Abstract
PURPOSE
We aimed to evaluate the findings and results from breast magnetic resonance imaging (MRI) examinations performed for problem-solving purposes due to inconclusive conventional imaging findings.
METHODS
Imaging findings, biopsy and follow-up results were retrospectively evaluated for breast MRI performed for problem-solving purposes at our department between January 2011 and December 2016 for cases whose mammography, tomosynthesis, or ultrasonography findings were inconclusive.
RESULTS
Lesions were identified in 414 of 986 problem-solving MRI examinations, and 13.3% of these lesions were diagnosed as malignant. A total of 124 lesions were additionally found by MRI, and 9.7% of these lesions were diagnosed as malignant. MRI produced false-negative results in four cases. In cases whose conventional imaging methods yielded indefinite results, the sensitivity, specificity, negative and positive predictive values of MRI were found to be 96.3%, 83%, 99.3%, and 46.5%, respectively. For the additional lesions identified, the sensitivity, specificity, negative and positive predictive values of MRI were found to be 91.7%, 69%, 98.7%, and 24%, respectively.
CONCLUSION
Breast MRI is a reliable problem-solving method for excluding malignancy that cannot be confirmed by conventional imaging. In such cases, additional findings from MRI may help identify new cancers that cannot be detected with conventional methods. However, it has moderately low specificity which may cause unnecessary biopsies, follow-ups, and anxiety to patients.
Breast magnetic resonance imaging (MRI) is the imaging method with the highest sensitivity in the detection of breast cancer. It is commonly used for different indications in breast imaging. However, the rates of false-positive results are high due to the relatively limited specificity of MRI. The specificity of breast MRI is variable depending on the respective indication for MRI (1, 2). Breast MRI is also an effective method to exclude cancer due to its high negative predictive values (1–4). One of the applications of breast MRI is as a problem-solving tool in cases where the existing clinical problem cannot be explained by conventional methods. Breast MRI is a useful tool for confirming the presence of a lesion and localize it when mammography and tomosynthesis findings are not adequate for evaluation, or the lesion is viewed from a single position and cannot be localized, or it is subtle and suspicious and cannot be detected by ultrasonography (US) (5–10). During the sonographic breast evaluation, it may be difficult to decide on the biopsy or follow-up for nonmass findings that are detected by US only and have no correlation in mammography. In clinical practice, problem-solving purposes involve a heterogeneous group of breast MRI indications. There are a few studies evaluating the efficacy of problem solving MRI compared with conventional imaging findings in the literature (11, 12). Since high sensitivity and relatively limited specificity may lead to false-positive results, the number of biopsies and follow-up recommendations can be unnecessarily high in these cases.
The purpose of this study was to review the findings of problem-solving breast MRI and to evaluate biopsy and follow-up results for indefinite findings identified by mammography, tomosynthesis, or breast US.
Methods
Approval was obtained from the institution’s ethics committee. Written informed consent was obtained from patients prior to any study-related procedure. In total, 986 of the 7867 breast MRI examinations performed at our department between January 2011 and December 2016 were for problem-solving purposes due to inconclusive conventional breast imaging findings. During this period, 43,221 mammography and 16,254 mammography and tomosynthesis examinations were performed at the breast imaging unit. Mammographic examinations of the women presenting to our department for mammography were for opportunistic screening or diagnostic purposes: 41% of the subjects were scanned for screening, and 59% for diagnostic purposes. At our department, a supplementary breast US scan is performed for all subjects whose mammogram shows dense breast tissue or abnormal findings. One-view (mediolateral oblique) tomosynthesis was performed in addition to diagnostic mammography and screening mammography, which reveals dense parenchyma or abnormal findings.
MRI examination was performed between days 7 and 14 of the menstrual cycle in premenopausal women and 4–6 weeks after stopping hormone replacement therapy in postmenopausal women who take hormone replacement. Indications for problem-solving breast MRI included architectural distortion and asymmetries observed in mammography or tomosynthesis that could not be confirmed to be real lesions, localized, or detected by US and subtle, nonmass lesions detected by US only. Microcalcifications and lesions accompanying microcalcifications were excluded from the study. Subjects with clinical complaints and physical examination findings who were evaluated to be normal according to conventional methods and for whom a problem-solving MRI was performed, as well as subjects who were newly diagnosed with breast cancer, were excluded from the study. Patients lost to follow-up and patients whose follow-up period was shorter than one year were also excluded. In total, 414 (42%) of the remaining 986 MRI examinations revealed MRI findings that were consistent with results from the previous mammography, tomosynthesis or US examinations. From the breast imaging unit’s digital archive and hospital electronic records, the patients’ clinical characteristics, physical examination findings and imaging findings were recorded. The results of biopsy and surgical excision, if any, were recorded.
