Abstract
Objective
To investigate the independent and combined associations of fitness and fatness with cardiometabolic risk factors in older Norwegian women and men.
Patients and Methods
We conducted a cross-sectional study of 505 women and 417 men aged 70 to 77 years enrolled in the Generation 100 study in Norway. Fitness was assessed as peak oxygen uptake and fatness as high body mass index (BMI; ≥25 kg/m2), waist circumference (WC) of 88 cm or greater for women and 102 cm or greater for men, and percent body fat (%BF) of 35% or greater and 25% or greater for women and men, respectively. High cardiometabolic risk was defined as the presence of 2 or more of the following risk factors: elevated triglyceride level, reduced high-density lipoprotein cholesterol concentration, elevated blood pressure, and elevated fasting glucose level or pharmacological treatment of these conditions.
Results
Receiver operating characteristic curve analyses identified fitness levels of less than 25.7 and less than 30.7 mL/kg per minute in women and men, respectively, as critical thresholds for having high cardiometabolic risk. Individuals with levels below these thresholds had an adjusted odds ratio of 2.77 (95% CI, 2.09-3.66) for having high cardiometabolic risk, while high BMI, WC, and %BF had odds ratios (95% CIs) of 3.58 (2.69-4.77), 3.06 (2.29-4.10), and 3.26 (2.47-4.30), respectively. In our combined analyses, being lean did not attenuate the cardiometabolic risk associated with low fitness, and combinations of low fitness and/or high BMI, WC, or %BF cumulatively increased cardiometabolic risk.
Conclusion
Low fitness and indication of fatness were independently and cumulatively associated with poor cardiometabolic health. Our results emphasize the importance of including both physical fitness and body fatness in the assessment of cardiometabolic risk and health promotion efforts in older adults.
Abbreviations and Acronyms: AUC, area under the curve; BMI, body mass index; BP, blood pressure; CV, cardiovascular; CVD, CV disease; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HTN, hypertension; OR, odds ratio; PA, physical activity; %BF, percent body fat; ROC, receiver operating characteristic; T2D, type 2 diabetes; TG, triglyceride; VO2peak, peak oxygen uptake; WC, waist circumference
Levels of physical activity (PA) and cardiorespiratory fitness (hereafter fitness) decline with age,1, 2 whereas the prevalence of overweight and obesity increases.3 As the population ages, medical expenditures associated with inactivity and obesity are therefore expected to increase, posing serious public health challenges.4 Cardiovascular (CV) disease (CVD) is the number one cause of death worldwide,5 with the highest prevalence in older adults.6 Combinations of cardiometabolic disturbances, such as dyslipidemia, elevated blood pressure (BP), and impaired glucose metabolism, have been identified as risk factors for CVD and all-cause and CVD mortality.6
Overweight and obesity are key contributors to the clustering of unfavorable cardiometabolic risk factor levels.7 Given the strong and consistent evidence that higher levels of fitness are associated with a lower risk of CVD morbidity and mortality,8, 9, 10, 11, 12, 13, 14, 15 fitness may be a relevant factor largely affecting the deleterious association between overweight, obesity, and CV health. In this context, the “fat but fit paradigm” has been suggested, which refers to individuals who despite being obese have a relatively high fitness level.7 Indeed, high fitness has been reported to attenuate most of the adverse effects of overweight and even mild obesity on all-cause and CVD mortality and morbidity.7, 8, 16, 17, 18, 19, 20, 21 Individuals who are fat but fit have lower mortality rates than lean but unfit individuals both in the general adult population11, 12, 13 and in older adults.10, 14, 22 However, whether high fitness can modify the deleterious association between body fatness and cardiometabolic risk factors has not been adequately addressed in older adults, and findings in the general population have been inconsistent.16 A few studies found that low fitness and indication of fatness carry similar risks for the prevalence of metabolic syndrome23, 24 or incidence of type 2 diabetes mellitus (T2D),25, 26, 27 whereas others found a greater magnitude of association between fatness and cardiometabolic risk factors.28, 29, 30 However, these studies have included few individuals older than 70 years and particularly few older women. Thus, the generalizability to the older population is limited.
Most previous studies used body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) as a crude estimate of fatness, and despite its advantages,31 it does not discriminate between body fat and lean body mass. In addition, estimated fitness or self-reported PA are commonly used as a proxy for directly measured fitness. Although fitness is associated with up to 70% of the variance in habitual PA,32 objectively measured fitness generally results in stronger associations with health outcomes than PA.9, 15, 16, 33
A better understanding of how fitness and fatness relate to other cardiometabolic risk factors has the potential to improve health promotion efforts. Accordingly, this study investigated the independent and combined associations of directly measured fitness and various indicators of body fatness with cardiometabolic risk factors in older Norwegian women and men.
Patients and Methods
Study Population
We used baseline data from the Generation 100 study, a randomized controlled clinical trial with the main objective of investigating the effect of 5 years of exercise training on morbidity and mortality in the older adult population.34 All inhabitants of Trondheim municipality, Norway, born between 1936 and 1942 (n=6966) were invited. A total of 1567 participants (790 women) accepted the invitation, complied with the inclusion criteria for Generation 100, and fulfilled baseline testing. Participants with known CVD or abnormal findings on electrocardiography during cardiopulmonary exercise testing (n=302), self-reported cancer during the preceding 2 years (n=24), and missing values on fitness (n=24), waist circumference (WC; n=4), percent body fat (%BF; n=5), and missing information on cardiometabolic risk factor composite score (n=286) were excluded. Overall, a total of 922 participants (505 women) were included in the current study.
The study was approved by the Regional Committee for Medical Research Ethics (REK Midt: 2013/1609) and complies with the Norwegian laws and the principles of the Declaration of Helsinki. All participants signed informed consent documents before participation.
