible that the surface area of GM increase with an increase in their size, resulting in larger number of EpH4 cells attached.
For the formation of EpH4/3T3L1 cells aggregates without FN-treated GM, the aggregates of 3T3L1 cells only and EpH4/3T3L1 cells were rapidly formed and the shape was spherical. However, for EpH4 cells only, the cell aggregates were formed slowly and the shape is not spherical (Fig. 4). This may be explained in terms of cells junctions and movement. It has been demonstrated that the movement of epithelial cells is lower than that of mesenchymal cells because epithelial cells has strong cell–cell junctions of tight junctions, gap junctions, and adherens junctions [54], [55]. In this study, it is conceivable that movement rate of mesenchymal 3T3L1 cells was high compared with that of epithelial EpH4 cells because of the strong epithelial cells junctions. The difference in the cells junctions and movement may result in the formation of non-spherical aggregates.
The inside localization of 3T3L1 cells and outside distribution of EpH4 cells were observed for in EpH4/3T3L1 cells aggregates without the GM (Fig. 8). It has been reported that mesenchymal 3T3-L1 cells have a higher mobility and self-organization potential than epithelial EpH4 cells. In this study, 3T3L1 cells were initially aggregated, followed by the distribution of EpH4 cells outside of 3T3-L1 cell aggregates. When human aortic fibroblast (HAF) cells were co-cultured with human umbilical vein endothelial cells (HUVEC), HAF cells were localized inside in HUVEC/HAF cells aggregates because HAF cells has a higher self-organization potential than HUVEC [56], [57]. On the other hand, for the EpH4/3T3L1 cells aggregates with FN-treated GM, the EpH4 and 3T3L1 cells were homogeneously distributed in the EpH4/3T3L1 cells aggregates (Fig. 5, Fig. 8). It is reported that cells-microspheres interactions were important for the formation of cell aggregates incorporating microspheres. For example, a larger number of microspheres in pluripotent stem cells-microspheres aggregates was present for gelatin microspheres than agarose microspheres because gelatin has higher cell adhesive properties than agarose for pluripotent stem cell [56]. It is highly conceivable that FM-treated GM functioned as a scaffold of both 3T3L1 and EpH4 cells, resulting in the inhibition of initial 3T3L1 self-organization and the consequent homogeneous distribution of EpH4 and 3T3L1 cells in EpH4/3T3L1 cells aggregates.
The ratio of l-lactic acid production to glucose consumption is a general measure of aerobic metabolism [52]. The lower the ratio is, the higher the aerobic metabolism of cells aggregates is. It is apparent from Fig. 7 that the lactic acid/glucose ratio of EpH4/3T3L1 cells aggregates cultured with FN-treated GM was significantly lower than that of aggregates without GM. We can say with certainly that the homogeneous incorporation of FN-treated GM into EpH4/3T3L1 cells aggregates increases the oxygen level, resulting in a lower ratio of l-lactic acid to glucose, in other words, the achievement of an aerobic culture condition. In addition, the presence of gelatin hydrogel microspheres distributed in the cell aggregate may increase oxygen permeation inside the aggregates because oxygen can be permeated through the water phase of hydrogel matrix [21]. As a results, in case of EpH4/3T3L1 cells aggregates with FN-treated GM, the larger number of live EpH4 and 3T3L1 cells in the cell aggregate incorporating GM was observed (Fig. 6). This may be because the presence of GM increases oxygen and nutrients permeability through the aggregate.
Preadipocyte 3T3L1 cells express the high levels of two essential basement membrane proteins, collagen type IV and laminin [48], [49]. The β-casein expression of EpH4 cells is required to allow the 3T3L1 cells to produce the basement membrane proteins collagen type IV and laminin [58], [59], [60]. It has been demonstrated that the laminin regulates the basal localization and activation of PI3K to sustain the STAT5 activation, followed by the β-casein expression [61], [62], [63]. The higher laminin and β-casein expression were observed for EpH4/3T3L1 cells aggregates with FN-treated GM (Fig. 9, Fig. 10, Fig. 11). In case of GM-absent EpH4/3T3L1 cells aggregates, 3T3L1 cells were localized inside EpH4/3T3L1 cells aggregates, but did not always function because of the lack of oxygen. On the other hand, for the GM-present EpH4/3T3L1 cells aggregates, 3T3L1 cells in EpH4/3T3L1 cell aggregates well functioned to secrets laminin due to better oxygen conditions, resulting in better EpH4-3T3L1 cells interactions mediated by laminin secreted from 3T3L1 cells. Consequently, the β-casein expression of EpH4 cells would become higher. The higher β-casein expression were observed for EpH4/3T3L1 cells aggregates with FN-treated GM50 than that of GM200. It is likely that the larger GM would physically impair aggregates with interaction between EpH4 and 3T3L1 cells. The mixing way of EpH4 cells, 3T3L1 cells, and GM also affected the level of β-casein expression (Fig. 8). Less contact of EpH4 cells with 3T3L1 cells was detected in EpH4/3T3L1 cells aggregates formed from 3T3L1 cells and EpH4 cells-attached GM. This may lead to a weaker EpH4-3T3L1 cells interactions and the consequent lower β-casein expression.
