Abstract
Background
The purpose of this study was to evaluate the clinical results following intra-articular knee injection of Stromal Vascular Fraction (SVF cell therapy).
Methods
This study involved 13 consecutive patients who had received SVF cell therapy at our clinic before November 2015 and completed the 6-month post-treatment follow-up period. For each treatment, approximately 200 mL or more of subcutaneous adipose tissue was harvested using tumescent liposuction technique and manual aspiration of tissue from the lower abdomen using a suction cannula under local anesthesia in the operating room. The adipose tissue harvested was processed using the Celution Centrifuge IV in the cell processing room of our clinic. These cells were injected into the articular cavity of both knees directly. Outcome was assessed on the basis of patient questionnaires using VAS for pain, the Japanese Knee Osteoarthritis Measure (JKOM), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).
Results
The 13 patients (26 knee joints), consisting of 2 men and 11 women, had a mean age of 74.5 ± 5.4 years. Eleven patients (9 women, 2 men) presented Grade IV knee OA according to the KL classification. The remaining two patients, both women, had Grade III. Pre-operative scores of JKOM, WOMAC, VAS, and BS-POP (for patients) were 55.9 ± 21.0, 49.6 ± 20.4, 72.7 ± 18.2, and 18.5 ± 2.0. No serious adverse events were reported. One month after injection of SVF, all the scores of JKOM, WOMAC, and VAS were significantly improved over baseline (P < 0.01). Ultimately, the scores were improved by an average of 35% over baseline for JKOM, 32% improvement in WOMAC, and 40% for pain (VAS).
Conclusions
Our approach is unique in that it occurred within the context of the recently enacted Japanese Regenerative Medicine Safety Act which is the first in the world.
Keywords: Stromal vascular fraction (SVF), Adipose-derived mesenchymal stem cell (MSC), Regenerative medicine safety act in Japan, Type II regenerative medicine provision plan, Knee osteoarthritis
1. Introduction
In Japan, the Act on the Safety of Regenerative Medicine (Regenerative Medicine Safety Act) came into effect as of November 25, 2014 under an institutional framework for promoting the implementation of regenerative medicine. This act, which covers clinical research and private practice, stipulates three risk-dependent standards and the procedures for notification of plans for regenerative medicine as well as the standards of cell culture and processing facilities and the licensing procedures to ensure the safety of regenerative medicine. In accordance with this law our clinic, which specializes in private practice, submitted a Type II regenerative medicine provision plan proposing use of intra-articular injection of adipose tissue-derived stromal vascular fraction (SVF) cells for knee osteoarthritis. Following review by the Certified Special Committee for Regenerative Medicine, this plan was accepted by the Director of the Regional Bureau of Health and Welfare. To our knowledge this is only the second such approval under the Regenerative Medicine Safety Act. In this study, we report the results from the first 13 patients treated under this approval.
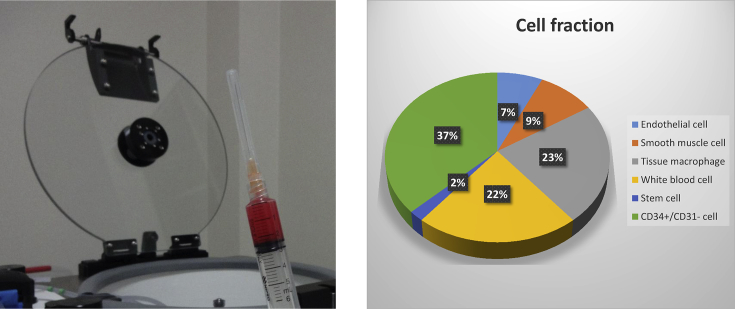
Interest in the clinical use of SVF began with the demonstration by Zuk et al., in 2001 [1] and 2002 [2] that SVF is a rich source of multipotent stem cells. These stem cells and other cells within the SVF have the potential to form a clinically valuable autologous cell therapy. SVF is obtained by enzymatic digestion of adipose tissue followed by centrifugation to remove mature adipocytes and processing reagents to prepare a population of cells that is comprised predominantly of stromal and vascular cells type (Fig. 1). The Celution Centrifuge IV (Cytori Therapeutics, K.K.) is an automated system that applies sterile, single use consumables, pharmaceutical grade enzyme, and a validated, software-controlled system to prepare SVF cells that are suitable for clinical use. This system (Fig. 2) has increasingly accelerated clinical application in Japan and around the world.
Fig. 1.