Mammography and tomosynthesis
A Mammomat Inspiration device (Siemens Medical) was used to perform standard two-view mammography and additional mammograms, where necessary (lateral and spot compression). Mediolateral oblique tomosynthesis was performed with the tube moving at a 25° angle arc. Projection images were reconstructed with a slice thickness of 1 mm. Mammography and tomosynthesis images were assessed on a workstation dedicated to mammography (MammoReport, Siemens Medical).
Ultrasonography
All US examinations were performed using an Acuson Antares unit (Siemens Medical) and a 6- to 13-MHz broad-band matrix transducer. Bilateral whole-breast and axillary US examinations were performed. Second-look US was performed for additional suspicious findings detected during reporting or if the lesions were identified only by MRI.
MRI parameters
Using a 1.5 T system (Achieva, Philips MS), the axial T1A spin-echo sequence was first scanned at the prone position using a dedicated 7-channel breast coil (TR/TE, 454/10 ms; FOV, 300; matrix, 432; slice thickness, 3 mm). Next, axial T2A short tau inversion recovery (STIR: TR/TE, 2000/173 ms; FOV, 300; matrix, 432; slice thickness, 2 mm) images were obtained. In the dynamic scan, the axial 3D T1A gradient-echo sequence (THRIVE: TR/TE, 7/3.4 ms; matrix: 352; FOV, 340; flip angle, 10°; slice thickness, 1 mm) scan was repeated 6 times consecutively, precontrast and postcontrast. Gadolinium contrast medium 0.1 mm/kg (gadoterate meglumine: Dotarem®, Guerbet; gadobutrol: Gadovist®, Bayer Healthcare; gadodiamide, Omniscan®, GE Healthcare) was administered intravenously using an automatic injector (Medrad Spectris Solaris EP, Bayer Radiology Solutions) at 2 mL/s, and then 10 mL of saline was used for flushing.
Image interpretation
Four radiologists with 14, 5, 3, and 2 years of experience in breast imaging evaluated the previous conventional imaging results and problem-solving MRI findings. Assessments were made with more experienced radiologists in consensus (5 and 14 years of experience). The mammography, tomosynthesis, and US findings were recorded. The mammography and tomosynthesis findings were classified as architectural distortion, asymmetrical opacity, asymmetry, and developing asymmetries. Nonmass US findings were classified as architectural distortion, focal hypoechoic focus-acoustic shadowing, and focal heterogeneity. Second-look US findings were grouped as mass and nonmass lesions. MRI findings were grouped as masses, nonmass enhancement, and foci. Lesions were classified according to BI-RADS (13).
Imaging-guided biopsy
Mammography or US-guided biopsy was performed for lesions that could be localized by mammography, US, or second-look US. MRI-guided biopsy was performed for MRI-only lesions. A marker was inserted in the biopsy site (Senomark UltraCor, Bard Biopsy Systems). Marker localization was documented by one-view mammography.
Histopathologic assessment
The results of all biopsies and surgical excisions, if any, were recorded. Histopathology results were followed up by the radiologists who performed the biopsy and were assessed for radiology-pathology concordance.
Radiologic follow-up
The 6-month and 1-year follow-up findings were recorded for patients who were followed up without biopsy and for those with a benign concordant diagnosis. Follow-up was planned with the imaging modality in which the lesion was detected and localized. Women followed up for less than one year were excluded from the study. The median follow-up time was 35 months (range, 14–60 months).
Statistical analysis
SPSS version 19.0 (IBM Corp.) was used in statistical analysis. Breast MRI examinations were considered positive if they showed a BI-RADS 4 or 5 finding. Breast MRI examinations were considered negative for BI-RADS 1, 2, 3 (negative, benign, or probably benign) MRI findings. The sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were calculated for problem-solving MRI for breast cancer in women with inconclusive findings on mammography, tomosynthesis, or US and for detection of cancer in any additional foci identified.
Results
In total, 414 of 986 MRI scans (42%) revealed findings that were consistent with those from conventional imaging. The median age was 51 years (range, 26–74 years). The time between conventional imaging and MRI was 0–67 days (median, 18 days). Of the women, 232 (56%) were premenopausal, and 182 (44%) were postmenopausal. Familial history of breast cancer was present in 54 subjects (13%), while 58 subjects (14%) had a personal history of breast cancer. There were no physical examination findings related with inconclusive imaging findings.
Mammographic breast density was BI-RADS a in 5%, BI-RADS b in 22%, BI-RADS c in 54%, and BI-RADS d in 19% of patients.