The LY294002 is known as a PI3K inhibitor [64]. To investigate the involvement of PI3K signaling pathway in the β-casein expression of EpH4 cells, the formation of EpH4/3T3L1 cells aggregates incorporating FN-treated GM was evaluated in the presence and absence of LY294002. As a result, the β-casein expression was inhibited by the LY294002 addition (Fig. 12). This findings indicates that the PI3K signaling pathway is required for the β-casein expression of EpH4 cells. It is reported that laminin regulates the PI3K basal localization and subsequent β-casein expression of EpH4 cells, but LY294002 did not completely inhibit the β-casein expression. This is because there was another prolactin receptor-STAT5 signaling pathway for the β-casein expression [62]. This pathway also contribute to this phenomenon. This phenomenon obtained in this study is similar to the results previously reported.
5. Conclusions
Upon co-culturing FN-treated GM, EpH4 cells, and 3T3L1 cells in PVA-coated U-bottomed wells, EpH4/3T3L1 cells aggregates with FN-treated GM were formed. The higher β-casein expression level of EpH4 cells in EpH4/3T3L1 cells aggregates in the presence of GM was observed than that of cells in the absence of GM. Laminin secretion was also promoted for the EpH4/3T3L1 cells aggregates cultured with FN-treated GM. It is concluded that the presence of FN-treated GM in the EpH4/3T3L1 cells aggregates gave a better condition to them, resulting in an enhanced generation of β-casein from EpH4 cells in the aggregates.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Thesleff I., Vainio S. Epithelial-mesenchymal interactions in tooth morphogenesis: the roles of extracellular matrix, growth factors, and cell surface receptors. J Craniofac Genet Dev Biol. 1991;11(4):229–237. [PubMed] [Google Scholar]
- 2.Cunha G.R., Hom Y.K. Role of mesenchymal-epithelial interactions in mammary gland development. J Mammary Gland Biol Neoplasia. 1996;1(1):21–35. doi: 10.1007/BF02096300. [DOI] [PubMed] [Google Scholar]
- 3.Minoo Parviz, King Richard J. Epithelial-mesenchymal interactions in lung development. Annu Rev Physiol. 1994;56:13–45. doi: 10.1146/annurev.ph.56.030194.000305. [DOI] [PubMed] [Google Scholar]
- 4.Aufderheide, Chiquet-Ehrismann R., Ekblom P. Epithelial-mesenchymal interactions in the developing kidney lead to expression of tenascin in the mesenchyme. J Cell Biol. 1987;105(1):599–608. doi: 10.1083/jcb.105.1.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botchkarevn Vladimir A., Kishimoto Jiro. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. JID Symp Proc. 2003;8(1):46–55. doi: 10.1046/j.1523-1747.2003.12171.x. [DOI] [PubMed] [Google Scholar]
- 6.Domenico R., Marcello S. Epithelial-mesenchymal interactions: a fundamental Developmental Biology mechanism. Int J Dev Biol. 2014;58:303–306. doi: 10.1387/ijdb.140143dr. [DOI] [PubMed] [Google Scholar]
- 7.Greer Rachel M., Prince Lawrence S. Epithelial-mesenchymal co-culture model for studying alveolar morphogenesis. Organogenesis. 2014;10(4):340–349. doi: 10.4161/org.29198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arakaki Makiko, Fukumoto Satoshi. Role of epithelial-stem cell interactions during dental cell differentiation. J Biol Chem. 2012;287(13):10590–10601. doi: 10.1074/jbc.M111.285874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai Norihiko, Tager Andrew M. vol. 1832. 2013. pp. 911–921. (Fibrosis of two: epithelial cell-fibroblast interactions in pulmonary fibrosis). 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim Sun-Ah, Kuh Hyo-Jeong. Co-culture of 3D tumor spheroids with fibroblasts as a model for epithelial–mesenchymal transition in vitro. Exp Cell Res. 2015;335:187–196. doi: 10.1016/j.yexcr.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Volponi Ana Angelova, Sharpe Paul T. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010;20(12):715–722. doi: 10.1016/j.tcb.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamir Eliah R., Ewald Andrew J. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Mol Cell Biol. 2014;15:647–664. doi: 10.1038/nrm3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okochi M., Takano S., Isaji Y., Senga T., Hamaguchi M., Honda H. Three dimensional cell culture array using magnetic force-based cell patterning for analysis of invasive capacity of BALB/3T3/v-src. Lab Chip. 2009;9(23):3378–3384. doi: 10.1039/b909304d. [DOI] [PubMed] [Google Scholar]