Left: Adipose tissue-derived stromal vascular fraction (SVF) extracted by Celution; Right: SVF cell fraction data provided by Cytori Therapeutics.
Fig. 2.

Celution centrifuge.
2. Subjects and method
This report describes 13 consecutive patients who had received intra-articular knee injection of SVF (SVF cell therapy) at our clinic before November 2015 and who had completed the 6-month post-treatment follow-up period. Patients had Grade III or IV knee OA according to the Kellgren–Lawrence (KL) grading system, as confirmed by clinical evaluation and MRI and/or X-ray. All patients responded inadequately to conservative treatment commonly provided at authorized insurance medical institutions in Japan. Specifically, they were recommended to undergo artificial joint replacement after poor response to oral medication for pain relief and hyaluronic acid injection. At this stage they approached our clinic to consider potential use of regenerative medicine approaches that might eliminate or delay the need for joint replacement surgery with the understanding that private practice was available by reservation only. We examined MRI knee images brought by all the patients (X-ray images, if any, were brought together). All patients received written information and consent forms for SVF cell therapy and fat harvesting. Written materials were supplemented with conversations with medical staff using MRI and/or X-ray images and a knee model. The patients who fully understood the explanation and answers to their questions provided signed informed consent and then were registered in this study. Patients with serious (systemic and local) complications such as infections and cancers were excluded as ineligible. This study also excluded patients with serious dysfunction evident on an overall assessment of laboratory tests (blood test).
For each treatment, approximately 200 mL or more of subcutaneous adipose tissue was harvested using tumescent liposuction technique and manual aspiration of tissue from the lower abdomen or the inside of the thigh using a suction cannula under local anesthesia in the operating room. This treatment caused one or two operative wounds with a size of approximately 1 cm. The adipose tissue harvested was processed using the Celution Centrifuge IV (Fig. 2, medical device notification number: 13B1 × 10155000013), in the cell processing room of our hospital in accordance with the manufacturer's instructions. Through this procedure, autologous SVF cells were produced without culture with simple button operation allowing separation and collection of SVF in a sterile single-use functionally-closed system, requiring approximately 2–2.5 h. These cells were injected into the articular cavity of both knees directly (2.5 mL per knee). While total SVF cell dose was not assessed for this cohort, processing 200 mL of adipose tissue typically yields 4–8 × 107 viable nucleated SVF cells for an estimated average dose of 3 × 107 SVF cells/knee. Post-treatment physical therapy was restricted to requesting that patients perform a target of 100 ‘bend-and-stretch’ exercise of the knees on the day of SVF injection and each day thereafter. Oral medication for pain relief and prophylactic antibiotics was prescribed for outpatient use for four and three days respectively. All patients received no other treatment or intervention during the evaluation period.
Outcome was assessed on the basis of patient questionnaires using Visual Analogue Scale (VAS) for pain, the Japanese Knee Osteoarthritis Measure (JKOM), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and the Brief Scale for Psychiatric Problems in Orthopedic Patients (BS-POP questionnaire for patients, pre-operative only) before SVF therapy [3], [4], [5], [6]. At 1 and 6 months after treatment with SVF, the patients revisited the hospital for clinical assessment and collection of adverse events, if any. Follow-up application of JKOM, WOMAC, and VAS tools was completed at home. For safety assessment, adverse events were to be specified, if any. Statistical analysis was performed using the Wilcoxon signed rank sum test with a significance level of less than 5%. Data for each item are represented as mean ± standard deviation.
3. Results
Patient characteristics are presented in Table 1. The 13 patients (26 knee joints), consisting of 2 men and 11 women, had a mean age of 74.5 ± 5.4(65–82) years. Eleven patients (9 women, 2 men) presented Grade IV knee OA according to the KL system. The remaining two patients, both women, had Grade III knee OA. Pre-operative scores of JKOM, WOMAC, VAS, and BS-POP (for patients) were 55.9 ± 21.0, 49.6 ± 20.4, 72.7 ± 18.2, and 18.5 ± 2.0, respectively (Table 1, Table 2).
Table 1.
Patient characteristics.
| Case number | 13 cases 26 knees |
| Sex (M/F) | 2/11 |
| Age (y.o.) | 74.5 ± 5.4 (65–82) |
| Height (cm) | 154.9 ± 7.3 (145–166) |
| Weight (kg) | 55.3 ± 6.6 (47–70) |
| BMI (kg/m2) | 23.0 ± 2.2 (19.0–28.4) |
| KL grade (grade III/IV) | 2/11 |
| BS-POP patients questionnaire (point) | 18.5 ± 2.0 |
BMI: body mass index; KL grade: Kellgren–Lawrence grading system; BS-POP: the brief scale for psychiatric problems in orthopedic patients.
