Abstract
Objective
To analyze contemporary trends in the incidence, management, and clinical outcomes of heart failure (HF) complications in patients hospitalized for ST-segment elevation myocardial infarction (STEMI) in the United States.
Patients and Methods
Using the 2003 through 2010 Nationwide Inpatient Sample databases, all patients with STEMI who were 18 years and older with acute HF were identified. Overall trends in the incidence of HF, coronary intervention, and in-hospital mortality were analyzed.
Results
Of 1,990,002 hospitalizations with a primary diagnosis of STEMI, 471,525 (23.7%) had HF complication (decreasing from 25.4% [95% CI, 25.3%-25.6%] in 2003 to 20.7% [95% CI, 20.5%-20.8%]) in 2010 (P trend<.001). The incidence of cardiogenic shock in patients with HF-complicated STEMI increased from 13.9% (95% CI, 13.6%-14.1%) to 22.6% (95% CI, 22.2%-23.0%) during this period (P trend<.001). From 2003 through 2010, the use of diagnostic coronary angiography and percutaneous coronary intervention increased in patients with HF-complicated STEMI from 44.3% to 62.1% and from 25.0% to 48.1%, respectively. In-hospital mortality decreased significantly in patients with HF-complicated STEMI (from 18.1% to 15.1%) and in subgroups of those with (from 42.4% to 29.9%) and without (from 14.1% to 10.8%) cardiogenic shock (all P trend<.001). The adjusted odds ratio (AOR) (per year) of death was 0.992 (95% CI, 0.988-0.997; P<.001), which changed significantly after additional adjustment for coronary intervention (AOR [per year], 1.012; 95% CI, 1.008-1.017; P<.001).
Conclusion
The incidence and in-hospital mortality of HF-complicated STEMI has decreased significantly during recent times along with increased use of percutaneous coronary intervention and diagnostic coronary angiography.
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; AMI, acute myocardial infarction; AOR, adjusted odds ratio; CABG, coronary artery bypass graft; CPI, consumer price index; CV, cardiovascular; dCA, diagnostic coronary angiography; HF, heart failure; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; IQR, interquartile range; LOS, length of stay; NIS, Nationwide Inpatient Sample; NRMI-2, National Registry of Myocardial Infarction-2; OR, odds ratio; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; UOR, unadjusted odds ratio
Heart failure (HF) is a commonly seen complication in patients with acute myocardial infarction (AMI), with an incidence ranging from 19% to 45%.1, 2, 3, 4, 5, 6, 7 Clearly, HF adversely affects survival, hospital readmissions, and quality of life of patients with AMI.1, 5, 6, 8, 9, 10 During the past decade, substantial improvements in AMI revascularization and medical therapies have occurred, along with tremendous increases in the burden of cardiovascular (CV) and non-CV comorbidities.11 Recent data describing national trends in the incidence, management, and clinical outcomes of HF-complicated ST-segment elevation myocardial infarction (STEMI) hospitalizations in a US population are limited. Note that previous studies have been limited to single community-based settings, smaller sample sizes, and descriptions of events from the 1970s to the early 2000s. In addition, the results are controversial.2, 4, 5, 12, 13 Hence, we studied a large sample to examine the national temporal trends in the incidence, CV interventions, and in-hospital outcomes associated with HF-complicated STEMI hospitalization using the 2003 through 2010 Nationwide Inpatient Sample (NIS) databases.
Patients and Methods
We performed this study using 2003 through 2010 data from the NIS, the largest publicly available all-payer database of US hospital inpatient stays. The NIS is an administrative data set sponsored by the Agency for Healthcare Research and Quality as a part of the Healthcare Cost and Utilization Project.14 The NIS includes all discharge records for the sampled hospitals and data for approximately 8 million inpatient stays from approximately 1000 hospitals in all participating states (n=45 in 2010). It is approximately a 20% stratified sample of all US hospitals, defined as “all non-Federal, short-term, general, and other specialty hospitals, excluding hospital units of institutions.”14 Each hospital visit is treated as an individual entry and is coded with 1 principal diagnosis, up to 24 secondary diagnoses, and 15 procedural diagnoses associated with that stay. Discharge weights, provided for each patient discharge record, were used to obtain national estimates for each year. The NIS database provides statistical sampling weights as a variable “DISCWT” that can be used to calculate expected hospitalization rates in the United States.14 The internal and external validity of the NIS database are maintained through annual data quality assessments and comparison with other databases, such as the National Hospital Discharge Survey and MedPAR (Medicare Provider and Analysis Review). These reports are published on the NIS website (http://www.hcup-us.ahrq.gov/db/nation/nis/nisrelatedreports.jsp).
We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (410.xx) listed as the primary diagnosis to identify patients with AMI from January 1, 2003, through December 31, 2010. Patients with the principal diagnosis of STEMI were then identified using ICD-9-CM codes 410.x1, except code 410.71, as used in other studies.15 We chose the principal diagnosis because it is considered the primary reason for hospital admission. All patients with a diagnostic code of 410.7x (non-STEMI) in any of the diagnostic fields were completely excluded to limit analysis to only STEMI hospitalizations. In administrative databases, the AMI diagnosis identified using ICD-9-CM codes has been found to have specificity of 99.5%, sensitivity of 72.4%, a negative predictive value of 96.1%, and a positive predictive value of 95.9%.16 Patients with HF-complicated STEMI hospitalization were then defined as patients with STEMI without a history of HF but with HF at the time of discharge identified using ICD-9-CM codes 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, and 428.x listed anywhere except the primary diagnosis. These ICD-9-CM codes have been previously validated and also used in administrative database studies to accurately identify patients with HF.16, 17, 18, 19, 20 We then identified patients with cardiogenic shock to create 2 groups: HF-complicated STEMI with and without cardiogenic shock. Coronary interventions were identified as percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), and diagnostic coronary angiography (dCA).