Clinical Examination
Clinical examinations were carried out between August 22, 2012, and June 30, 2013, by trained personnel and followed standardized routines described in detail previously.34 Briefly, body mass, %BF, visceral fat, and muscle mass were measured using bioelectrical impedance (Inbody 720, Biospace Co, Ltd); WC was measured at the uppermost border of the iliac crest around the abdomen. Gas exchange ergorespirometry (MetaMax II, Cortex Biophysik GmbH) was used to determine peak oxygen uptake (VO2peak). The test protocol and equipment were identical to those used in previously published studies from our group.34, 35 Participants were breathing into an appropriately sized mask (Hans Rudolph, Inc), which was connected to the gas analyzer. Heart rate was monitored using the Accurex RS300X SD device (Polar Electro Oy). Calibration of the MetaMax II included barometric pressure (daily before testing), volume (between every test with a 3-L syringe [5530 series, Hans Rudolph, Inc]), and gas analyzer (between every fourth test or if ambient air measurements indicated that additional calibration was required) using ambient air and a reference gas containing 5% carbon dioxide and 15% oxygen (Scott Medical Products). Participants were encouraged to avoid handrail grasp or if necessary exclusively use handrail grasp for balance. Following a 10-minute warm-up and familiarization period, inclination or speed were increased by either 2% or 1 km/h, respectively, every 1 to 2 minutes until voluntary exhaustion. Finally, VO2peak was calculated as the average of the 3 highest consecutive 10-second measurements of oxygen consumption.
For blood measurements, the participants were asked to arrive in a fasting state, abstain from exercise training, caffeine, nicotine, and alcohol 12 hours before the clinical examination, and continue their regular medication routines. Participants fasting for less than 8 hours were excluded from the analysis of fasting glucose level (n=286). Previously described questionnaires34 provided information about smoking and alcohol consumption, the use of prescriptive medication (for hypertension [HTN], T2D, and dyslipidemia), health status, prevalence of CVD (including myocardial infarction, angina pectoris, heart failure, atrial fibrillation, other heart diseases, and stroke) and cancer. The presence of cardiometabolic risk factors was defined on the basis of diagnostic criteria for the metabolic syndrome36: elevated triglyceride (TG) level (≥1.7 mmol/L) or pharmacological treatment for dyslipidemia; reduced high-density lipoprotein cholesterol (HDL-C) level (<1.3 mmol/L in women and <1.0 mmol/L in men) or pharmacological treatment for dyslipidemia; elevated BP (systolic ≥130 mm Hg and/or diastolic ≥85 mm Hg) or pharmacological treatment for HTN; and elevated fasting glucose level (≥100 mg/dL [to convert to mmol/L, multiply by 0.0555]) or pharmacological treatment for T2D. High cardiometabolic risk was defined as the presence of 2 or more of these risk factors. Because WC is strongly correlated with other indices of fatness, WC was not considered in the definition of high cardiometabolic risk to avoid biasing the results against the higher fatness groups, as suggested by others.7
Statistical Analyses
Descriptive data are presented as mean ± SD for continuous variables and percentages for categorical variables. Receiver operating characteristic (ROC) curve analysis was used to estimate the optimal thresholds of fitness, BMI, WC, and %BF that best identify individuals with high cardiometabolic risk (ie, ≥2 cardiometabolic risk factors present).37 All combinations of sensitivity and 1 − specificity that can be realized by incrementally changing the cutoff values of fitness, BMI, WC, and %BF were summarized as the areas under the ROC curves (AUC) with 95% CIs for each parameter. Optimal thresholds for each variable were estimated by the Youden Index37, 38 as the value producing the combination of sensitivity and specificity closest to 1 (ie, perfect test).
According to the thresholds derived from the ROC curve analyses, low fitness was classified as less than 25.7 mL/kg per minute and less than 30.7 m/kg per minute for women and men, respectively. Because the ROC-derived thresholds for BMI, WC, and %BF were located close to commonly used and extensively validated diagnostic thresholds, we used a BMI of 25 kg/m2 or higher, WC of 88 cm or greater for women and 102 cm or greater for men, and %BF of 35% or more and 25% or more for women and men, respectively39 as thresholds for fatness status.
Logistic regression analyses were used to estimate adjusted odds ratios (ORs) and 95% CIs for having high cardiometabolic risk according to independent and combined categories of fitness, BMI, WC, and %BF. To ensure sufficient power, analyses for women and men were pooled. The pattern and effect sizes of the association between fitness, fatness, and high cardiometabolic risk in sex-specific analyses were very similar to those observed in the pooled analyses, and no significant interactions between sex and other covariates were observed (all P>.05).
Analyses were adjusted for age, sex, smoking habits, alcohol intake, and PA. Categories were nonsmoker/smoker and non–heavy drinker/heavy drinker (>7 and >10 alcohol U/wk in women and men, respectively). Habitual PA was assessed objectively by Actigraph GT3X+ accelerometer over the course of 7 consecutive days, as described in detail previously.40 An accumulated time of 150 min/wk or more spent in moderate- to vigorous-intensity PA was considered as meeting PA recommendations based on the current American College of Sports Medicine/American Heart Association guidelines for older adults.41 To assess the robustness of our findings, all analyses were additionally run with single cardiometabolic risk factors (ie, elevated TG level, reduced HDL-C level, elevated BP, and elevated fasting glucose level) as outcome variables. To examine the robustness of the fitness thresholds, additional ROC curve analyses were run for subgroups according to levels of PA, education, alcohol consumption, no prescription medication use, and use of at least one prescription medication. Furthermore, because glycated hemoglobin (HbA1c) has been proposed to be a more accurate glycemic indicator than fasting plasma glucose level,42 main analyses were additionally performed using HbA1c levels of 6% or higher43 or pharmacological treatment of diabetes instead of elevated fasting glucose level as an outcome variable.
All statistical tests were 2-sided, and P<.05 was considered statistically significant. The statistical analyses were conducted using SPSS predictive analytics software, version 23, statistical package for social sciences (IBM).
Results
The characteristics of the 922 study participants are presented in Table 1. The mean age was 73.0 years for women and 72.9 years for men, with 54.8% of the sample (505) being women, 44.5% of all participants (410) being overweight (BMI, 25.0-29.9 kg/m2), and 11.3% (104) being obese (BMI, ≥30 kg/m2). The mean VO2peak was 26.5±5.0 mL/kg per minute in women and 31.9±6.5 mL/kg per minute in men. Overall, 41.6% of women (210) and 49.6% of men (207) were at high cardiometabolic risk (ie, ≥2 risk factors present); 25.1% of the women (127) and 29.7% of the men (124) were pharmacologically treated for HTN, 3.2% of the women (16) and 8.6% of the men (36) for T2D, and 5.7% of the women (29) and 7.0% of the men (29) for dyslipidemia.