Table 2.
JKOM, WOMAC, VAS score (point).
| Prior to injection | Post 1 month | Post 6 month | |
|---|---|---|---|
| JKOM | 55.9 ± 21.0 | 43.0 ± 17.4* | 36.5 ± 21.9*,** |
| WOMAC | 49.6 ± 20.4 | 37.9 ± 17.7* | 33.8 ± 20.9*,** |
| VAS | 72.7 ± 18.2 | 49.2 ± 21.1* | 43.5 ± 24.1*,** |
JKOM: the Japanese knee osteoarthritis measure; WOMAC: the Western Ontario and McMaster Universities Osteoarthritis Index; VAS: visual analogue scale for pain.
*Significant difference between pre-Op and 1 month, 6 months (P < 0.01).
**No significant difference between 1 month and 6 months (P > 0.05).
No serious adverse events (as defined by the International Conference of Harmonisation guidelines) were reported. Pain and swelling at the injection and fat harvesting sites that lasted for a few days was observed and all resolved within two weeks with the usual dose of the pain reliever described above. There were no reports of other potential treatment-related adverse events such as reduced range of motion of the knee, fat embolism, deep venous thrombosis, sepsis caused by intra-articular infection, adhesion of the knee associated with SVF injection, or superficial infection or intra-articular bleeding at the injection sites in the knee.
One month after injection of SVF, all the scores of JKOM, WOMAC, and VAS were significantly improved over baseline (Table 2). This improvement was sustained at the six month visit. A small numerical improvement in each score was observed between the one month and the six month follow-up visit, however, this difference was not statistically significant. Ultimately, the scores were improved by an average of 35% over baseline for JKOM, 32% improvement in WOMAC, and 40% for pain (VAS).
4. Discussion
Between 2001 and 2002, Zuk et al. first reported that a large amount of stem cells are present in subcutaneous fat [1], [2] and proposed the use of SVF containing uncultured adipose-derived stem cells for regenerative medicine. Treatment with adipose-derived cells has the following advantages, which are not provided by bone marrow-derived cells: easier liposuction under local anesthesia, higher frequency of stem cell-like cells than in bone marrow, anti-inflammatory effects, no need for culture, and short-time processing. Many basic researchers [7], [8], [9], [10] have suggested the potential of bone marrow cells or adipose-derived SVF for the treatment of OA. In particular, the studies in rabbits have demonstrated that SVF injection repaired the cartilage as assessed by X-ray or histological evaluation [9], [10] and suggested that the tissues are repaired by stem cells contained in SVF and cytokines and growth factors produced by these cells. The expectation that a similar repair is also observed in human combined with the development of an automated SVF separation system has increasingly accelerated clinical application and clinical trials of SVF cell therapy. The results of the present study involving private practice are consistent with other reports.
However, our approach is unique in that it occurred within the context of the recently enacted Japanese Regenerative Medicine Safety Act which is the first in the world to apply multi-tiered approach to review and approval of regenerative medicine [11]. This act specifies the level of documentation and data required for review by the Regional Bureau of Health and Welfare in charge of the relevant provision plan, together with the regenerative medicine provision plan and the opinion issued after the review by the Certified Committee for Regenerative Medicine designated by the Ministry of Health, Labour and Welfare. Treatment of knee OA with intra-articular injection of SVF is a Type II regenerative medicine technology in which SVF obtained by enzyme treatment and centrifugation of adipose tissue harvested from patients themselves is injected into the articular cavity of the affected knee of the patients themselves. Ensuring the safety of SVF is discussed according to the specifications of the Regenerative Medicine Safety Act. The current study applied the Celution System which combines an automated device and single-use consumable with two enzymes (Celase®, Intravase®). These enzymes do not contain raw materials of ruminant origin and are sterile. In addition, they are diluted and removed during SVF preparation and, therefore, residual enzymes in autologous SVF prepared using this system are considered as negligible. In the United States, a phase II clinical study of intra-articular injection of SVF in patients with knee OA has been just completed (NCT02326961) [12]. According to the manufacturer Cytori Therapeutics, no serious adverse event, infection, hospitalization, or problem with fat harvesting has been reported from all 94 patients who are being followed up. Although the patients in the present study experienced pain and swelling at the injection and fat harvesting sites for a few days, no serious adverse event was noted. As applied in the present study, SVF cell therapy has an extremely low risk of contamination and infection because, unlike SVF prepared by manual digestion of certain other semi-automated approaches, the SVF in the current study was prepared using a single use, sterile consumable with GMP-quality enzymatic reagent and an automated system that has been validated to ensure that residual enzyme levels are below determined safety limits [17]. This product can be injected on the day of preparation and does not require culture or additional processing, and thus offers a high level of safety. On this basis and a package of related preclinical data, the current study was approved under the Regenerative Medicine Safety Act.