We initially studied the overall trends in the proportion of HF-complicated STEMI hospitalizations. Then an analysis was conducted for the HF with cardiogenic shock and HF without cardiogenic shock subgroups. We also examined the trends in cardiac interventions (PCI, CABG, and dCA). The primary outcome of interest was all-cause in-hospital mortality, defined as “died” during the hospitalization encounter in the NIS database. We used the median length of stay (LOS) and the consumer price index (CPI)–adjusted median hospital cost (in 2010 US dollars) as secondary outcomes. Costs were inflation adjusted using the US Bureau of Labor Statistics' CPI, with 2010 as the index base.
Baseline patient characteristics used included demographic characteristics (age, sex, race, primary expected payer, weekday vs weekend admission, and median household income for patient zip code), clinical comorbidities (such as diabetes mellitus with and without complications, smoking, dyslipidemia, hypertension, obesity, alcohol abuse, atrial fibrillation, known coronary artery disease, previous AMI, carotid artery disease, peripheral vascular disease, liver disease, acute renal failure, and chronic pulmonary disease); and in-hospital procedures (PCI, CABG, and dCA). A list of ICD-9-CM and Clinical Classifications Software codes used to identify comorbidities and in-hospital procedures is provided in the Supplemental Table (available online at http://www.mcpiqojournal.org). Hospital characteristics, such as hospital region (northeast, midwest, south, and west), bed size (small, medium, and large), location (rural, urban), and teaching status, were also included.
For trend analysis, we used the Mantel-Haenszel χ2 test of linear association for categorical variables and linear regression for continuous variables. To determine whether there was temporal variability from year to year in the incidence of HF-complicated STEMI, coronary intervention, and in-hospital mortality, we used unadjusted and multivariable-adjusted logistic regression models to determine the odds of developing HF complication, undergoing coronary intervention, or dying during hospitalization each year relative to 2003. We used 2 types of regression models. Model 1 was adjusted for all the demographic characteristics, hospital characteristics, and clinical comorbidities, and model 2 was adjusted for all the covariates (as in model 1) along with coronary intervention. Model 2 was used everywhere to obtain adjusted odds ratios (AORs) except when mentioned otherwise. In both models, we entered calendar year as a continuous variable to obtain unadjusted ORs and AORs (per year) for the overall temporal trend and then as a categorical variable (with 2003 as the reference year) to determine whether there was temporal variability from year to year. Temporal trends in median LOS and median CPI-adjusted hospital charges were also examined.
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 23.0 (IBM Corp). All P values were 2 sided, with a significance threshold of P<.001. A lower-than-usual P value threshold was selected to correct for the effects of a large sample size as well as inflation of type I error because of repeated testing using a large number of variables. Categorical variables are expressed as percentages and continuous variables as mean ± SD or median (interquartile range [IQR]) as appropriate. The OR and 95% CI are used to report the results of logistic regression.
Results
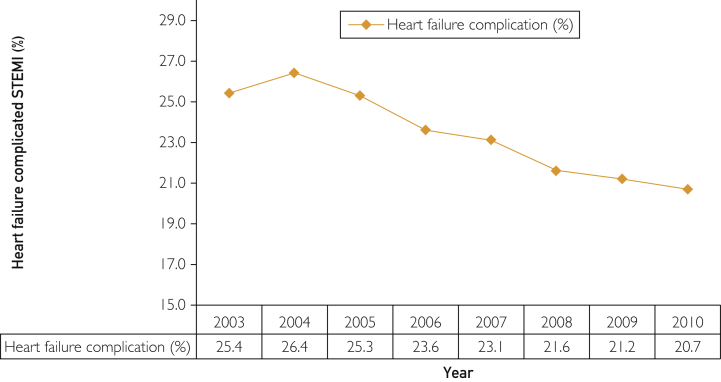
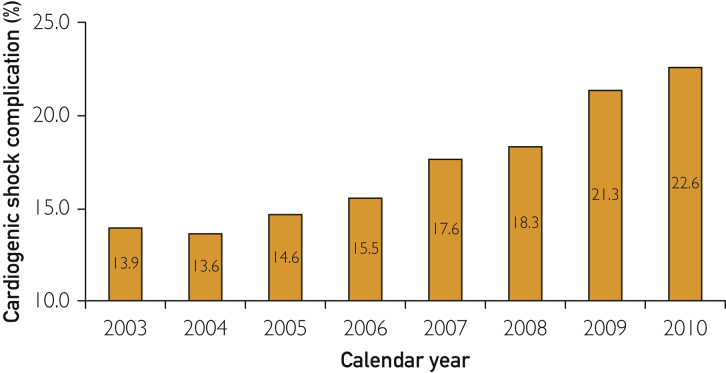
From January 1, 2003, through December 31, 2010, 1,990,002 STEMI hospitalizations with a primary diagnosis of STEMI in patients 18 years and older were identified. The overall incidence of HF complication in patients admitted with STEMI across 8 years was 23.7% (n=471,525), with a decrease in the proportion of patients from 25.4% in 2003 to 20.7% in 2010 (AOR [per year], 0.987; 95% CI, 0.985-0.989; P<.001) (Figure 1). In patients with HF-complicated STEMI, the incidence of cardiogenic shock was 16.5% (n=77,627), and the proportion of these patients increased from 13.9% (95% CI, 13.6%-14.1%) in 2003 to 22.6% (95% CI, 22.2%-23.0%) in 2010 (AOR [per year], 1.093; 95% CI, 1.088-1.098; P<.001) (Figure 2).
Figure 1.
Trends in the incidence rates of heart failure–complicated ST-segment elevation myocardial infarction (STEMI). Heart failure (%) was calculated as the number of patients with HF complication divided by the number of patients with STEMI per year × 100 (P trend<.001).
Figure 2.
Trends in the incidence of cardiogenic shock complication in heart failure (HF)–complicated ST-segment elevation myocardial infarction (STEMI). Cardiogenic shock (%) was calculated as the number of patients with cardiogenic shock divided by the number of patients with HF-complicated STEMI per year × 100 (P trend<.001).