Table 1.
| Variable | Women (n=505) | Men (n=417) |
|---|---|---|
| Age (y) | 73.0±2.1 | 72.9±2.1 |
| Peak oxygen uptake (mL/kg/min) | 26.5±5.0 | 31.9±6.5 |
| Body mass index (kg/m2) | 25.4±3.7 | 26.3±3.3 |
| Underweight (<18.5) | 5 (1.0) | 3 (0.7) |
| Normal weight (18.5-24.9) | 251 (49.7) | 149 (35.7) |
| Overweight (25.0-29.9) | 192 (38.0) | 218 (52.3) |
| Obese class I (30.0-34.9) | 50 (9.9) | 41 (9.8) |
| Obese class II and III (≥35.0) | 7 (1.4) | 6 (1.4) |
| Waist circumference (cm) | 89.6±10.9 | 98.0±9.7 |
| Percent body fat | 34.5±6.9 | 25.3±6.5 |
| Muscle mass (kg) | 23.6±2.6 | 33.7±3.7 |
| Triglycerides (mmol/L) | 1.12±0.58 | 1.19±0.60 |
| Elevatedc | 96 (19.0) | 94 (22.5) |
| High-density lipoprotein cholesterol (mmol/L) | 1.92±0.51 | 1.55±0.41 |
| Reducedc | 78 (15.4) | 45 (10.8) |
| Blood pressure (mm Hg) | ||
| Systolic | 133±18 | 132±16 |
| Diastolic | 73±9 | 77±9 |
| Elevatedc | 301 (59.6) | 266 (63.8) |
| Fasting glucose (mg/dL) | 5.54±0.58 | 5.87±0.79 |
| Elevatedc | 169/369 (45.8) | 196/307 (63.8) |
| High cardiometabolic riskc | 210 (41.6) | 207 (49.6) |
| Current smoker | 38/488 (7.8) | 37/406 (9.1) |
| Heavy drinkerd | 40/483 (8.3) | 34/404 (8.4) |
| Moderate to vigorous physical activity (min/d) | 19.3±19.8 | 19.2±20.8 |
| Meeting physical activity recommendationse | 146/409 (35.7) | 112/331 (33.8) |
Data are presented as No. (percentage) of participants or mean ± SD.
SI conversion factors: To convert glucose values to mmol/L, multiply by 0.0555.
Elevated triglyceride level defined as ≥1.7 mmol/L or pharmacological treatment of dyslipidemia; reduced high-density lipoprotein cholesterol level defined as <1.3 mmol/L in women and <1.0 mmol/L in men or pharmacological treatment of dyslipidemia; elevated blood pressure defined as systolic blood pressure ≥130 mm Hg and/or diastolic blood pressure ≥85 mm Hg or pharmacological treatment of hypertension; elevated fasting glucose level defined as ≥100 mg/dL or pharmacological treatment of diabetes; high cardiometabolic risk defined as ≥2 of the 4 risk factors (triglyceride, high-density lipoprotein cholesterol, blood pressure, and fasting glucose levels).
Heavy drinker was defined as >7 alcohol U/wk for women and >10 alcohol U/wk for men.
Defined as ≥150 min/wk of moderate and vigorous physical activity assessed by accelerometer.
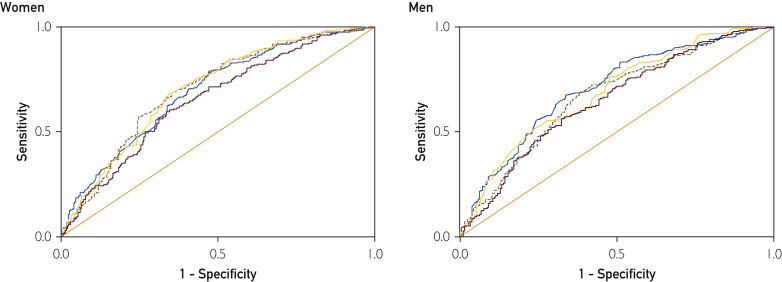
The ROC curve analyses revealed significant discriminatory accuracy of fitness, BMI, WC, and %BF to identify individuals with high cardiometabolic risk, with AUC for all parameters being significantly greater than 0.5 (Table 2; all P<.001). The Figure presents the ROC curves for fitness and fatness. The curves have similar shapes and AUC with overlapping 95% CI, suggesting that fitness and fatness have similar discriminatory utility in identifying individuals with high cardiometabolic risk.
Table 2.
| Variable | Area under the curve (95% CI) | Optimal threshold | Sensitivity | Specificity |
|---|---|---|---|---|
| VO2peak (mL/kg/min) | ||||
| Women | 0.65 (0.60-0.70) | 25.7 | 0.60 | 0.66 |
| Men | 0.65 (0.61-0.70) | 30.7 | 0.57 | 0.68 |
| Body mass index (kg/m2) | ||||
| Women | 0.70 (0.66-0.75) | 25.1 | 0.68 | 0.65 |
| Men | 0.69 (0.64-0.74) | 25.1 | 0.76 | 0.52 |
| Waist circumference (cm) | ||||
| Women | 0.70 (0.66-0.75) | 88.6 | 0.70 | 0.63 |
| Men | 0.67 (0.62-0.72) | 96.6 | 0.69 | 0.62 |
| Percent body fat | ||||
| Women | 0.69 (0.65-0.74) | 33.3 | 0.78 | 0.53 |
| Men | 0.71 (0.66-0.76) | 25.3 | 0.68 | 0.66 |
HDL = high-density lipoprotein; ROC = receiver operating characteristic; VO2peak = peak oxygen uptake.