Of 414 lesions, 157 (38%) showed architectural distortion, 73 (18%) showed asymmetrical opacity in one-view mammography, 61 (15%) showed focal asymmetrical opacity in two-view mammography, and 64 (15%) developed asymmetrical opacity. Fifty-nine (14%) women had subtle, nonmass findings detected by US only. Of the 157 architectural distortions, 85 were detected by tomosynthesis only and had no US correlation. The remaining 72 architectural distortions were visible by one-view mammography but were not clearly localized in the other view. Of the 73 asymmetrical opacities detected in one-view mammography, 34 were detected by one-view mediolateral oblique tomosynthesis only, and 39 were detected by mammography only. Additionally, 14 of 73 asymmetrical opacities were detected by one-view mammography, 18 of 61 asymmetrical opacities were detected by two-view mammography, and 14 of 64 developed asymmetrical opacities showed subtle, nonmass findings that could not be confirmed to be primary lesions.
In 28 of 59 nonmass lesions detected by US only, the lesion shape changed with probe position, and none had mammography correlation. Twenty-four focal architectural distortions, 21 focal acoustic shadowing-hypoechoic foci and 14 focal heterogeneities were identified.
The median size of the lesions, as detected by MRI, was 17 mm (range, 4–62 mm). In total, 223 (54%) masses, 131 (32%) nonmass enhancement, and 60 (14%) foci were found. Of these lesions, 299 (72%) were classified as BI-RADS 2–3 and 115 (28%) were classified as BI-RADS 4–5 (Figs. 1–3).
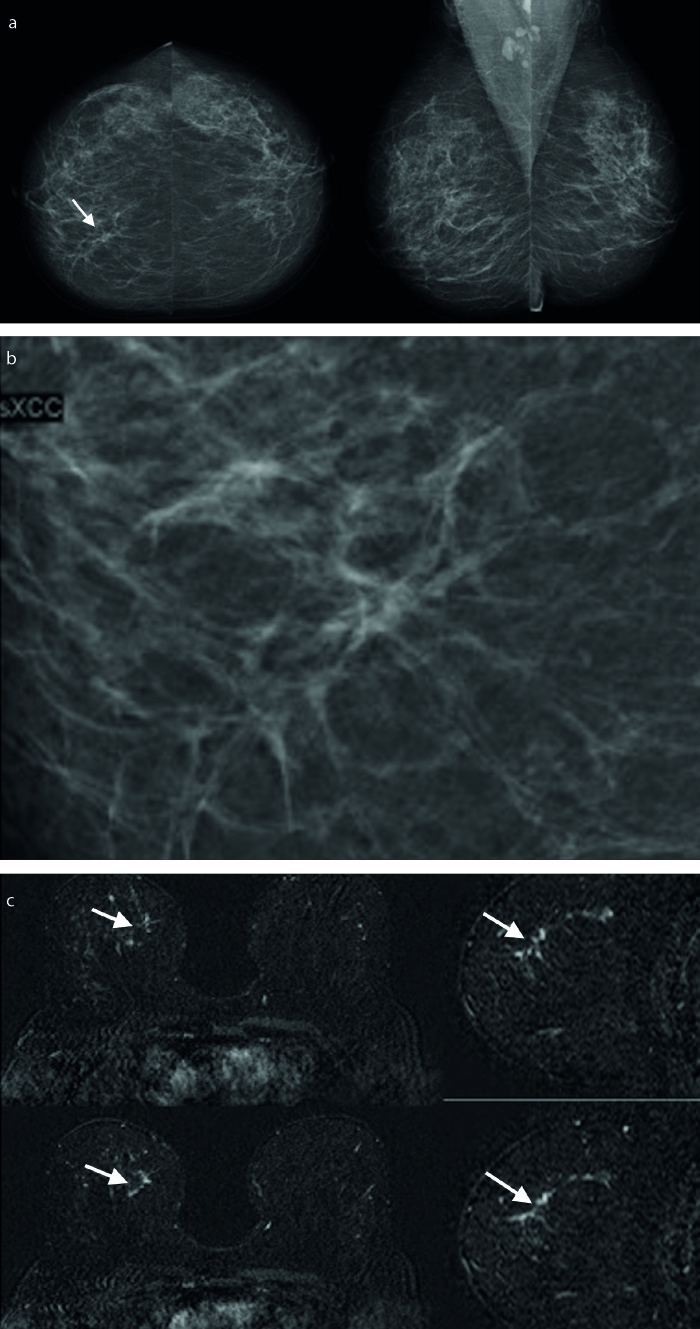
Figure 1. a–c.
A 54-year-old asymptomatic woman. A subtle architectural distortion and asymmetry were seen in right cranial-caudal mammogram (a, arrow) and spot compression mammogram (b). US examination was normal (not shown). Axial and reformat sagittal postcontrast subtraction MRI revealed nonmass enhancement (arrows) (c). Invasive lobular carcinoma was diagnosed after MRI-guided 10G vacuum-assisted biopsy.