Baseline demographic characteristics, hospital characteristics, and clinical comorbidities are presented in Table 1 for patients who experienced acute HF while hospitalized for STEMI during the study period. The mean ± SD age decreased from 73.4±13.4 years to 71.5±14.2 years (P trend<.001). From 2003 through 2010, there was an increase in the proportion of men; white individuals constituted the highest proportion of patients; and the prevalence of all comorbidities increased, except chronic pulmonary disease and atrial fibrillation, for which the prevalence decreased (all P trend<.001).
Table 1.
Baseline Demographic Characteristics, Hospital Characteristics, and Comorbidities of Patients With Heart Failure–Complicated ST-Segment Elevation Myocardial Infarctiona
| Variable | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Overall |
|---|---|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||||
| No. of cases (weighted) | 78,845 | 76,901 | 67,023 | 62,870 | 52,839 | 48,588 | 44,111 | 40,348 | 471,525 |
| Age (y), mean ± SD | 73.4±13.4 | 73.6±13.2 | 73.9±13.5 | 73.2±13.8 | 72.9±14.1 | 72.5±14.2 | 71.7±14.2 | 71.5±14.2 | 73.0±13.8 |
| Female sex (%) | 49.1 | 48.4 | 48.3 | 47.2 | 46.8 | 46.2 | 44.9 | 43.5 | 47.2 |
| Race (%) | |||||||||
| White | 77.9 | 79.6 | 81.1 | 80.7 | 78.5 | 79.6 | 77.5 | 76.9 | 79.1 |
| African American | 8.3 | 8.5 | 6.7 | 7.4 | 8.2 | 7.5 | 8.1 | 8.8 | 7.9 |
| Hispanic | 8.4 | 6.6 | 7.1 | 6.9 | 7.2 | 5.8 | 6.7 | 7.6 | 7.1 |
| Asian or Pacific Islander | 2.8 | 2.1 | 1.9 | 1.9 | 2.2 | 2.7 | 2.7 | 2.8 | 2.4 |
| Native American | 0.1 | 0.3 | 0.3 | 0.6 | 0.8 | 1.0 | 0.8 | 0.8 | 0.5 |
| Other | 2.5 | 2.9 | 2.9 | 2.4 | 3.0 | 3.4 | 4.2 | 3.1 | 3.0 |
| Primary expected payer (%) | |||||||||
| Medicare | 73.5 | 72.5 | 72.5 | 71.3 | 68.7 | 67.7 | 65.7 | 63.9 | 70.2 |
| Medicaid | 4.5 | 4.3 | 4.6 | 4.5 | 5.0 | 5.1 | 5.5 | 6.8 | 4.9 |
| Private insurance | 17.2 | 17.7 | 17.5 | 17.7 | 19.6 | 19.6 | 21.0 | 20.8 | 18.6 |
| Self-pay | 2.9 | 3.4 | 3.3 | 3.9 | 3.8 | 4.6 | 5.2 | 5.5 | 3.9 |
| No charge | 0.2 | 0.3 | 0.4 | 0.3 | 0.4 | 0.4 | 0.3 | 0.4 | 0.3 |
| Other | 1.7 | 1.8 | 1.8 | 2.3 | 2.4 | 2.6 | 2.3 | 2.6 | 2.1 |
| Median household income (%) | |||||||||
| 0-25th percentile | 27.8 | 29.9 | 29.3 | 28.8 | 30.1 | 30.1 | 29.1 | 29.0 | 29.2 |
| 26th-50th percentile | 29.0 | 28.9 | 27.4 | 28.0 | 26.9 | 29.5 | 28.2 | 28.0 | 28.3 |
| 51st-75th percentile | 24.7 | 21.7 | 24.1 | 23.6 | 23.0 | 21.9 | 23.2 | 24.4 | 23.3 |
| 76th-100th percentile | 18.4 | 19.5 | 19.2 | 19.6 | 20.0 | 18.5 | 19.4 | 18.5 | 19.1 |
| Weekend admission (%) | 26.3 | 26.0 | 25.3 | 26.1 | 26.9 | 26.9 | 27.3 | 28.0 | 26.4 |
| Elective admission (%) | 8.6 | 7.7 | 7.8 | 7.6 | 7.6 | 6.6 | 5.9 | 6.6 | 7.5 |
| Hospital characteristics | |||||||||
| Region (%) | |||||||||
| Northeast | 14.2 | 18.0 | 18.5 | 17.2 | 19.6 | 18.2 | 18.4 | 19.1 | 17.7 |
| Midwest | 26.7 | 24.2 | 24.1 | 24.7 | 25.3 | 23.1 | 22.6 | 24.7 | 24.6 |
| South | 39.6 | 38.8 | 39.3 | 39.5 | 36.4 | 39.6 | 39.8 | 35.6 | 38.7 |
| West | 19.5 | 19.0 | 18.1 | 18.6 | 18.7 | 19.0 | 19.2 | 20.6 | 19.0 |
| Bed size (%) | |||||||||
| Small | 11.2 | 12.0 | 11.3 | 15.7 | 12.5 | 14.6 | 11.5 | 12.3 | 12.6 |