- 14.Mohan N., Nair P.D., Tabata Y. A 3D biodegradable protein based matrix for cartilage tissue engineering and stem cell differentiation to cartilage. J Mater Sci Mater Med. 2009;1(20Suppl):S49–S60. doi: 10.1007/s10856-008-3481-7. [DOI] [PubMed] [Google Scholar]
- 15.Benton G., George J., Kleinman H.K., Arnaoutoya I.P. Advancing science and technology via 3D culture on basement membrane matrix. J Cell Physiol. 2009;221(1):18–25. doi: 10.1002/jcp.21832. [DOI] [PubMed] [Google Scholar]
- 16.Hanjaya-Putra D., Gerecht S. Vascular engineering using human embryonic stem cells. Biotechnol Prog. 2009;25(1):2–9. doi: 10.1002/btpr.129. [DOI] [PubMed] [Google Scholar]
- 17.Nelson L.J., Walker S.W., Hayes P.C., Plevris J.N. Low-shear modelled microgravity environment maintains morphology and differentiated functionality of primary porcine hepatocyte cultures. Cells Tissues Organs. 2010;192(2):125–140. doi: 10.1159/000308893. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda J., Sakai Y., Nakazawa K. Novel hepatocyte culture system developed using microfabrication and collagen/polyethylene glycol microcontact printing. Biomaterials. 2006;27(7):1061–1070. doi: 10.1016/j.biomaterials.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Lin R.Z., Chang H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3(9–10):1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 20.Kellner K., Liebsch G., Wolfbeis O.S., Blunk T., Schulz M.B., Klimant I. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol Bioeng. 2002;80(1):73–83. doi: 10.1002/bit.10352. [DOI] [PubMed] [Google Scholar]
- 21.Compan V., Guzman J., Riande E. A potentiostatic study of oxygen transmissibility and permeability through hydrogel membranes. Biomaterials. 1998;19(23):2139–2145. doi: 10.1016/s0142-9612(98)00113-6. [DOI] [PubMed] [Google Scholar]
- 22.Tajima Shuhei, Tabata Yasuhiko. Preparation and functional evaluation of cell aggregates incorporating gelatin microspheres with different degradabilities. J Tissue Eng Regen Med. 2013;10:801–811. doi: 10.1002/term.1469. [DOI] [PubMed] [Google Scholar]
- 23.Zekorn D. Intravascular retention, dispersal, excretion and break-down of gelatin plasma substitutes. Bibl Haematol. 1969;33:131–140. doi: 10.1159/000384835. [DOI] [PubMed] [Google Scholar]
- 24.Narita A., Takahara M., Ogino T., Fukushima S., Kimura Y., Tabata Y. Effect of gelatin hydrogel incorporating fibroblast growth factor 2 on human meniscal cells in an organ culture model. Knee. 2009;16(4):285–289. doi: 10.1016/j.knee.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi Y., Yamamoto M., Tabata Y. Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and beta-tricalcium phosphate. Biomaterials. 2005;26(17):3587–3596. doi: 10.1016/j.biomaterials.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 26.Hori Y., Inoue S., Hirano Y., Tabata Y. Effect of culture substrates and fibroblast growth factor addition on the proliferation and differentiation of rat bone marrow stromal cells. Tissue Eng. 2004;10(7–8):995–1005. doi: 10.1089/ten.2004.10.995. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe M., Jo J., Radu A., Kaneko M., Tabata Y., Flake A.W. A tissue engineering approach for prenatal closure of myelomeningocele with gelatin sponges incorporating basic fibroblast growth factor. Tissue Eng Part A. 2010;16(5):1645–1655. doi: 10.1089/ten.TEA.2009.0532. [DOI] [PubMed] [Google Scholar]
- 28.Akagawa Y., Kubo T., Hayashi K., Doi K., Matsumura A., Koretake K. Initial bone regeneration around fenestrated implants in Beagle dogs using basic fibroblast growth factor–gelatin hydrogel complex with varying biodegradation rates. J Prosthodont Restor. 2008;53(1):41–47. doi: 10.1016/j.jpor.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Hiraoka Y., Yamashiro H., Yasuda K., Kimura Y., Inamoto T., Tabata Y. In situ regeneration of adipose tissue in rat fat pad by combining a collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Tissue Eng. 2006;12(6):1475–1487. doi: 10.1089/ten.2006.12.1475. [DOI] [PubMed] [Google Scholar]