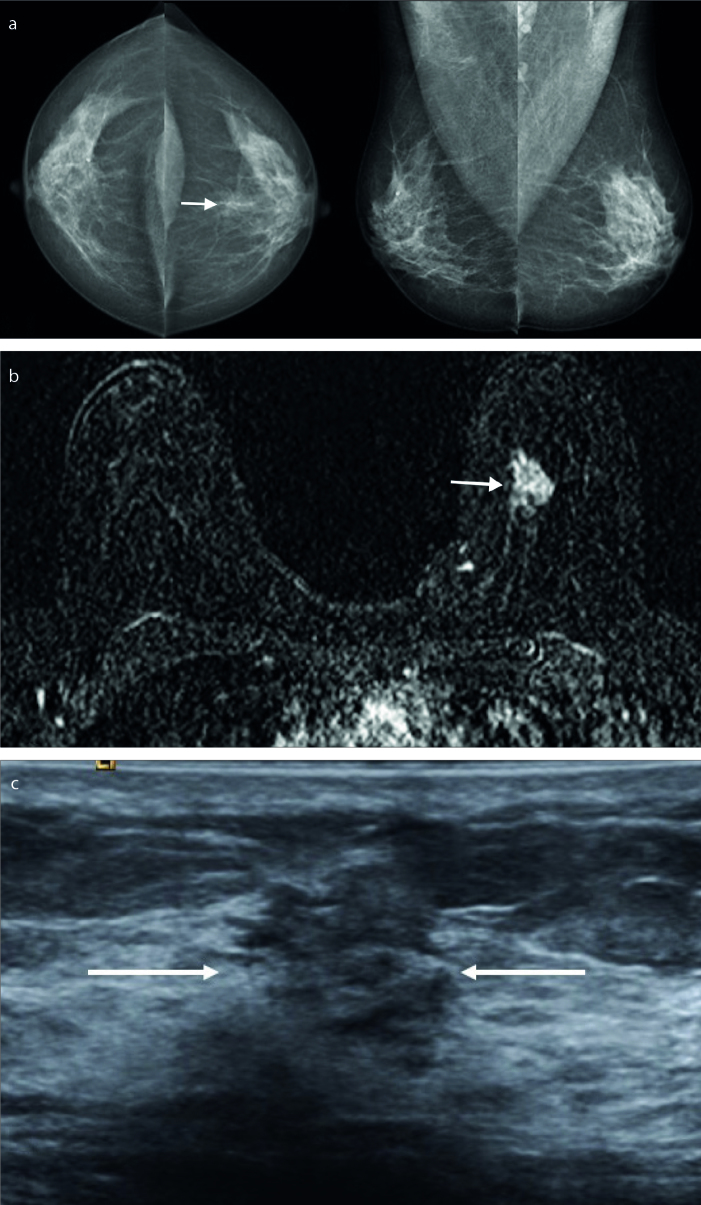
Figure 2. a–c.
A 47-year-old asymptomatic woman. An asymmetrical opacity was seen in left breast retroareolar region (a, arrow). US examination was normal (not shown). Axial postcontrast subtraction MRI revealed a mass (b, arrow). Second look US was performed and a subtle nonmass lesion was seen in the same location of left breast (arrows). Invasive ductal carcinoma was diagnosed after US-guided 14G core-needle biopsy (c).
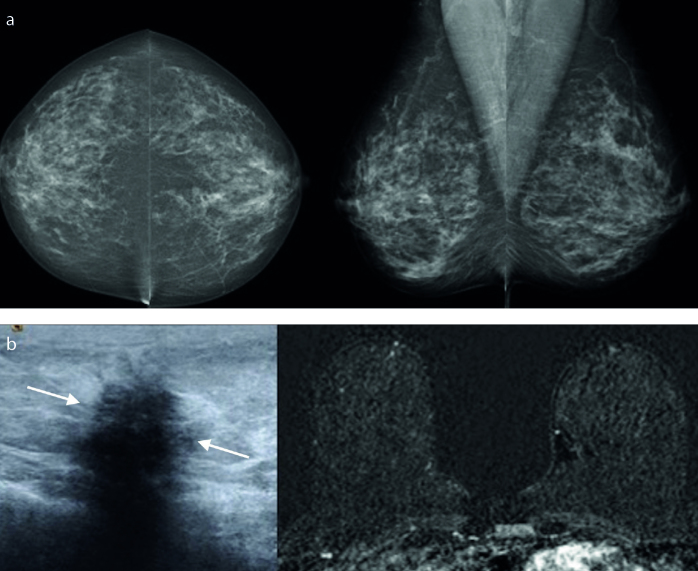
Figure 3. a, b.
A 51-year-old asymptomatic woman. No visible abnormality was seen on bilateral cranial-caudal and mediolateral-oblique mammograms (a). US examination revealed a nonmass focal hypoechoic lesion in right breast (arrows). Axial postcontrast subtracted MRI was normal (b). Fibrocystic changes was diagnosed after US-guided 14G core-needle biopsy.