| Medium | 25.0 | 24.1 | 24.1 | 24.3 | 25.9 | 22.9 | 22.4 | 20.3 | 23.9 |
| Large | 63.8 | 63.9 | 64.7 | 59.9 | 61.5 | 62.4 | 66.1 | 67.3 | 63.5 |
| Urban location (%) | 80.3 | 83.6 | 82.5 | 83.7 | 83.3 | 84.1 | 86.9 | 86.6 | 83.5 |
| Teaching hospital (%) | 40.1 | 40.3 | 34.7 | 41.3 | 43.8 | 40.9 | 44.8 | 43.9 | 40.8 |
| Clinical comorbidities (%)b | |||||||||
| Smoking | 14.6 | 14.6 | 15.9 | 17.5 | 20.1 | 23.2 | 28.9 | 29.3 | 19.3 |
| Alcohol abuse | 1.8 | 2.1 | 2.1 | 2.2 | 2.8 | 2.6 | 2.8 | 3.1 | 2.3 |
| Hypertension | 50.7 | 50.9 | 52.4 | 54.0 | 56.5 | 60.7 | 62.6 | 63.5 | 55.3 |
| Dyslipidemia | 23.5 | 25.9 | 28.5 | 31.0 | 36.1 | 40.2 | 46.6 | 49.2 | 33.1 |
| Diabetes (uncomplicated) | 27.7 | 27.5 | 27.2 | 27.6 | 28.9 | 29.2 | 29.5 | 30.5 | 28.3 |
| Diabetes (complicated) | 6.1 | 6.1 | 6.1 | 6.1 | 6.3 | 5.7 | 7.1 | 6.7 | 6.2 |
| Obesity | 5.1 | 5.2 | 5.8 | 6.3 | 6.7 | 9.0 | 10.5 | 11.0 | 7.0 |
| Atrial fibrillationc | 23.5 | 24.3 | 25.2 | 24.6 | 23.8 | 21.7 | 22.7 | 23.5 | 23.8 |
| Previous myocardial infarction | 7.3 | 7.4 | 7.2 | 7.8 | 8.7 | 8.6 | 10.5 | 10.6 | 8.2 |
| Coronary artery disease | 67.8 | 68.5 | 70.2 | 73.0 | 74.3 | 77.4 | 79.8 | 80.4 | 67.8 |
| Carotid artery disease | 0.9 | 0.8 | 0.9 | 1.0 | 1.2 | 1.4 | 1.9 | 2.0 | 1.2 |
| Peripheral vascular disease | 8.6 | 8.9 | 8.8 | 9.3 | 10.6 | 11.5 | 12.9 | 11.6 | 9.9 |
| Chronic pulmonary disease | 25.1 | 25.8 | 27.9 | 27.2 | 26.1 | 24.2 | 25.1 | 24.6 | 25.9 |
| Fluid and electrolyte disorder | 22.4 | 23.2 | 23.9 | 25.6 | 26.2 | 27.2 | 29.5 | 30.2 | 25.4 |
| Renal failure (chronic) | 11.1 | 11.6 | 14.1 | 20.7 | 22.4 | 21.0 | 22.4 | 23.3 | 17.3 |
| Liver disease | 0.7 | 0.8 | 0.8 | 0.9 | 1.1 | 1.0 | 1.0 | 1.1 | 0.9 |
The Mantel-Haenszel χ2 test of linear association was used for categorical variables, and linear regression was used for continuous variables. All P values are <.001.
Comorbidities were extracted using the International Classification of Diseases, Ninth Revision, Clinical Modification and Clinical Classifications Software codes.
The exact values were 23.52% in 2003 and 23.51% in 2010.
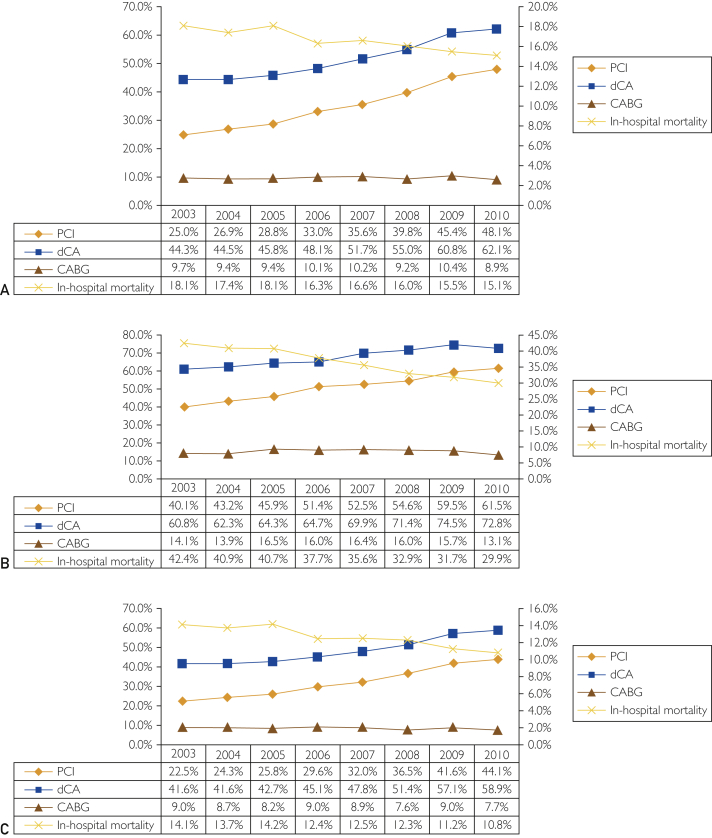
Figure 3 describes the temporal changes in the use of dCA, PCI, and CABG in patients with acute HF with STEMI and subgroups based on cardiogenic shock as a coexisting complication. Use of dCA and PCI increased from 44.3% in 2003 to 62.1% in 2010 (unadjusted OR [per year], 1.116; 95% CI, 1.113-1.119; P<.001) and from 25.0% in 2003 to 48.1% in 2010 (unadjusted OR [per year], 1.164; 95% CI, 1.161-1.167; P<.001), respectively, whereas CABG procedures decreased from 9.7% in 2003 to 8.9% in 2010 (unadjusted OR [per year], 1.000, 95% CI, 0.996-1.004; P=.99). Even after adjustment for demographic characteristics, hospital characteristics, and comorbidities, a similar trend in the utilization rates of PCI (AOR [per year], 1.174; 95% CI, 1.170-1.178; P<.001), dCA (AOR [per year], 1.126; 95% CI, 1.122-1.130; P<.001), and CABG procedures (AOR [per year], 0.979; 95% CI, 0.974-0.984; P<.001) was observed (Table 2). Similar trends were present in the subgroups with and without cardiogenic shock for the use of invasive coronary interventions.
Figure 3.