High cardiometabolic risk defined as the presence of at least 2 of the following 4 risk factors: elevated triglyceride level (≥1.7 mmol/L) or pharmacological treatment of dyslipidemia; reduced HDL cholesterol (<1.3 mmol/L in women and <1.0 mmol/L in men) or pharmacological treatment of dyslipidemia; elevated blood pressure (systolic ≥130 mm Hg and/or diastolic ≥85 mm Hg) or pharmacological treatment of hypertension; and elevated fasting glucose level (≥100 mg/dL [to convert to mmol/L, multiply by 0.0555]) or pharmacological treatment of diabetes.
Figure.
Receiver operating characteristic curves for cardiorespiratory fitness (brown line), body mass index (yellow line), waist circumference (brown dashed line), and percent body fat (blue line) for the prediction of high cardiometabolic risk in 505 women and 417 men aged 70 to 77 years. High cardiometabolic risk was defined as the presence of at least 2 of the following 4 risk factors: elevated triglyceride level (≥1.7 mmol/L) or pharmacological treatment of dyslipidemia; reduced high-density lipoprotein cholesterol level (<1.3 mmol/L in women and <1.0 mmol/L in men) or pharmacological treatment of dyslipidemia; elevated blood pressure (systolic ≥130 mm Hg and/or diastolic ≥85 mm Hg) or pharmacological treatment of hypertension; and elevated fasting glucose level (≥100 mg/dL [to convert to mmol/L, multiply by 0.0555]) or pharmacological treatment of diabetes.
For fitness as a discriminatory test of high cardiometabolic risk, the optimal pairs of true-positive (sensitivity) and false-positive (1 − specificity) rates were 0.60 and 0.34, respectively, in women and 0.57 and 0.32, respectively, in men. The fitness values at these points were 25.7 mL/kg per minute in women and 30.7 mL/kg per minute in men (Table 2). Optimal fitness thresholds to discriminate participants with elevated TG levels, reduced HDL-C levels, elevated BP, and elevated fasting glucose levels in women were similar (range, 24.5-26.2 mL/kg per minute in women and 30.6-31.1 mL/kg per minute in men; Supplemental Table 1, available online at http://www.mcpiqojournal.org).
Fitness levels lower than 25.7 mL/kg per minute in women and lower than 30.7 mL/kg per minute in men were associated with 2.8-fold higher odds of having high cardiometabolic risk (OR, 2.77; 95% CI, 2.09-3.66) after adjustment for age, sex, smoking, alcohol consumption, and PA (Table 3, model 1; P<.001). This association persisted after additional adjustment for BMI (Table 3, model 2), WC, and %BF (data not shown), suggesting an independent association between fitness and high cardiometabolic risk.
Table 3.
| Variable | Model 1, OR (95% CI) | Model 2, OR (95% CI) |
|---|---|---|
| Low peak oxygen uptake | ||
| Women (<25.7 mL/kg/min) | 2.89 (1.96-4.24) | 2.12 (1.41-3.20) |
| Men (<30.7 mL/kg/min) | 2.71 (1.79-4.12) | 2.04 (1.31-3.18) |
| Pooled | 2.77 (2.09-3.66) | 2.06 (1.53-2.77) |
| High body mass index | ||
| Women (≥25 kg/m) | 3.86 (2.62-5.70) | 3.17 (2.11-4.75) |
| Men (≥25 kg/m) | 3.33 (2.16-5.12) | 2.72 (1.73-4.28) |
| Pooled | 3.58 (2.69-4.77) | 2.94 (2.18-3.97) |
| High waist circumference | ||
| Women (≥88 cm) | 3.74 (2.51-5.58) | 3.03 (2.00-4.59) |
| Men (≥102 cm) | 2.44 (1.57-3.78) | 1.79 (1.11-2.87) |
| Pooled | 3.06 (2.29-4.10) | 2.39 (1.75-3.25) |
| High percent body fat | ||
| Women (≥35%) | 3.02 (2.06-4.43) | 2.29 (1.51-3.46) |
| Men (≥25%) | 3.60 (2.38-5.44) | 2.92 (1.85-4.62) |
| Pooled | 3.26 (2.47-4.30) | 2.54 (1.87-3.45) |
HDL = high-density lipoprotein; OR = odds ratio.
High cardiometabolic risk defined as the presence of at least 2 of the following 4 risk factors: elevated triglyceride level (≥1.7 mmol/L) or pharmacological treatment of dyslipidemia; reduced HDL cholesterol (<1.3 mmol/L in women and <1.0 mmol/L in men) or pharmacological treatment of dyslipidemia; elevated blood pressure (systolic ≥130 mm Hg and/or diastolic ≥85 mm Hg) or pharmacological treatment of hypertension; and elevated fasting glucose level (≥100 mg/dL [to convert to mmol/L, multiply by 0.0555]) or pharmacological treatment of diabetes. Odds ratios are compared to the reference categories of either high peak oxygen uptake, low body mass index, low waist circumference, or low percent body fat. Model 1: Adjusted for age, smoking status, alcohol consumption, and physical activity (meeting/not meeting recommendation for moderate to vigorous physical activity). Pooled analyses for women and men are additionally adjusted for sex. Model 2: Adjusted as model 1 plus body mass index (entered as categorical variable) for peak oxygen uptake and peak oxygen uptake (entered as categorical variable) for body mass index, waist circumference, and percent body fat.
All 3 indices of fatness were significantly and positively associated with cardiometabolic risk (Table 3, model 1; all P<.001). High BMI (≥25 kg/m) was associated with a 3.6-fold higher odds of having high cardiometabolic risk (OR, 3.58; 95% CI, 2.69-4.77), while high WC (≥88 cm in women and ≥102 cm in men) and high %BF (≥35% in women and ≥25% in men) had 3.1-fold (OR, 3.06; 95% CI, 2.29-4.10) and 3.3-fold (OR, 3.26; 95% CI, 2.47-4.30) higher odds of having high cardiometabolic risk, respectively. The association between indices of fatness and high cardiometabolic risk persisted after additional adjustment for fitness. (Table 3, model 2).