Imaging-guided biopsy was performed for 115 lesions suspected of malignancy and 21 BI-RADS 3 lesions (biopsy indications of the BI-RADS 3 lesions: personal history of breast cancer, discordance between conventional imaging and MRI findings, multidisciplinary meeting decisions). Fifty-five lesions (13.3%) were diagnosed as malignant, and 81 (86.7%) were diagnosed as benign. Additionally, 34 of 157 architectural distortions (21.6%), 7 of 73 asymmetries (9.5%), 5 of 64 developing asymmetries (8%), 4 of 61 focal asymmetries (6.5%), and 4 of 59 lesions detected by US only (7%) were diagnosed as malignant. The distribution of conventional imaging findings by histopathologic diagnosis is summarized in Table 1. The BI-RADS scores and histopathologic diagnoses of MRI-detected lesions are summarized in Table 2.
Table 1.
Distribution of MRI-positive conventional imaging findings by histopathologic results
| Conventional imaging finding | Histopathologic diagnosis | Total | |
|---|---|---|---|
| Benign, n (%) | Malignant, n (%) | ||
| Architectural distortiona | 123 (78) | 34 (22) | 157 |
| Asymmetrical opacity in one-view mammographyb | 66 (90.5) | 7 (9.5) | 73 |
| Asymmetrical opacity in two-view mammography | 57 (93) | 4 (7) | 61 |
| Developing asymmetry | 59 (92) | 5 (8) | 64 |
| Nonmass lesion detected by US only | 55 (93) | 4 (7) | 59 |
| Total | 360 (87) | 54 (13) | 414 |
MRI, magnetic resonance imaging; US, ultrasonography.
85 lesions detected by tomosynthesis only and 72 lesions detected by mammography only.
34 lesions detected by tomosynthesis only and 39 lesions detected by mammography only.
Table 2.
Distribution of MRI-detected lesions by BI-RADS and histopathologic diagnosis
| MRI finding | BI-RADS 3 | BI-RADS 4–5 | Total | ||
|---|---|---|---|---|---|
|
|
|
||||
| Benign, n (%) | Malignant, n (%) | Benign, n (%) | Malignant, n (%) | ||
| Mass | 161 (72.2) | 1 (0.4) | 32 (14.4) | 29 (13) | 223 |
|
| |||||
| NME | 81 (62) | 0 (0) | 27 (21) | 23 (17) | 131 |
|
| |||||
| Focus | 56 (93.3) | 1 (1.7) | 2 (3.3) | 1 (1.7) | 60 |
|
| |||||
| Total | 298 (72) | 2 (0.5) | 61 (14.7) | 53 (12.8) | 414 |
MRI, magnetic resonance imaging; BI-RADS, breast imaging-reporting and data system; NME, nonmass enhancement.
The sensitivity, specificity, NPV, and PPV values of MRI were 96.3%, 83%, 99.3%, and 46.5%, respectively. In 78 subjects with concordant benign histopathologic results, no suspicious changes were observed in the follow-up with US or MRI. For 186 BI-RADS 3 lesions followed up without biopsy, the lesions were found to be stable in a mean follow-up period of 35 months.
An additional 124 lesions were found either in the same or opposite breast in 117 of 414 MRI scans (28%). Of these 124 lesions, 22 (18%) were in the same quadrant as the index lesion, 57 (46%) were in a different quadrant, and 45 (36%) were in the opposite breast. Of the 124 lesions, 78 (63%) were BI-RADS 3, and 46 (37%) were BI-RADS 4–5. The median lesion size was 14 mm (range, 4–47 mm). A biopsy was performed for 46 suspicious lesions and 9 BI-RADS 3 lesions; 11 were diagnosed as malignant. In a patient followed up after a concordant benign diagnosis, the biopsy was repeated due to an increased lesion size at the 6-month mammography and MRI, and the patient was diagnosed with invasive ductal cancer. Of the additional lesions identified by MRI, 9.7% were diagnosed as malignant. Two lesions diagnosed by biopsy as atypical ductal hyperplasia and one lesion diagnosed as a radial scar were diagnosed as ductal carcinoma in situ following surgical excision. No suspicious changes were observed in 69 lesions followed up without biopsy. The distribution of additional lesions by BI-RADS and histopathologic results are summarized in Table 3. The sensitivity, specificity, NPV, and PPV of MRI to detect cancers in additional lesions were 95%, 64.4%, 98.5%, and 34%, respectively.
Table 3.
Distribution of MRI-detected additional lesions by BI-RADS and histopathologic results
| Histopathologic diagnosis | BI-RADS 2–3, n (%) | BI-RADS 4–5, n (%) | Total, n (%) |
|---|---|---|---|
| Benign | 77 (98.7) | 35 (76) | 112 (90.3) |
| Malignant | 1 (1.3) | 11 (24) | 12 (9.7) |
| Total | 78 | 46 | 124 |
MRI, magnetic resonance imaging; BI-RADS, breast imaging-reporting and data system.