Trends in procedure use (percutaneous coronary intervention [PCI], diagnostic coronary angiography [dCA], and coronary artery bypass graft [CABG]) in patients with heart failure (HF)–complicated ST-segment elevation myocardial infarction (STEMI) (A), patients with HF-complicated STEMI with cardiogenic shock (B), and patients with HF-complicated STEMI without cardiogenic shock (C) (left y-axis). Procedure use (%) was calculated as the number of patients who underwent the procedure (PCI, dCA, CABG) divided by the number of patients with HF-complicated STEMI, HF-complicated STEMI with cardiogenic shock, or HF-complicated STEMI without cardiogenic shock, respectively, per year × 100 (all P trend<.001). Trends in in-hospital mortality in patients with HF-complicated STEMI, HF-complicated STEMI with cardiogenic shock, or HF-complicated STEMI without cardiogenic shock, respectively (right y-axis). In-hospital mortality (%) was calculated as the number of patients who died in the hospital divided by the number of patients with HF-complicated STEMI, HF-complicated STEMI with cardiogenic shock, or HF-complicated STEMI without cardiogenic shock, respectively, per year × 100 (all P trend<.001).
Table 2.
In-hospital Cardiovascular Interventions and Mortalitya
| Variable | Odds ratio (per year) (95% CI) | P value |
|---|---|---|
| Overall HF-complicated STEMI hospitalizations | ||
| PCI | ||
| UOR | 1.164 (1.161-1.167) | <.001 |
| AORb | 1.174 (1.170-1.178) | <.001 |
| CABG | ||
| UOR | 1.000 (0.996-1.004) | .99 |
| AORb | 0.979 (0.974-0.984) | <.001 |
| dCA | ||
| UOR | 1.116 (1.113-1.119) | <.001 |
| AORb | 1.126 (1.122-1.130) | <.001 |
| In-hospital mortality | ||
| UOR | 0.970 (0.967-0.973) | <.001 |
| AOR-1b | 0.992 (0.988-0.997) | <.001 |
| AOR-2c | 1.012 (1.008-1.017) | <.001 |
| HF-complicated STEMI hospitalizations without cardiogenic shock | ||
| PCI | ||
| UOR | 1.133 (1.126-1.140) | <.001 |
| AORb | 1.172 (1.168-1.177) | <.001 |
| CABG | ||
| UOR | 0.986 (0.981-0.991) | <.001 |
| AORb | 0.966 (0.960-0.972) | <.001 |
| dCA | ||
| UOR | 1.109 (1.106-1.112) | <.001 |
| AORb | 1.124 (1.119-1.128) | <.001 |
| In-hospital mortality | ||
| UOR | 0.957 (0.953-0.961) | <.001 |
| AOR-1b | 0.977 (0.971-0.982) | <.001 |
| AOR-2c | 1.001 (.996-1.007) | .63 |
| HF-complicated STEMI hospitalizations with cardiogenic shock | ||
| PCI | ||
| UOR | 1.160 (1.156-1.163) | <.001 |
| AORb | 1.138 (1.129-1.147) | <.001 |
| CABG | ||
| UOR | 1.003 (0.994-1.011) | .50 |
| AORb | 0.992 (0.982-1.003) | .16 |
| dCA | ||
| UOR | 1.098 (1.091-1.106) | <.001 |
| AORb | 1.097 (1.087-1.106) | <.001 |
| In-hospital mortality | ||
| UOR | 0.922 (0.916-0.928) | <.001 |
| AOR-1b | 0.931 (0.923-0.938) | <.001 |
| AOR-2c | 0.955 (0.949-0.963) | <.001 |
AOR = adjusted odds ratio; CABG = coronary artery bypass graft; dCA = diagnostic coronary angiography; HF = heart failure; PCI = percutaneous coronary intervention; STEMI = ST-segment elevation myocardial infarction; UOR = unadjusted odds ratio.
Model 1 is adjusted for patient demographic characteristics, hospital characteristics, and clinical comorbidities.
Model 2 is adjusted for patient demographic characteristics, hospital characteristics, clinical comorbidities, and invasive coronary intervention.
Trends for In-hospital Mortality
In-hospital mortality in the overall cohort of patients with acute HF-complicated STEMI was 16.9%. A decreasing trend for in-hospital mortality was seen from 18.1% in 2003 to 15.1% in 2010 (unadjusted OR [per year], 0.970; 95% CI, 0.967-0.973; P <.001) (Figure 3A). When adjusted for demographic characteristics, hospital characteristics and comorbidities, there was a declining trend in in-hospital mortality from 2003 to 2010 in patients with STEMI (AOR [per year], 0.992; 95% CI, 0.988-0.997; P<.001). After additional adjustment for cardiac intervention, the effect of year on death was significantly attenuated (AOR [per year], 1.012; 95% CI, 1.008-1.017; P<.001) (Table 2). Similar results were present in the subgroup without cardiogenic shock, and the AOR for the cardiogenic shock subgroup did not change significantly after adjustment (Table 2).
Median LOS and Hospitalization Costs
Table 3 represents median LOS and CPI-adjusted hospitalization cost for the overall cohort and subgroups. The median (IQR) LOS and CPI-adjusted hospital cost for the overall cohort with acute HF–complicated STEMI were 5 (3-9) days and $39,717 ($16,646-$82,184), respectively. Both median LOS and hospitalization cost for the subgroup with HF-complicated STEMI with cardiogenic shock were significantly higher than for those without cardiogenic shock (P<.001). Although the median LOS remained constant for the overall group and the individual subgroups, CPI-adjusted hospitalization cost increased across 8 years (2003-2010) (Table 3).
Table 3.