Significant associations between fitness or fatness and single cardiometabolic risk factors (ie, elevated TG, reduced HDL-C, elevated BP, and elevated glucose levels) support these results (Supplemental Table 2, available online at http://www.mcpiqojournal.org). Effect sizes were similar to those observed for the association between fitness, fatness, and the cardiometabolic risk composite score.
In combined analyses (Table 4), the highest odds of having high cardiometabolic risk were observed in combined categories of both low fitness and high BMI, WC, or %BF. Participants with low fitness and high BMI had 6.1-fold higher odds of having high cardiometabolic risk (OR, 6.13; 95% CI, 4.23-8.80) compared with individuals with high fitness and low BMI. However, having normal BMI, WC, and %BF did not eliminate the cardiometabolic risk associated with low fitness, and similarly, having high fitness did not attenuate the risk associated with fatness. Specifically, lean individuals with low fitness and fit individuals with indication of fatness still had significantly higher odds of having high cardiometabolic risk than lean individuals with high fitness (all P<.05).
Table 4.
Adjusted Odds Ratiosa (95% CIs) for High Cardiometabolic Riskb According to Combined Categories of Fitness and Fatness
| Variable | Peak oxygen uptakec |
|
|---|---|---|
| High | Low | |
| Body mass indexd | ||
| Low | 1.00 (Reference) | 2.55 (1.57-4.12) |
| High | 3.36 (2.29-4.94) | 6.13 (4.23-8.80) |
| Waist circumferencee | ||
| Low | 1.00 (Reference) | 3.21 (2.12-4.85) |
| High | 3.59 (2.36-5.44) | 4.90 (3.40-7.04) |
| Percent body fatf | ||
| Low | 1.00 (Reference) | 3.01 (1.84-4.90) |
| High | 3.45 (2.32-5.11) | 4.80 (3.41-6.77) |
Odds ratios adjusted for sex, age, smoking status, alcohol consumption, and physical activity.
High cardiometabolic risk defined as the presence of at least 2 of the following 4 risk factors: elevated triglyceride level (≥1.7 mmol/L) or pharmacological treatment of dyslipidemia; reduced high-density lipoprotein cholesterol (<1.3 mmol/L in women and <1.0 mmol/L in men) or pharmacological treatment of dyslipidemia; elevated blood pressure (systolic ≥130 mm Hg and/or diastolic ≥85 mm Hg) or pharmacological treatment of hypertension; and elevated fasting glucose level (≥100 mg/dL [to convert to mmol/L, multiply by 0.0555]) or pharmacological treatment of diabetes.
Low peak oxygen uptake defined as <25.7 mL/kg/min in women and <30.7 mL/kg/min in men.
High body mass index defined as ≥25 kg/m2.
High waist circumference defined as ≥88 cm in women and ≥102 cm in men.
High percent body fat defined as ≥35% in women and 25% in men.
A similar pattern was observed for the associations of combined categories of fitness and fatness and the odds of having elevated TG, reduced HDL-C, and elevated BP levels (Supplemental Table 3, available online at http://www.mcpiqojournal.org). The association between high BMI, WC, or %BF and elevated fasting glucose level, however, was somewhat stronger than for low fitness. Specifically, lean individuals did not have significantly increased odds of having elevated fasting glucose levels associated with low fitness (P=.09), while the association between BMI, WC, %BF, and elevated fasting glucose remained significant in fit individuals (P=.002).
Additional ROC curve analyses with fitness as a discriminatory test of high cardiometabolic risk in subgroups of (1) meeting and not meeting PA recommendations, (2) higher education and no higher education, (3) low and high alcohol consumption, and (4) no prescription medication use and use of at least one prescription medication yielded optimal fitness thresholds similar to those observed for the total sample (Supplemental Table 4, available online at http://www.mcpiqojournal.org). Optimal fitness thresholds varied from 25 to 28 mL/kg per minute in women and from 30 to 34 mL/kg per minute in men, with AUC for all parameters being significantly greater than 0.5 (P<.05). Supplemental analyses using HbA1c levels of 6% or higher43 or pharmacological treatment of diabetes yielded results similar to those observed for elevated fasting glucose level as outcome variable. Individuals with low fitness, high BMI, high WC, or high %BF had ORs (95% CIs) of 1.83 (1.07-2.22), 1.91 (1.28-2.86), 2.27 (1.53-3.36), and 1.67 (1.14-2.45), respectively, for having high cardiometabolic risk.
Discussion
The primary findings of this study were that fitness and fatness were independently associated with cardiometabolic risk factors and that a combination of low fitness and either high BMI, WC, or %BF cumulatively increased the cardiometabolic risk. These associations persisted after accounting for possible confounders, including PA, smoking habits, and alcohol consumption. Moreover, our results suggest critical fitness thresholds for having high cardiometabolic risk in older adults, derived from the currently largest data set of directly measured fitness by gas exchange in older adults worldwide.
Health Criterion Thresholds for Cardiorespiratory Fitness
Most of the previously published studies defined low fitness on the basis of sample distributions (eg, lowest 20%) and/or used indirect estimates of fitness.10, 11, 12, 14, 15, 23, 26, 27 In this study, we identified and validated theoretical fitness thresholds of 25.7 mL/kg per minute in women and 30.7 mL/kg per minute in men, which had significant discriminatory accuracy for identification of individuals at high cardiometabolic risk. The generated ROC curve yielded a significant AUC, with acceptable magnitude similar to other studies using comparable methodology.25, 44 Furthermore, the fitness thresholds for having high cardiometabolic risk documented robustness to the single components of the composite outcome, such as elevated TG, reduced HDL-C, elevated BP, and partly elevated fasting glucose levels. The 3 indices of fatness displayed discriminatory accuracy similar to that observed for fitness, and ROC curves generated thresholds that were located close to frequently used diagnostic criteria and guidelines,39 further supporting our results. Health criterion thresholds for age-specific fitness could potentially be implemented in future health promotion efforts as a simple guideline for lifestyle modifications.