Of 572 MRI-negative patients, 21 were lost to follow-up. Of the remaining 551 women, 164 were included for the 6-month and then the one-year follow-up. The remaining women were called for a routine yearly follow-up. A biopsy was performed due to the increased size of asymmetry in one-view mammography at the one-year check-up, and the patient was diagnosed with microinvasive ductal cancer. In one patient, increased density with focal architectural distortion with a 4 mm diameter at this localization was detected at the 6-month follow-up; the patient was diagnosed with invasive lobular cancer by biopsy. A patient for whom a 6-month follow-up was recommended due to dense breast tissue in mammography and retroareolar, hypoechoic focus with a 4 mm diameter in US presented with sudden-onset bloody nipple discharge after 10 months. No changes were detected with mammography and US, and the patient was diagnosed with mucinous cancer following biopsy of the lesion. Repeated MRI examination revealed a well-defined mass in this location. The rate of cancer detection was 0.5% in subjects who had no breast MRI-detected lesions. No changes were found in radiologic follow-up in the remaining women.
Discussion
The purpose of this study was to evaluate the contribution of problem-solving MRI to radiologic assessment in women with inconclusive, nonlocalizable findings detected by conventional breast imaging methods.
Additional mammograms (e.g., mediolateral, spot compression, or exaggerated lateral-medial) and tomosynthesis are useful in confirming and localizing the lesion in mammography-detected inconclusive findings (14–16). Studies have shown that tomosynthesis increases cancer detection rates with 3D cross-sectional imaging and helps to clarify mammography findings that do not reveal whether a real lesion exists (17, 18). In the case of architectural distortion or asymmetry detected by tomosynthesis only, MRI can be a useful problem-solving tool if the findings cannot be correlated to US (19, 20). Mammography-detected unclear findings may be associated with technical factors (e.g., compression or positioning), glandular tissue configuration, asymmetry or density. First, it should be understood whether or not the finding indicates a real lesion. In cases where additional mammograms or tomosynthesis are not conclusive and cancer may not be excluded, breast US is an important complementary imaging method. Breast US offers fast, cheap and reliable assessment and biopsy guidance (21, 22). If there is no US correlation for such lesions, problem-solving breast MRI is a convenient method for further evaluation (23).
Similarly, MRI can be a problem-solving tool to confirm the presence of lesions for subtle, nonmass findings that are detected by breast US only and have no mammography or tomosynthesis correlation. In this study, cancer was detected in 7% of the subjects with MRI-confirmed lesions who had nonmass, unclear US findings that could not exclude cancer; all but one of the remaining lesions had disappeared, diminished or remained stable during the follow-up. While one lesion was stable in the US follow-up, a biopsy was performed due to bloody nipple discharge, and the lesion was diagnosed as malignant. In the literature that could be accessed, previous studies evaluating the use of problem-solving MRI did not make an assessment of lesions detected by US only. The most important difference in this study is that MRI was shown to be a useful problem-solving method also in nonmass, unclear findings detected by US only. Biopsy is an option for the second step for such findings detected by US. However, the decision to perform biopsy is made difficult when US-detected lesions are nonmass, inconclusive lesions and cannot be confirmed by other imaging methods. In such cases, the use of MRI before biopsy is the subject of a new investigation that is worthy of discussion.
In the literature, the rate of cancer detection by problem-solving breast MRI is between 5.2% and 26.3%. Sardanelli et al. (9) detected cancer in 26.3% of 19 patients evaluated by problem-solving MRI. Lee et al. (24) detected cancer in 10.5% of 86 patients. In a study by Moy et al. (25), cancer was detected in 5.2% of 115 patients. The rate of cancer detection was 13.5% in 111 patients in a recently published study by Spick et al. (11) and 13.6% in 294 patients in the study by Giess et al. (12). In our study, this rate was 13.3% in 414 patients. The reasons for such different results may include heterogeneous indications, differences in patient selection (e.g., selection according to imaging findings or the solution of a clinical problem) and differences in patient characteristics (e.g., diagnostic or screening examinations, age, the presence of risk factors, or breast density). In the study by Giess et al. (12), three cases were reported where problem-solving MRI yielded false-negative results. There were no false-negative results in the other studies (23–25). In our study, four false-negative cases were found.
An important difference between this study and similar studies is that the findings detected not only by mammography but also by tomosynthesis only or by US only were evaluated.
Considering the sensitivity, specificity, NPV, and PPV values calculated for MRI in similar studies, MRI appears as a good problem-solving tool with high sensitivity and NPV values. In their study evaluating the contribution of problem-solving breast MRI, Spick et al. (11) reported 100% sensitivity, 88.5% specificity, 100% NPV and 57.7% PPV. In the study by Giess et al. (12), sensitivity, specificity, NPV, and PPV were 92.5%, 62.4%, 97.8%, and 31.9%, respectively. In our study, these values were 96.4%, 83%, 99.3%, and 46.5%, respectively. The common conclusion among a few similar studies is that MRI is a reliable imaging method for excluding malignancy in case of inconclusive findings from conventional breast imaging methods.