Median Length of Stayand Consumer Price Index-Adjusted Cost of Hospitalization
| Year | Median length of stay (d), median (IQR) | Consumer Price Index-adjusted cost of hospitalization (2010 USD), median (IQR) |
|---|---|---|
| Overall HF-complicated STEMI hospitalizations | ||
| 2003 | 5 (3-9) | 34,810 (15674-73,800) |
| 2004 | 5 (3-9) | 37,874 (16,684-79,994) |
| 2005 | 5 (3-9) | 38,051 (16,096-83,149) |
| 2006 | 5 (3-9) | 43,034 (17,728-86,537) |
| 2007 | 5 (3-9) | 45,051 (19,673-89,965) |
| 2008 | 5 (3-8) | 48,144 (21,114-93060) |
| 2009 | 5 (3-9) | 56,499 (25,117-109,714) |
| 2010 | 5 (3-8) | 61,899 (27,422-119,511) |
| HF-complicated STEMI hospitalizations with cardiogenic shock | ||
| 2003 | 8 (3-13) | 74,927 (32,346-140,144) |
| 2004 | 8 (3-13) | 83,637 (37,656-148,923) |
| 2005 | 7 (3-13) | 85,184 (37,732-154,984) |
| 2006 | 8 (4-13) | 88,727 (47,150-157,856) |
| 2007 | 7 (3-13) | 90,330 (47,218-161,475) |
| 2008 | 7 (3-12) | 92,867 (48,108-163,252) |
| 2009 | 8 (4-14) | 112,209 (60,694-204,285) |
| 2010 | 8 (4-13) | 116,976 (60,590-200,649) |
| HF-complicated STEMI hospitalizations without cardiogenic shock | ||
| 2003 | 5 (3-9) | 31,196 (14,540-64,601) |
| 2004 | 5 (3-8) | 33,811 (15,647-68,717) |
| 2005 | 5 (3-8) | 33,569 (14,949-70,910) |
| 2006 | 5 (3-8) | 37,181 (16,011-74,208) |
| 2007 | 5 (3-8) | 38,976 (17,395-76,218) |
| 2008 | 5 (3-8) | 41,990 (18,814-78,382) |
| 2009 | 5 (3-8) | 48,003 (20,987-87,968) |
| 2010 | 4 (3-7) | 51,292 (23,029-96,054) |
HF = heart failure; IQR = interquartile range; STEMI = ST-segment elevation myocardial infarction.
Discussion
In this large real-world US population–based observational study, we found decreasing rates of HF complication in STEMI hospitalizations from 2003 through 2010 despite a significant increase in the CV and non-CV comorbidity burden. The proportion of patients with HF-complicated STEMI with cardiogenic shock increased significantly. There was a temporal increase in PCI and dCA use, in-hospital survival, and median hospital charges in patients with STEMI with acute HF complication. The temporal changes in cardiac interventions likely played an important role in mediating the secular decrease in in-hospital mortality of patients with STEMI with acute HF complication.
Previous studies have reported incidence rates of HF-complicated AMI hospitalization ranging from 19% to 40%.2, 4, 5, 6, 12, 13 The results with respect to temporal trends from different studies are controversial. A report from the National Registry of Myocardial Infarction-2 (NRMI-2) reported an overall incidence of 19.1% of HF complication in 190,518 STEMI admissions from 1994 to 1998.6 Researchers from the Worcester Heart Attack Study reported an overall incidence of 32.4% in 11,061 patients with AMI, with a decline in the incidence of HF complication from 35.4% in 1975 to 25.8% in 2005.5 Gerber et al21 also observed a temporal declining trend in HF complication (in person-years) in 2596 patients with AMI from 10.8 in 1990 to 1996 to 9.8 in 2004 to 2010. In contrast, other studies, such as those from Olmsted County and the Framingham Heart Study, have reported conflicting data on events from the 1970s to the early 2000s.2, 4, 12, 13 Although the Framingham Heart Study reported increases in post-MI HF incidence at both 30 days (from 10% in 1970-1979 to 23.1% in 1990-1999) and 5 years (from 27.6% in 1970-1979 to 31.9% in 1990-1999), a study from Olmsted County reported a reduction in the incidence at 28 days (from 27% in 1979-1984 to 23% in 1990-1994) and 5 years (from 40% in 1979-1984 to 33% in 1990-1994). Potential reasons for these dissimilarities from the present study can be attributed to differences in the study sample size, patient characteristics, regional differences, HF definitions, different timelines for HF inclusion (admission only vs in-hospital vs 30 days or 5 years), inclusion of all patients with AMI (non-STEMI and STEMI vs STEMI only in the present study), and the advancements in invasive and medical interventions for both AMI and HF in recent years.