Fitness and Cardiometabolic Risk
The observation that fitness had a strong and independent inverse association with cardiometabolic health in older adults is in line with findings from several other studies that have documented the protective effect of high fitness on CVD morbidity and mortality9, 10, 11, 12, 13, 14, 15 and cardiometabolic risk factors associated with CVD.26, 27, 29, 45, 46, 47, 48, 49 Our data expand these observations by illustrating the association between fitness and cardiometabolic risk factors in the largest data set of directly measured fitness in older adults (≥70 years). In particular, we also included a high number of older women. Furthermore, we found that having normal BMI, WC, and %BF did not attenuate the deleterious association between low fitness and cardiometabolic risk. Specifically, lean individuals still had higher odds of having high cardiometabolic risk associated with low fitness, suggesting that being lean per se might not be enough to ensure optimal CV health if participants did not have a certain level of fitness. Thus, having high age-specific fitness is a key factor for good cardiometabolic health in older adults, regardless of BMI, body composition, and other lifestyle factors.
Combined Associations of Fitness and Fatness With Cardiometabolic Risk
High body fatness is one of the most important contributors to the clustering of adverse cardiometabolic risk factors and metabolic syndrome.7 Our findings support this observation but additionally highlight a mediating effect of fitness. Within strata of normal or high BMI, WC, and %BF, higher levels of fitness were associated with lower odds of having cardiometabolic risk factors. Although low fitness and indication of body fatness carried approximately the same risk, a combination of both had a cumulative effect on cardiometabolic risk in our study.
Relatively few studies have examined the combined associations of fitness and fatness with cardiometabolic risk factors associated with CVD. In some, low fitness is found to have approximately the same risk as fatness for prevalence of metabolic syndrome23, 24 or incidence of T2D.25, 26, 27 We expand these observations to apply to older adults as well. Our findings are novel because this is the first study to assess the independent and combined associations of fitness and fatness with cardiometabolic risk factors specifically in older adults, using direct measures of fitness and different indices of body fatness, namely BMI, WC, and %BF.
Other studies, however, found a greater magnitude of association between fatness and poor cardiometabolic health than low fitness.28, 29, 30 The inconsistent evidence on this topic may reflect complex genetic, biological, and behavioral interactions that affect disease progression and subsequent morbidity and mortality in epidemiological studies. Findings may also be influenced by methodology, such as the use of different indices of fitness and fatness, as well as the categorization into discrete predictor variables despite their continuous nature.
Body fatness has consistently been associated with adverse cardiometabolic risk factor outcomes, even after accounting for fitness.16, 23, 24, 28, 29, 30 This association was also found in the present study and contrasts data from most previous studies with mortality outcomes. The fat but fit paradigm suggests that having high fitness may attenuate most of the adverse effects of obesity on all-cause and CVD morbidity and mortality.16, 17, 18, 19, 20, 21, 22 Furthermore, overweight and even mild obesity may be associated with better survival, especially in older populations.20, 22 Together these findings raise the possibility of a disparate effect of fitness and fatness on risk factors for CVD as compared with CVD events and especially mortality in an older cohort.22
Exercise training and PA are the common recommendation to increase fitness and reduce fatness, and promotion of exercise and PA would potentially reduce cardiometabolic risk as well as CVD morbidity and mortality.17, 18, 20, 21, 22, 50, 51, 52 However, in the current study, adjustment for PA did not substantially change the associations between low fitness and fatness and cardiometabolic risk, indicating that meeting the current PA recommendations41 per se may not be sufficient to ensure optimal cardiometabolic health.53 Instead, a greater focus may be placed on the desired outcome of PA (eg, enhanced fitness and/or weight control).
Study Limitations
Our sample was limited to individuals who were able to perform a maximal cardiopulmonary exercise test, and most of the participants were white, well educated, and from middle to upper socioeconomic strata.34 A comparison of participants and nonparticipants has been made in the protocol paper of the Generation 100 study.34 Although our participants' levels of fitness corresponded well to those reported in an independent Norwegian population (the Nord-Trøndelag Health Study 3),1 they are higher than those reported in an US population.54 Thus, our sample may be more fit than other populations of older adults. Furthermore, subclinical disease, which is highly prevalent in older adults,55 might affect study outcomes. Conversely, our study population was diverse and included both healthy individuals and those with comorbidities. Furthermore, supplementary analyses indicate that the fitness thresholds presented in the current study were relatively robust across populations with different characteristics, including subgroups with presumably poorer health than observed in the total sample of this study.
The use of fasting glucose level as an indicator of impaired glycemic control may be a limitation of our study. The association between fitness and fasting glucose level was less robust than that observed for elevated TG, reduced HDL-C, and elevated BP levels, indicating a possible disparate effect of fitness and fatness on the different cardiometabolic risk factors used in this study. Furthermore, HbA1c has been proposed to be a more accurate glycemic indicator than fasting plasma glucose level.42 However, additional sensitivity analyses using elevated HbA1c levels instead of elevated glucose levels as outcome variable yielded the same conclusions as presented in this study, although the strength of associations was slightly attenuated.
Finally, the cross-sectional design does not allow us to determine whether fitness and fatness prospectively predict development of cardiometabolic risk factors, and the causal interplay between fitness, fatness, and cardiometabolic health and consequent effects on CVD events or mortality warrants further investigation in older adults.
Conclusion
Our findings illustrate that low fitness and indication of body fatness are independently and cumulatively associated with cardiometabolic risk factors in older adults. Our results also emphasize the importance of including both fitness and body fatness in the assessment of cardiometabolic risk and health promotion efforts in older adults. In such assessments, fitness levels below 25.7 mL/kg per minute in women and 30.7 mL/kg per minute in men may represent critical thresholds for poor cardiometabolic health in older adults.
Acknowledgments
The cardiopulmonary tests were performed at the core facility NeXt Move, Norwegian University of Science and Technology. We thank the Clinical Research Facility at St. Olavs Hospital for excellent assistance during the testing periods and all personnel for contributions to the collection of data. We are also indebted to the participants of the Generation 100 study.
The funding organizations had no role in the design and execution of the study, in the collection, analysis, and interpretation of the data or in the preparation, review, or approval of the manuscript.
Footnotes
Grant Support: This study was funded by grants from the K. G. Jebsen Foundation, the Research Council of Norway, and the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology.