The raw material used for preparation of SVF (adipose tissue obtained from patients) was harvested using tumescent liposuction, which is widely performed in the area of cosmetic surgery (more than one million cases a year around the world). The volume of harvested adipose tissue was small (∼100 mL) Several clinical researchers have published case series with SVF in the knee. Koh et al. have published three papers comprising a total of 73 patients treated with SVF prepared manually using enzymatic digestion [13], [14], [15] supplemented with platelet-rich plasma. In the first two papers subjects also underwent concomitant arthroscopic intervention (e.g.: synovectomy, reduction of osteophytes, and/or meniscal repair) which may have contributed to, or confounded, outcome. Through these studies this group has reported significant improvement in pain scores, MRI imaging, and in surgeon's assessment at the time of second look arthroscopy. The 37% improvement in pain score reported by Koh et al. (2012) at three months of follow-up is similar to that in our study (40%). The two year follow-up showed greater improvement in pain (58%) while the improvement in WOMAC score at two years (39%) was similar to that in our study at six months (32%). Another group [16] has reported a very large series of over 1100 patients. Results showed improvement in pain however, this study was complicated by the fact that the SVF cells were not characterized and that two different methods of preparing SVF were used one of which did not use enzymatic digestion and which, in the absence of any characterization showing otherwise, cannot be considered to be SVF [Markarian CF, Frey GZ, Silveira MD, et al. (2014) Biotechnol Lett. 36(4):693–702; Bourin P, Bunnell, BA, Casteilla L, et al. (2013) Cytotherapy. 15(6):641–8]. Further, while many patients received cells by intra-articular injection some were treated by injection into the synovium. Unlike our study, the approaches of Michalek et al. and Koh et al. did not mandate the nature of post-treatment physiotherapy although it should be noted that, as our approach was applied in the context of clinical practice rather than a formal clinical trial, we did not monitor compliance with the mandated stretching exercises. Further, like our own experience, none of these studies applied a randomized, blinded trial design which is important given the very well-recognized placebo effect in osteoarthritis of the knee. Nonetheless, in the light of the apparent safety and feasibility reported in these studies SVF cell therapy is considered as appropriate because the advantages from the therapy are fully expected to outweigh the disadvantages.
Koh et al. (2013) have suggested that cell dose is important in outcome in osteoarthritis. According to the catalog provided by Cytori Therapeutics the total cell count of SVF obtained from 100 mL of adipose tissue averages approximately 2 × 107 to 3 × 107 cells/5 mL It is also important to recognize that SVF prepared by different methods can have different composition and properties [17]. Cytori Therapeutics has reported the mean proportion of each type of the main cell types contained in SVF: 37% with CD34+/CD31− cells, 23% with tissue macrophages, 22% with white blood cells, 9% with smooth muscle cells, 7% with endothelial cells, and 2% with stem cells (Fig. 1) [18]. A variety of compositions of cells and a great individual difference in the proportion of each cell type, which are both due to uncultured and unprocessed SVF, make it difficult to elucidate the mechanism of action, and more specifically, to determine the type of cells that exert an effect and the degree of their effect. Compared with products with uniform properties, such as pharmaceuticals and artificial joints manufactured in conventional facilities, it is more difficult to maintain a uniform quality level because an individual difference leads to the difference in quality of final products. One advantage of an automated system such as that used in our study is that by applying a software-controlled approach, it improves standardization and reproducibility. This is further enhanced by the use of a sterile fluid path and pharmaceutical quality enzymes (which were not specified in any of the reports cited above). At the time of informed consent, the individual difference should be explained to patients more sufficiently than before to obtain their understanding and consent.