During the past 2 decades, we have witnessed tremendous improvements in the secondary prevention and management of coronary heart disease. The increased awareness for the modification of CV disease risk factors through healthy lifestyle changes and use of medications, such as aspirin, statins (including at intensive doses), β-blockers, angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers, and dual antiplatelet therapies, has resulted in regression of the incidence and severity of acute ischemic coronary events.22, 23 In addition, clear guidelines with respect to triage, interfacility transfer, reperfusion strategies, protocols (such as door-to-balloon time), and use of invasive coronary interventions have led to infarct size reductions, cardiac function improvements, and improvements in overall clinical outcomes in patients with STEMI.24, 25 All of the previously mentioned factors might have contributed to the reduction in the incidence and mortality of patients with STEMI with acute HF complication. Although information related to medications, hospital transfers, and time variables, such as door-to-balloon time, are unavailable in this study, we observed a significant decrease in acute HF-complicated STEMI, from 25.4% in 2003 to 20.7% in 2010, even after broad adjustments for multiple factors, such as patient demographic characteristics, hospital characteristics, clinical comorbidities, and coronary intervention. Notably, we also found increasing rates of cardiogenic shock complication in patients with HF-complicated STEMI. Although given the administrative nature of the database, exact description behind this surprising trend is not possible, but we believe it can possibly be related to reimbursement issues, early and increased recognition of cardiogenic shock, along with increasing use of medical interventions such as β-blockers, opioids, ACE inhibitors, diuretics, and PCI, as highlighted in previous studies.26, 27, 28, 29
The overall incidence of acute mortality in patients with HF-complicated STEMI was 16.9%, with a temporal decline in mortality from 2003 to 2010, similar to those reported by earlier research.5, 6, 30 Previous studies based on the Global Registry of Acute Coronary Events (GRACE), the NRMI-2, and the Worcester Heart Attack Study have reported in-hospital mortality rates in such patient populations of 12% to 21%. Interestingly, despite encouraging survival trends in the study, patients with acute HF-complicated STEMI had markedly higher hospital mortality than rates reported in the general STEMI population.24, 31 Likewise, patients with HF-complicated AMI have previously been found to be significantly undertreated with lifesaving therapies, such as aspirin, statins, β-blockers, heparin, PCI, and dCA, at the time of initial presentation or during hospitalization.5, 6, 30, 32 Even in the present study, the utilization rates of PCI (33.5%), CABG (9.7%), and dCA (50.1%) were much lower in patients with STEMI with acute HF diagnosis than those reported in overall patients with STEMI.31 The undertreatment of patients with HF-complicated AMI has been plausibly attributed to the older age and higher comorbidity burden compared with patients who did not develop acute HF complication.5, 33 Also, although the present findings of underuse of invasive interventions are consistent with those reported by others, note that we were unable to stratify the medical facilities based on their invasive procedure capacity and, hence, the numbers might be an underrepresentation than for accredited “chest pain” centers.5
It is critical to emphasize that enough literature exists supporting the role of therapies such as β-blockers, reperfusion strategies, and revascularization in improving clinical outcomes in patients with HF-complicated AMI.24, 30, 32, 34 At the same time, the increasing trend of cardiogenic shock in patients with HF-complicated STEMI in the present study highlights the need for clinical presentation–specific guidelines for the use of medications such as ACE inhibitors, opioids, and diuretics, as addressed for β-blocker use in HF-complicated STEMI in the most recent guidelines.24 In the present study, the declining trend of mortality in patients with HF-complicated STEMI became significantly attenuated after adjustment for temporal changes of invasive coronary intervention, possibly reflecting their critical role in mortality reduction, as reported previously by Shah et al35 in an overall STEMI population. The fact that HF-complicated AMI has markedly higher mortality but is surprisingly undertreated deserves special attention, as is also highlighted in studies from the NRMI-2 and GRACE investigators.6, 30 This has been emphasized in the most recent 2013 American College of Cardiology/American Heart Association STEMI guidelines that recommend performing emergency revascularization and dCA as a class I (level of evidence: B) recommendation for the treatment of patients with severe acute HF after STEMI, irrespective of the time delay from onset of AMI, which is a major change from previous guidelines.24, 36 Because the present study is limited to 2010 and earlier, it will be interesting to study the changes in the management and outcomes of patients with HF-complicated STEMI after guideline introduction.
This study has certain limitations secondary to the retrospective observational nature of the study and being based on an administrative-type database. Therefore, this study lacks certain potentially important clinical information, such as angiographic details, ejection fraction, medication use, biomarkers, HF phenotypic expression and subcategories (ie, systolic vs diastolic), and door-to-balloon times, and is prone to coding errors. The NIS database does not allow defining the temporal relationship of the development of HF, ie, present on admission, with that developing during hospitalization. Given that the NIS database consists of approximately 20% of all US hospitalizations and the national estimates are created using discharge weight estimates, it may not fully reflect all national hospitalizations. Another important limitation was the inability to assess the transfer status of patients because this information was missing in most patients (60% for transfer in and 90% for transfer out). Also, observations in the NIS are at the level of the hospitalization rather than the individual. Hence, addressing individual-specific issues, such as underuse of invasive coronary interventions, is not possible. Last, outcomes are limited to in-hospital events, and the exact cause of death is not available. Nevertheless, the NIS provided an unequaled statistical power and important insight into the real-world data to study the changes in incidence and management related to this critically ill subgroup of patients with STEMI. Hence, this analysis should also be seen as a call out for further research based on databases such as the ACTION Registry–Get With The Guidelines, which provide more details to account for the effects of medications, accreditation status of “chest pain” centers, clinical parameters, and time variables.
Conclusion
In this large nationwide study, we observed a temporal decline in the overall adjusted incidence of acute HF complication in US STEMI hospitalizations. There have been favorable trends in the use of PCI and dCA, with an overall decline in mortality in this high-risk patient group.
Footnotes
Potential Competing Interests: The authors report no competing interests.