Potential Competing Interests: Dr Wisløff has minor ownership interests in Mio Global. Dr Lavie has published a book on the obesity paradox with potential royalties. The rest of the authors report no competing interests.
Supplemental Online Material
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Aspenes S.T., Nilsen T.I., Skaug E.A., et al. Peak oxygen uptake and cardiovascular risk factors in 4631 healthy women and men. Med Sci Sports Exerc. 2011;43(8):1465–1473. doi: 10.1249/MSS.0b013e31820ca81c. [DOI] [PubMed] [Google Scholar]
- 2.Fleg J.L., Morrell C.H., Bos A.G., et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 3.Fakhouri T.H., Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of obesity among older adults in the United States, 2007-2010. NCHS Data Brief. 2012;(106):1–8. [PubMed] [Google Scholar]
- 4.Carlson S.A., Fulton J.E., Pratt M., Yang Z., Adams E.K. Inadequate physical activity and health care expenditures in the United States. Prog Cardiovasc Dis. 2015;57(4):315–323. doi: 10.1016/j.pcad.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozaffarian D., Benjamin E.J., Go A.S., et al. American Heart Association Statistics Committee and Stroke Statistics Committee Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [published correction appears in Circulation. 2016;133(15):e599] [DOI] [PubMed] [Google Scholar]
- 7.Ortega F.B., Lavie C.J., Blair S.N. Obesity and cardiovascular disease. Circ Res. 2016;118(11):1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 8.Ross R., Blair S.N., Arena R., et al. American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health. Council on Clinical Cardiology. Council on Epidemiology and Prevention. Council on Cardiovascular and Stroke Nursing. Council on Functional Genomics and Translational Biology. Stroke Council Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign; a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 9.Myers J., McAuley P., Lavie C.J., Despres J.P., Arena R., Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis. 2015;57(4):306–314. doi: 10.1016/j.pcad.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Sui X., LaMonte M.J., Laditka J.N., et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298(21):2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei M., Kampert J.B., Barlow C.E., et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282(16):1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 12.Lee C.D., Blair S.N., Jackson A.S. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69(3):373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 13.Farrell S.W., Fitzgerald S.J., McAuley P.A., Barlow C.E. Cardiorespiratory fitness, adiposity, and all-cause mortality in women. Med Sci Sports Exerc. 2010;42(11):2006–2012. doi: 10.1249/MSS.0b013e3181df12bf. [DOI] [PubMed] [Google Scholar]
- 14.McAuley P., Pittsley J., Myers J., Abella J., Froelicher V.F. Fitness and fatness as mortality predictors in healthy older men: the Veterans Exercise Testing Study. J Gerontol A Biol Sci Med Sci. 2009;64(6):695–699. doi: 10.1093/gerona/gln039. [DOI] [PubMed] [Google Scholar]
- 15.Lee D.C., Sui X., Ortega F.B., et al. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br J Sports Med. 2011;45(6):504–510. doi: 10.1136/bjsm.2009.066209. [DOI] [PubMed] [Google Scholar]
- 16.Fogelholm M. Physical activity, fitness and fatness: relations to mortality, morbidity and disease risk factors; a systematic review. Obes Rev. 2010;11(3):202–221. doi: 10.1111/j.1467-789X.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- 17.Barry V.W., Baruth M., Beets M.W., Durstine J.L., Liu J., Blair S.N. Fitness vs. fatness on all-cause mortality: a meta-analysis. Prog Cardiovasc Dis. 2014;56(4):382–390. doi: 10.1016/j.pcad.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 18.McAuley P.A., Beavers K.M. Contribution of cardiorespiratory fitness to the obesity paradox. Prog Cardiovasc Dis. 2014;56(4):434–440. doi: 10.1016/j.pcad.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Ricketts T.A., Sui X., Lavie C.J., Blair S.N., Ross R. Addition of cardiorespiratory fitness within an obesity risk classification model identifies men at increased risk of all-cause mortality. Am J Med. 2016;129(5):536. doi: 10.1016/j.amjmed.2015.11.015. e13-e20. [DOI] [PubMed] [Google Scholar]
- 20.Lavie C.J., De Schutter A., Parto P., et al. Obesity and prevalence of cardiovascular diseases and prognosis—the obesity paradox updated. Prog Cardiovasc Dis. 2016;58(5):537–547. doi: 10.1016/j.pcad.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Lavie C.J., McAuley P.A., Church T.S., Milani R.V., Blair S.N. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63(14):1345–1354. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Jahangir E., De Schutter A., Lavie C.J. Low weight and overweightness in older adults: risk and clinical management. Prog Cardiovasc Dis. 2014;57(2):127–133. doi: 10.1016/j.pcad.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Church T.S., Finley C.E., Earnest C.P., Kampert J.B., Gibbons L.W., Blair S.N. Relative associations of fitness and fatness to fibrinogen, white blood cell count, uric acid and metabolic syndrome. Int J Obes Relat Metab Disord. 2002;26(6):805–813. doi: 10.1038/sj.ijo.0802001. [DOI] [PubMed] [Google Scholar]
- 24.Kim S., Kim J.Y., Lee D.C., Lee H.S., Lee J.W., Jeon J.Y. Combined impact of cardiorespiratory fitness and visceral adiposity on metabolic syndrome in overweight and obese adults in Korea. PLoS One. 2014;9(1):e85742. doi: 10.1371/journal.pone.0085742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katzmarzyk P.T., Craig C.L., Gauvin L. Adiposity, physical fitness and incident diabetes: the Physical Activity Longitudinal Study. Diabetologia. 2007;50(3):538–544. doi: 10.1007/s00125-006-0554-3. [DOI] [PubMed] [Google Scholar]