New lesions not detected by conventional methods may also be detected by breast MRI due to its high sensitivity. In this study, additional lesions were found by MRI either in the same or opposite breast in 28% of subjects; 37% of these additional lesions were classified as BI-RADS 4 and 5, however 9.7% were diagnosed with cancer. Additional lesions were not evaluated in some of the studies assessing the clinical benefit of problem-solving MRI. Different cancer detection rates were reported in studies in which additional lesions were also evaluated. In a study by Moy et al. (25), additional lesions were identified by MRI in 15.7% of subjects, and all were diagnosed as benign. Lee et al. (24) reported that cancer was detected in 8.3% of additional lesions identified by MRI. In the study by Giess et al. (12), the rate of cancer detection in MRI-identified additional lesions was 17.1%.
One of the limitations of this study is possible inter-reader variability. Lower specificity of problem-solving MRI may also depend on different experience of readers and different types of lesions. Baltzer et al. (26) reported that low reader experience and nonmass lesion type negatively affect diagnostic accuracy of MRI. In their study, Marino et al. (27) showed that the “Tree scoring system” reduces inter-reader variability related to reader experience and improves diagnostic accuracy in non-expert readers. Another important limitation of this study concerns deciding whether biopsy is necessary for inconclusive, unclear conventional imaging findings while evaluating them retrospectively. In daily clinical practice, it is difficult to decide on follow-up or further assessment for such findings and make a BI-RADS classification. While knowing the suspicion level of the conventional finding makes it easier to determine the radiologic approach for recommending and evaluating problem-solving MRI, we believe that it cannot be performed with full accuracy in routine clinical practice. It seems practical to report such examinations as BI-RADS 0. Another limitation of this study is that 21% of the subjects had a follow-up period of less than two years.
If lesions are found following problem-solving MRI, a general BI-RADS assessment followed by biopsy or follow-up decision is required. If the lesion is considered “probably benign”, radiologic follow-up should be planned with the simplest method possible that allows clear assessment of the lesion. Problem-solving purposes may indicate a very large and heterogeneous group of indications in daily practice. The use of problem-solving MRI is a highly debated topic and is not accepted unanimously as an MRI indication (6). MRI is an imaging method that may lead to an unnecessarily high number of biopsies and follow-ups with false-positive results while excluding malignancy due to its high sensitivity and relatively limited specificity. In this study, 12 cases of cancer were detected that could not have been identified without MRI, while biopsy was performed with benign results in 43 women, and 69 women were followed up by MRI. MRI produced false-negative results in four subjects with cancer. In assessing the findings of problem-solving MRI, false negativity should also be considered, and biopsy should be performed despite negative MRI if there is a strong suspicion of cancer with the primary findings.
In conclusion, problem-solving breast MRI is a reliable method for excluding malignancy due to high NPV values in cases of suspicious findings that cannot be confirmed or localized by conventional imaging methods. Problem-solving breast MRI should be performed in the presence of precise indications so that cancer can be excluded without unnecessarily increasing the number of biopsies and follow-ups.
Main points.
Lesions were identified in 414 of 986 problem-solving magnetic resonance imaging (MRI) examinations, and 13.3% of these lesions were diagnosed as malignant.
The sensitivity, specificity, negative predictive value, and positive predictive value of MRI for inconclusive conventional imaging findings were found to be 96.3%, 83%, 99.3%, and 46.5%, respectively.
Breast MRI is a reliable problem-solving method for excluding malignancy that cannot be confirmed by conventional imaging.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Peters NH, Borel Rinkes IH, Zuithoff NP, et al. Metaanalysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246:116–124. doi: 10.1148/radiol.2461061298. [DOI] [PubMed] [Google Scholar]
- 2.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26:3248–3258. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 3.Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008;148:671–679. doi: 10.7326/0003-4819-148-9-200805060-00007. [DOI] [PubMed] [Google Scholar]
- 4.Kuhl CK. Current status of breast MR imaging. Part 2. Clinical applications. Radiology. 2007;244:672–691. doi: 10.1148/radiol.2443051661. [DOI] [PubMed] [Google Scholar]
- 5.ACR practice guideline for the performance of contrast-enhanced Magnetic Resonance Imaging (MRI) of the breast. Available from: http://www.acr.org/~/media/2a0eb28eb59041e2825179afb72ef624.pdf.