Supplemental Online Material
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Gerber Y., Weston S.A., Enriquez-Sarano M., et al. Mortality associated with heart failure after myocardial infarction: a contemporary community perspective. Circ Heart Fail. 2016;9(1):e002460. doi: 10.1161/CIRCHEARTFAILURE.115.002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hellermann J.P., Goraya T.Y., Jacobsen S.J., et al. Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol. 2003;157(12):1101–1107. doi: 10.1093/aje/kwg078. [DOI] [PubMed] [Google Scholar]
- 3.Chen J., Hsieh A.F., Dharmarajan K., Masoudi F.A., Krumholz H.M. National trends in heart failure hospitalization after acute myocardial infarction for Medicare beneficiaries: 1998-2010. Circulation. 2013;128(24):2577–2584. doi: 10.1161/CIRCULATIONAHA.113.003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velagaleti R.S., Pencina M.J., Murabito J.M., et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118(20):2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McManus D.D., Chinali M., Saczynski J.S., et al. 30-year trends in heart failure in patients hospitalized with acute myocardial infarction. Am J Cardiol. 2011;107(3):353–359. doi: 10.1016/j.amjcard.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu A.H., Parsons L., Every N.R., Bates E.R., Second National Registry of Myocardial Infarction Hospital outcomes in patients presenting with congestive heart failure complicating acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI-2) J Am Coll Cardiol. 2002;40(8):1389–1394. doi: 10.1016/s0735-1097(02)02173-3. [DOI] [PubMed] [Google Scholar]
- 7.Desta L., Jernberg T., Lofman I., et al. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction: the SWEDEHEART Registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Fail. 2015;3(3):234–242. doi: 10.1016/j.jchf.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Weir R.A., McMurray J.J., Velazquez E.J. Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance. Am J Cardiol. 2006;97(10A):13F–25F. doi: 10.1016/j.amjcard.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Lewis E.F., Li Y., Pfeffer M.A., et al. Impact of cardiovascular events on change in quality of life and utilities in patients after myocardial infarction: a VALIANT study (valsartan in acute myocardial infarction) JACC Heart Fail. 2014;2(2):159–165. doi: 10.1016/j.jchf.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Najafi F., Dobson A.J., Hobbs M., Jamrozik K. Temporal trends in the frequency and longer-term outcome of heart failure complicating myocardial infarction. Eur J Heart Fail. 2007;9(9):879–885. doi: 10.1016/j.ejheart.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Roger V.L., Weston S.A., Gerber Y., et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121(7):863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg R.J., Spencer F.A., Yarzebski J., et al. A 25-year perspective into the changing landscape of patients hospitalized with acute myocardial infarction (the Worcester Heart Attack Study) Am J Cardiol. 2004;94(11):1373–1378. doi: 10.1016/j.amjcard.2004.07.142. [DOI] [PubMed] [Google Scholar]
- 13.Spencer F.A., Meyer T.E., Goldberg R.J., et al. Twenty year trends (1975-1995) in the incidence, in-hospital and long-term death rates associated with heart failure complicating acute myocardial infarction: a community-wide perspective. J Am Coll Cardiol. 1999;34(5):1378–1387. doi: 10.1016/s0735-1097(99)00390-3. [DOI] [PubMed] [Google Scholar]
- 14.Healthcare Cost and Utilization Project . Agency for Healthcare Research and Quality; Rockville, MD: 2014. Overview of the National (Nationwide) Inpatient Sample (NIS) [Google Scholar]
- 15.Agarwal M., Agrawal S., Garg L., et al. Effect of chronic obstructive pulmonary disease on in-hospital mortality and clinical outcomes after ST-segment elevation myocardial infarction. Am J Cardiol. 2017;119(10):1555–1559. doi: 10.1016/j.amjcard.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan H., Li B., Saunders L.D., et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424–1441. doi: 10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blecker S., Paul M., Taksler G., Ogedegbe G., Katz S. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61(12):1259–1267. doi: 10.1016/j.jacc.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosamond W.D., Chang P.P., Baggett C., et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5(2):152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goff D.C., Jr., Pandey D.K., Chan F.A., Ortiz C., Nichaman M.Z. Congestive heart failure in the United States: is there more than meets the I(CD code)? the Corpus Christi Heart Project. Arch Intern Med. 2000;160(2):197–202. doi: 10.1001/archinte.160.2.197. [DOI] [PubMed] [Google Scholar]
- 20.Birman-Deych E., Waterman A.D., Yan Y., Nilasena D.S., Radford M.J., Gage B.F. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 21.Gerber Y., Weston S.A., Berardi C., et al. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol. 2013;178(8):1272–1280. doi: 10.1093/aje/kwt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Keefe J.H., Carter M.D., Lavie C.J. Primary and secondary prevention of cardiovascular diseases: a practical evidence-based approach. Mayo Clin Proc. 2009;84(8):741–757. doi: 10.4065/84.8.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith S.C., Jr., Benjamin E.J., Bonow R.O., et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011;58(23):2432–2446. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 24.O'Gara P.T., Kushner F.G., Ascheim D.D., et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 25.Spencer F., Scleparis G., Goldberg R.J., Yarzebski J., Lessard D., Gore J.M. Decade-long trends (1986 to 1997) in the medical treatment of patients with acute myocardial infarction: a community-wide perspective. Am Heart J. 2001;142(4):594–603. doi: 10.1067/mhj.2001.117776. [DOI] [PubMed] [Google Scholar]
- 26.Kolte D., Khera S., Aronow W.S., et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3(1):e000590. doi: 10.1161/JAHA.113.000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babaev A., Frederick P.D., Pasta D.J., et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294(4):448–454. doi: 10.1001/jama.294.4.448. [DOI] [PubMed] [Google Scholar]
- 28.Meine T.J., Roe M.T., Chen A.Y., et al. Association of intravenous morphine use and outcomes in acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. Am Heart J. 2005;149(6):1043–1049. doi: 10.1016/j.ahj.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds H.R., Hochman J.S. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117(5):686–697. doi: 10.1161/CIRCULATIONAHA.106.613596. [DOI] [PubMed] [Google Scholar]
- 30.Steg P.G., Dabbous O.H., Feldman L.J., et al. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE) Circulation. 2004;109(4):494–499. doi: 10.1161/01.CIR.0000109691.16944.DA. [DOI] [PubMed] [Google Scholar]
- 31.Fox K.A., Steg P.G., Eagle K.A., et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999-2006. JAMA. 2007;297(17):1892–1900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 32.Spencer F.A., Meyer T.E., Gore J.M., Goldberg R.J. Heterogeneity in the management and outcomes of patients with acute myocardial infarction complicated by heart failure: the National Registry of Myocardial Infarction. Circulation. 2002;105(22):2605–2610. doi: 10.1161/01.cir.0000017861.00991.2f. [DOI] [PubMed] [Google Scholar]
- 33.Dziewierz A., Siudak Z., Rakowski T., et al. In-hospital management and mortality in elderly patients with non-ST-segment elevation acute coronary syndromes treated in centers without on-site invasive facilities. Cardiol J. 2008;15(5):451–457. [PubMed] [Google Scholar]
- 34.Freemantle N., Cleland J., Young P., Mason J., Harrison J. β-Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318(7200):1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah R.U., Henry T.D., Rutten-Ramos S., Garberich R.F., Tighiouart M., Bairey Merz C.N. Increasing percutaneous coronary interventions for ST-segment elevation myocardial infarction in the United States: progress and opportunity. JACC Cardiovasc Interv. 2015;8(1):139–146. doi: 10.1016/j.jcin.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Antman E.M., Anbe D.T., Armstrong P.W., et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) Circulation. 2004;110(5):588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.