- 26.Sui X., Hooker S.P., Lee I.M., et al. A prospective study of cardiorespiratory fitness and risk of type 2 diabetes in women. Diabetes Care. 2008;31(3):550–555. doi: 10.2337/dc07-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee D.C., Sui X., Church T.S., Lee I.M., Blair S.N. Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care. 2009;32(2):257–262. doi: 10.2337/dc08-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz V.A., Player M.S., Mainous A.G., III, Carek P.J., Geesey M.E. Competing impact of excess weight versus cardiorespiratory fitness on cardiovascular risk. Am J Cardiol. 2006;98(11):1468–1471. doi: 10.1016/j.amjcard.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 29.Racette S.B., Evans E.M., Weiss E.P., Hagberg J.M., Holloszy J.O. Abdominal adiposity is a stronger predictor of insulin resistance than fitness among 50-95 year olds. Diabetes Care. 2006;29(3):673–678. doi: 10.2337/diacare.29.03.06.dc05-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christou D.D., Gentile C.L., DeSouza C.A., Seals D.R., Gates P.E. Fatness is a better predictor of cardiovascular disease risk factor profile than aerobic fitness in healthy men. Circulation. 2005;111(15):1904–1914. doi: 10.1161/01.CIR.0000161818.28974.1A. [DOI] [PubMed] [Google Scholar]
- 31.Ortega F.B., Sui X., Lavie C.J., Blair S.N. Body mass index, the most widely used but also widely criticized index: would a criterion standard measure of total body fat be a better predictor of cardiovascular disease mortality? Mayo Clin Proc. 2016;91(4):443–455. doi: 10.1016/j.mayocp.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paffenbarger R.S., Jr., Blair S.N., Lee I.M., Hyde R.T. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25(1):60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 33.DeFina L.F., Haskell W.L., Willis B.L., et al. Physical activity versus cardiorespiratory fitness: two (partly) distinct components of cardiovascular health? Prog Cardiovasc Dis. 2015;57(4):324–329. doi: 10.1016/j.pcad.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Stensvold D., Viken H., Rognmo Ø., et al. A randomised controlled study of the long-term effects of exercise training on mortality in elderly people: study protocol for the Generation 100 study. BMJ Open. 2015;5(2):e007519. doi: 10.1136/bmjopen-2014-007519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wisløff U., Støylen A., Loennechen J.P., et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 36.Alberti K.G., Eckel R.H., Grundy S.M., et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 37.Bewick V., Cheek L., Ball J. Statistics review 13: receiver operating characteristic curves. Crit Care. 2004;8(6):508–512. doi: 10.1186/cc3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fluss R., Faraggi D., Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 39.Bray G.A. Fat distribution and body weight. Obes Res. 1993;1(3):203–205. doi: 10.1002/j.1550-8528.1993.tb00613.x. [editorial] [DOI] [PubMed] [Google Scholar]
- 40.Sandbakk S.B., Nauman J., Zisko N., et al. Sedentary time, cardiorespiratory fitness, and cardiovascular risk factor clustering in older adults—the Generation 100 Study. Mayo Clin Proc. 2016;91(11):1525–1534. doi: 10.1016/j.mayocp.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 41.Nelson M.E., Rejeski W.J., Blair S.N., et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 42.Martínez-Vizcaíno V., Cavero-Redondo I., Álvarez-Bueno C., Rodríguez-Artalejo F. The accuracy of diagnostic methods for diabetic retinopathy: a systematic review and meta-analysis. PLoS One. 2016;11(4):e0154411. doi: 10.1371/journal.pone.0154411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz J.R., Ortega F.B., Rizzo N.S., et al. High cardiovascular fitness is associated with low metabolic risk score in children: the European Youth Heart Study. Pediatr Res. 2007;61(3):350–355. doi: 10.1203/pdr.0b013e318030d1bd. [DOI] [PubMed] [Google Scholar]
- 45.Wei M., Gibbons L.W., Mitchell T.L., Kampert J.B., Lee C.D., Blair S.N. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med. 1999;130(2):89–96. doi: 10.7326/0003-4819-130-2-199901190-00002. [published correction appears in Ann Intern Med. 1999;131(5):394] [DOI] [PubMed] [Google Scholar]
- 46.Liu J., Sui X., Lavie C.J., et al. Effects of cardiorespiratory fitness on blood pressure trajectory with aging in a cohort of healthy men. J Am Coll Cardiol. 2014;64(12):1245–1253. doi: 10.1016/j.jacc.2014.06.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park Y.M., Sui X., Liu J., et al. The effect of cardiorespiratory fitness on age-related lipids and lipoproteins. J Am Coll Cardiol. 2015;65(19):2091–2100. doi: 10.1016/j.jacc.2015.03.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parto P., Lavie C.J., Swift D., Sui X. The role of cardiorespiratory fitness on plasma lipid levels. Expert Rev Cardiovasc Ther. 2015;13(11):1177–1183. doi: 10.1586/14779072.2015.1092384. [DOI] [PubMed] [Google Scholar]
- 49.Kokkinos P. Cardiorespiratory fitness, exercise, and blood pressure. Hypertension. 2014;64(6):1160–1164. doi: 10.1161/HYPERTENSIONAHA.114.03616. [DOI] [PubMed] [Google Scholar]
- 50.Lavie C.J., Arena R., Swift D.L., et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117(2):207–219. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vuori I.M., Lavie C.J., Blair S.N. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88(12):1446–1461. doi: 10.1016/j.mayocp.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 52.Sallis R., Franklin B., Joy L., Ross R., Sabgir D., Stone J. Strategies for promoting physical activity in clinical practice. Prog Cardiovasc Dis. 2015;57(4):375–386. doi: 10.1016/j.pcad.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Moholdt T., Wisløff U., Lydersen S., Nauman J. Current physical activity guidelines for health are insufficient to mitigate long-term weight gain: more data in the fitness versus fatness debate (the HUNT study, Norway) Br J Sports Med. 2014;48(20):1489–1496. doi: 10.1136/bjsports-2014-093416. [DOI] [PubMed] [Google Scholar]
- 54.Kaminsky L.A., Arena R., Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the Fitness Registry and the Importance of Exercise National Database. Mayo Clin Proc. 2015;90(11):1515–1523. doi: 10.1016/j.mayocp.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuller L.H., Arnold A.M., Psaty B.M., et al. 10-Year follow-up of subclinical cardiovascular disease and risk of coronary heart disease in the Cardiovascular Health Study. Arch Intern Med. 2006;166(1):71–78. doi: 10.1001/archinte.166.1.71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.