- 30.Igai H., Chang S.S., Gotoh M. Regeneration of canine tracheal cartilage by slow release of basic fibroblast growth factor from gelatin sponge. ASAIO J. 2006;52(1):86–91. doi: 10.1097/01.mat.0000196513.97411.3d. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto T., Yamamoto Y., Gotoh M., Tabata Y. Slow release of bone morphogenetic protein 2 from a gelatin sponge to promote regeneration of tracheal cartilage in a canine model. J Thorac Cardiovasc Surg. 2004;127(2):329–334. doi: 10.1016/j.jtcvs.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Kimura Y., Tabata Y. Controlled release of stromal-cell-derived factor-1 from gelatin hydrogels enhances angiogenesis. J Biomater Sci Polym Ed. 2010;21(1):37–51. doi: 10.1163/156856209X410193. [DOI] [PubMed] [Google Scholar]
- 33.Esaki J., Marui A., Tabata Y. Controlled release systems of angiogenic growth factors for cardiovascular diseases. Expert Opin Drug Deliv. 2007;4(6):635–649. doi: 10.1517/17425247.4.6.635. [DOI] [PubMed] [Google Scholar]
- 34.Tabata Y., Nagano A., Ikada Y. Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Eng. 1999;5(2):127–138. doi: 10.1089/ten.1999.5.127. [DOI] [PubMed] [Google Scholar]
- 35.Patel Z.S., Yamamoto M., Ueda H., Tabata Y. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008;4(5):1126–1138. doi: 10.1016/j.actbio.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kushibiki T., Tomoshige R., Iwanaga K., Tabata Y. Controlled release of plasmid DNA from hydrogels prepared from gelatin cationized by different amine compounds. J Control Release. 2006;112(2):249–256. doi: 10.1016/j.jconrel.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Ichinohe N., Kuboki Y., Tabata Y. Bone regeneration using titanium nonwoven fabrics combined with fgf-2 release from gelatin hydrogel microspheres in rabbit skull defects. Tissue Eng Part A. 2008;14(10):1663–1671. doi: 10.1089/ten.tea.2006.0350. [DOI] [PubMed] [Google Scholar]
- 38.Patel Z.S., Ueda H., Yamamoto M., Tabata Y. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm Res. 2008;25(10):2370–2378. doi: 10.1007/s11095-008-9685-1. [DOI] [PubMed] [Google Scholar]
- 39.Tabata Y., Hijikata S., Muniruzzaman M., Ikada Y. Neovascularization effect of biodegradable gelatin microspheres incorporating basic fibroblast growth factor. J Biomater Sci Polym Ed. 1999;10(1):79–94. doi: 10.1163/156856299x00298. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira L., Squier T., Park H., Choe H., Kohane D.S., Langer R. Human Embryoid bodies containing nano and microparticle delivery vehicles. Adv Mater. 2008;20(12):2285–2291. [Google Scholar]
- 41.Chung H.J., Park T.G. Injectable cellular aggregates prepared from biodegradable porous microspheres for adipose tissue engineering. Tissue Eng Part A. 2009;15(6):1391–1400. doi: 10.1089/ten.tea.2008.0344. [DOI] [PubMed] [Google Scholar]
- 42.Bratt-Leal A.M., Carpenedo R.L., Ungrin M.D., Zandstra P.W., McDevitt T.C. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials. 2011;32(1):48–56. doi: 10.1016/j.biomaterials.2010.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banerjee M.R. Responses of mammary cells to hormones. Int Rev Cytol. 1976;47:1–97. doi: 10.1016/s0074-7696(08)60086-8. [DOI] [PubMed] [Google Scholar]
- 44.Rosen J.M., Freeman C.S. Mechanism of action of prolactin in the mammary gland. In: Jaffe R.B., editor. Prolactin. Elsevier Science Publishing Co. Inc.; New York: 1980. pp. 85–126. [Google Scholar]
- 45.Topper Y.J., Freeman C.S. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980;60:1044–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- 46.Juergens W.G., Stockdale F.E., Topper Y.J., Elias J.J. Hormone dependent differentiation of mammary gland in vitro. Proc Natl Acad Sci USA. 1965;54:629–633. doi: 10.1073/pnas.54.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ono M., Oka T. The differential actions of cortisol on the accumulation of a-lactalbumin and casein in mid-pregnant mouse mammary gland in culture. Cell. 1980;19:473–480. doi: 10.1016/0092-8674(80)90522-x. [DOI] [PubMed] [Google Scholar]