While the current study was prospective, the fact that it involved private practice not covered by health insurance, made it infeasible to include an appropriate control group (a single-institution, open-label, uncontrolled study). Our clinic does not provide medical treatments covered by health insurance, including direct observation of affected sites by arthroscopy. If no particular adverse event is noted, patients need to be referred back to a neighboring hospital as needed after the 6-month follow-up period (after the completion of provision of regenerative medicine) to receive only treatments covered by insurance, which is a challenge for SVF cell therapy in private practice. Since SVF cell therapy is a new approach, collecting the data on medium- and long-term results is also an issue in the future. These data will be followed up by fixed-point observation in the follow-up survey (including delivery and collection of the questionnaires of JKOM, WOMAC, and VAS and MRI scanning) to the extent possible. The manufacturer of the Celution System has conducted a 94 patient randomized, double blind, placebo-controlled study in osteoarthritis. Data are expected to be made public in 2017. The fact that the current study used the same cell preparation system used in this trial will allow direct comparison of results obtained in the context of private practice in Japan with those of a controlled clinical trial in the USA.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001 Apr;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 2.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002 Dec;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akai M., Doi T., Fujino K., Iwaya T., Kurosawa H., Nasu T. An outcome measure for Japanese people with knee osteoarthritis. J Rheumatol. 2005 Aug;32(8):1524–1532. [PubMed] [Google Scholar]
- 4.Bellamy N., Buchanan W.W., Goldsmith C.H., Campbell J., Stitt L.W. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988 Dec;15(12):1833–1840. [PubMed] [Google Scholar]
- 5.Hashimoto H., Hanyu T., Sledge C.B., Lingard E.A. Validation of a Japanese patient-derived outcome scale for assessing total knee arthroplasty: comparison with Western Ontario and McMaster Universities osteoarthritis index (WOMAC) J Orthop Sci. 2003;8(3):288–293. doi: 10.1007/s10776-002-0629-0. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida K., Sekiguchi M., Otani K., Mashiko H., Shiota H., Washita T. A validation study of the brief scale for psychiatric problems in orthopaedic patients (BS-POP) for patients with chronic low back pain (verification of reliability, validity, and reproducibility) J Orthop Sci. 2011 Jan;16(1):7–13. doi: 10.1007/s00776-010-0012-4. [DOI] [PubMed] [Google Scholar]
- 7.Black L.L., Gaynor J., Gahring D., Adams C., Aron D., Harman S. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther. 2007 Winter;8(4):272–284. [PubMed] [Google Scholar]
- 8.Black L.L., Gaynor J., Adams C., Dhupa S., Sams A.E., Taylor R. Effects of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008 Fall;9(3):192–200. [PubMed] [Google Scholar]
- 9.Desando G., Cavallo C., Sartoni F., Martini L., Parrilli A., Veronesi F. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res Ther. 2013 Jan 29;15(1):R22. doi: 10.1186/ar4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toghraie F., Razmkhah M., Gholipour M.A., Faghih Z., Chenari N., Torabi Nezhad S. Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch Iran Med. 2012 Aug;15(8):495–499. [PubMed] [Google Scholar]
- 11.About Regenerative Medicine. Ministry of Health, Labour and Welfare web site: http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iryou/saisei_iryou/ [in Japanese].
- 12.Cytori Therapeutics (sponsor): Celution prepared adipose derived regenerative cells in the treatment of osteoarthritis of the knee: a double-blind, placebo controlled, multi-center safety and feasibility study. CrinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02326961.
- 13.Koh Y.G., Choi Y.J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902–907. doi: 10.1016/j.knee.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Koh Y.G., Jo S.B., Kwon O.R., Suh D.S., Lee S.W., Park S.H. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013 Apr;29(4):748–755. doi: 10.1016/j.arthro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Koh Y.G., Choi Y.J., Kwon S.K., Kim Y.S., Yeo J.E. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015 May;23(5):1308–1316. doi: 10.1007/s00167-013-2807-2. [DOI] [PubMed] [Google Scholar]
- 16.Michalek J., Moster R., Lukac L., Proefrock K., Petrasovic M., Rybar J. Autologous adipose tissue-derived stromal vascular fraction cells application in patients with osteoarthritis. Cell Transpl. 2015 Jan 20 doi: 10.3727/096368915X686760. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Aronowitz J.A., Ellenhorn J.D. Adipose stromal vascular fraction isolation: a head-to-head comparison of four commercial cell separation systems. Plast Reconstr Surg. 2013 Dec;132(6):932e–939e. doi: 10.1097/PRS.0b013e3182a80652. [DOI] [PubMed] [Google Scholar]
- 18.Lin K., Matsubara Y., Masuda Y., Togashi K., Ohno T., Tamura T. Characterization of adipose tissue-derived cells isolated with the Celution system. Cytotherapy. 2008;10(4):417–426. doi: 10.1080/14653240801982979. [DOI] [PubMed] [Google Scholar]