- 6.Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer. 2010;46:1296–1316. doi: 10.1016/j.ejca.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: Guidelines from the European Society of Breast Imaging. Eur Radiol. 2008;18:1307–1318. doi: 10.1007/s00330-008-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CH. Problem solving MR imaging of the breast. Radiol Clin N Am. 2004;42:919–934. doi: 10.1016/j.rcl.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Sardanelli F, Mealni E, Ottonello C, et al. Magnetic resonance imaging of the breast in characterizing positive or uncertain mammographic findings. Cancer Detect Prev. 1998;22:39–42. doi: 10.1046/j.1525-1500.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 10.Olsen ML, Morton MJ, Stan DL, Pruthi S. Is there a role for magnetic resonance imaging in diagnosing palpable breast masses when mammogram and ultrasound are negative? J Women’s Health. 2012;21:1149–1154. doi: 10.1089/jwh.2012.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spick C, Szolar DH, Preidler KW, Tillich M, Reittner P, Baltzer PA. Breast MRI used as a problem-solving tool reliably excludes malignancy. Eur J Radiol. 2015;84:61–64. doi: 10.1016/j.ejrad.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Giess CS, Chikarmane SA, Sippo DA, Birdwell RL. Clinical utility of breast MRI in the diagnosis of malignancy after inconclusive or equivocal mammographic diagnostic evaluation. AJR Am J Roentgenol. 2017;208:1–8. doi: 10.2214/AJR.16.16751. [DOI] [PubMed] [Google Scholar]
- 13.BI-RADS: Breast Imaging Reporting and Data System, Atlas. 5th Edition. ACR; Reston, VA, USA: 2013. [Google Scholar]
- 14.Sickles EA. The spectrum of breast asymmetries: imaging features, work-up, management. Radiol Clin N Am. 2007;45:765–771. doi: 10.1016/j.rcl.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Chesebro AL, Winkler NS, Birdwell RL, Giess CS. Developing asymmetries at mammography: a multimodality approach to assessment and management. Radiographics. 2016;36:322–334. doi: 10.1148/rg.2016150123. [DOI] [PubMed] [Google Scholar]
- 16.Giess CS, Frost EP, Birdwell RL. Interpreting one-view mammographic findings: minimizing callbacks while maximizing cancer detection. Radiographics. 2014;34:928–940. doi: 10.1148/rg.344130066. [DOI] [PubMed] [Google Scholar]
- 17.Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267:47–56. doi: 10.1148/radiol.12121373. [DOI] [PubMed] [Google Scholar]
- 18.Conant EF. Clinical implementation of digital breast tomosynthesis. Radiol Clin North Am. 2014;52:499–518. doi: 10.1016/j.rcl.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taskin F, Durum Y, Soyder A, Unsal A. Review and management of breast lesions detected with breast tomosynthesis but not visible on mammography and ultrasonography. Acta Radiol. 2017;58:1442–1447. doi: 10.1177/0284185117710681. [DOI] [PubMed] [Google Scholar]
- 20.Durand MA, Wang S, Hooley RJ, et al. Tomosynthesis-detected architectural distortion: management algorithm with radiologic-pathologic correlation. Radiographics. 2016;36:311–321. doi: 10.1148/rg.2016150093. [DOI] [PubMed] [Google Scholar]
- 21.Mendelson EB. Problem-solving ultrasound. Radiol Clin North Am. 2004;42:909–918. doi: 10.1016/j.rcl.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Uematsu T. Ultrasonographic findings of missed breast cancer: pitfalls and pearls. Breast Cancer. 2014;21:10–19. doi: 10.1007/s12282-013-0498-7. [DOI] [PubMed] [Google Scholar]
- 23.Bennani-Baiti B, Bennani-Baiti N, Baltzer PA. Diagnostic performance of breast magnetic resonance imaging in noncalcified equivocal breast findings: results from a systematic review and meta-analysis. PLoS One. 2016;11:e0160346. doi: 10.1371/journal.pone.0160346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CH, Smith RC, Levine JA, Troiano RN, Tocino I. Clinical usefulness of MR imaging of the breast in the evaluation of the problematic mammogram. AJR Am J Roentgenol. 1999;173:1323–1329. doi: 10.2214/ajr.173.5.10541112. [DOI] [PubMed] [Google Scholar]
- 25.Moy L, Elias K, Patel V, et al. Is breast MRI helpful in the evaluation of inconclusive mammographic findings? AJR Am J Roentgenol. 2009;193:986–993. doi: 10.2214/AJR.08.1229. [DOI] [PubMed] [Google Scholar]
- 26.Baltzer PAT, Kaiser WA, Dietzel M. Lesion type and reader experience affect the diagnostic accuracy of breast MRI: A multiple reader ROC study. Eur J Radiol. 2015;84:86–91. doi: 10.1016/j.ejrad.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Marino MA, Clauser P, Woitek R, Wengert GJ, et al. A simple scoring system for breast MRI interpretation: does it compensate for reader experience? Eur Radiol. 2016;26:2529–2537. doi: 10.1007/s00330-015-4075-7. [DOI] [PubMed] [Google Scholar]