- 48.Aratani Yasuaki, Kitagawa Yasuo. Enhanced synthesis and secretion of type IV collagen and entactin during adipose conversion of 3T3-Ll cells and production of unorthodox laminin complex. J Biol Chem. 1988;263(31):16163–16169. [PubMed] [Google Scholar]
- 49.Campbell Jonathan J., Watson Christine J. A multifunctional 3D Co-Culture system for studies of mammary tissue morphogenesis and stem cell biology. PLoS One. 2011;6(9):e25661. doi: 10.1371/journal.pone.0025661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reichmann Ernst, Ball R., Groner B., Friis R.R. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J Cell Biol. 1988;108:1127–1138. doi: 10.1083/jcb.108.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rao J., Otto W.R. Fluorimetric DNA assay for cell growth estimation. Anal Biochem. 1992;207(1):186–192. doi: 10.1016/0003-2697(92)90521-8. [DOI] [PubMed] [Google Scholar]
- 52.Nam J.H., Ermonval M., Sharfstein S.T. Cell attachment to microcarriers affects growth, metabolic activity, and culture productivity in bioreactor culture. Biotechnol Prog. 2007;23(3):652–660. doi: 10.1021/bp070007l. [DOI] [PubMed] [Google Scholar]
- 53.Johansson S., Syineng G., Wennerberg K., Armulik A., Lohikangas L. Fibronectin-integrin interactions. Front Biosci. 1997;1(2):d126–d146. doi: 10.2741/a178. [DOI] [PubMed] [Google Scholar]
- 54.Theveneau Eric, Mayor Roberto. Collective cell migration of epithelial and mesenchymal cells. Cell Mol Life Sci. 2013;70:3481–3492. doi: 10.1007/s00018-012-1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hartsock Andrea, Nelson W. James. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778(3):660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du D., Xu F., Yu L., Zhang C., Lu X., Yuan H. The tight junction protein, occludin, regulates the directional migration of epithelial cells. Dev Cell. 2010;18(1):52–63. doi: 10.1016/j.devcel.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Fennema E., Rivron N., Rouwkema J., Bitterswiik C., Boer J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013;33(2):108–115. doi: 10.1016/j.tibtech.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 58.LiangliI Ming, Bissell Mina J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci. 1987;84:136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seely Keith A., Aggeler Judith. Modulation of milk protein synthesis through alteration of the cytoskeleton in mouse mammary epithelial cells cultured on a reconstituted basement membrane. J Cell Physiol. 1991;146:117–130. doi: 10.1002/jcp.1041460116. [DOI] [PubMed] [Google Scholar]
- 60.Novaro V., Roskelley C.D., Bissel M.J. Collagen-IV and laminin-1 regulate estrogen receptor α expression and function in mouse mammary epithelial cells. J Cell Sci. 2003;116(Pt 14):2975–2986. doi: 10.1242/jcs.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Somasiri A., Wu C., Ellchuk T., Turley S., Roskelley C.D. Phosphatidylinositol 3-Kinase is required for adherens junction-dependent mammary epithelial cell spheroid formation. Differentiation. 2000;66:116–125. doi: 10.1046/j.1432-0436.2000.660206.x. [DOI] [PubMed] [Google Scholar]
- 62.Zoubianne G.S., Valentijn A., Lowe E.T., Akhtar N., Bagley S., Gilmore A.P. A role for the cytoskeleton in prolactin-dependent mammary epithelial cell differentiation. J Cell Sci. 2004;117(2):271–280. doi: 10.1242/jcs.00855. [DOI] [PubMed] [Google Scholar]
- 63.Xu R., Spencer V.A., Groesser D.L., Bissell M.J. Laminin regulates PI3K basal localization and activation to sustain STAT5 activation. Cell Cycle. 2010;9(21):4315–4322. doi: 10.4161/cc.9.21.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gharbi S.L., Zvelebil M.J., Shuttleworth S.J., Hancox T., Saghir N., Timms J.F. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404(Pt 1):15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]