Abstract
PURPOSE
We aimed to evaluate the imaging features of bile duct adenoma (BDA) on ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI).
METHODS
Retrospective search in our institution database was performed for histologically confirmed BDA. Their imaging studies before histologic confirmation were reviewed. The search identified seven adults (mean age, 52.9 years) with histologically proven single BDA each. US (n=3), CT (n=5), and MRI (n=3) were performed before histologic confirmation. Additionally, a systematic English literature review for BDA and reported imaging findings since 2000 was also conducted using the following search criteria “bile duct adenoma, peribiliary hamartoma, biliary adenoma, CT, ultrasound, MRI” (date range: 01/01/2000 through 08/31/2016). The imaging findings of those cases reported were summarized and compared with our series.
RESULTS
All seven individual nodules were well circumscribed. Five lesions were located in the right hepatic lobe and two in the left hepatic lobe. On US, lesions appeared hypoechoic (n=2) and hyperechoic (n=1). BDA was hypodense on unenhanced CT images (n=1). On MRI, BDA were hypointense on T1 (n=3), hyperintense on T2 (n=3), and hyperintense on diffusion-weighted images (n=2). On contrast-enhanced CT and MRI, BDAs showed arterial phase hyperenhancement that persisted on portal venous/delayed phase images.
CONCLUSION
BDA demonstrates characteristic arterial phase hyperenhancement that persisted into the portal venous and delayed phases on CT and MRI, which may be useful in differentiating from other hepatic lesions.
Bile duct adenoma (BDA) is a rare epithelial bile duct neoplasm. BDAs are clinically significant in that they may be misdiagnosed as malignant neoplasms or as other benign lesions based on their histologic and imaging findings. Accurate diagnosis of BDA is potentially further complicated by inconsistency in the literature related to nomenclature and controversy regarding its cell of origin and pathogenesis (1).
BDA is composed of a small aggregate of noncystic bile ductules associated with varying degrees of inflammation and fibrosis (2, 3). BDAs are different from the more commonly recognized biliary hamartomas (Von Meyenberg Complexes), which are composed of irregularly dilated bile ducts embedded in fibrous stroma (4). It is also worth noting that the histologically defined entity of BDA has been described in the literature using a broad range of names or terms including names that refer to other distinct pathologic entities such as: peribiliary gland hamartoma, benign cholangioma, cholangioadenoma, biliary microhamartoma, bile duct adenoma, biliary hamartoma, and biliary adenofibroma (1, 5). For the purpose of clarity and taking into consideration the cell of origin we will refer to this entity as BDA throughout the manuscript.
BDA is typically a small, solitary, peripheral or subcapsular lesion with a mean diameter of 5.8 mm (1–20 mm) (2). Grossly, BDA is a well-circumscribed grayish-white nodule on hepatic surface (6). Given their small size and location, these neoplasms are usually detected as an incidental finding at the time of surgery or at autopsy and they have been considered a benign process with limited growth potential (2). However, one case report suggests that BDA may have malignant potential with intrahepatic cholangiocarcinoma arising from BDAs (7). While there has been considerable controversy relating to its pathogenesis, it is likely that BDAs represent a true neoplasm and that they should no longer be designated as reactive processes or hamartomas (8).
There is a paucity of literature regarding the imaging features of BDAs. The majority of the radiologic literature is composed of case reports. Interpretation of the radiologic literature describing BDAs is further complicated by the aforementioned potentially confusing and frequently inconsistent nomenclature. Prospective BDA detection is likely difficult due to their small size, subcapsular location and overlapping imaging features and may be thought to represent other lesions such as metastases. A misdiagnosis of liver metastasis instead of BDA might result in a patient being subjected to unnecessary diagnostic procedures and interventions such as chemotherapy and surgery with multiple potential side effects and risks (2, 9). Additionally, the limited available imaging literature is likely due to the neoplasm’s asymptomatic presentation. However, with improved imaging techniques and ever increasing number of radiologic investigations, it is likely that radiologists will encounter more of these lesions in their clinical practice. Because of this, it is important to be familiar with the imaging features of this benign neoplasm as it can have overlapping features with focal hepatic lesions and clinically significant pathologic entities. Specifically, this study sought to describe the imaging features of BDAs on ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) from our series and those from literature.
Methods
This is an institutional review board approved (ID: 15-004925), HIPPA-compliant retrospective study. The need to obtain informed consent was waived. Our institutional pathology database was searched over a 16-year period (January 1, 2000 to August 31, 2016), for histologically confirmed cases of BDA using keywords: “bile duct adenoma”, “peribiliary hamartoma”, or “biliary adenoma”.
The search yielded 135 patients with a diagnosis of BDA. A total of 70 patients were excluded as BDAs were detected incidentally at the time of surgery (e.g., through visual inspection or palpation) and/or without preoperative imaging. An additional 58 patients were excluded as there were multiple lesions seen on both preoperative imaging and at surgery/pathology specimens without any definitive landmark descriptions making it difficult to correlate lesions on imaging to those lesions found at surgery/pathology specimens. A total of seven patients with single histologically confirmed BDA formed the final study cohort.
The histopathologic specimens were retrospectively analyzed by a board-certified pathologist (G.P.R.) for confirmation of diagnosis of BDA. The preoperative imaging studies were reviewed.
Imaging features were reviewed by one board-certified radiologist for US (W.M.V.), CT (J.A.C.) and MRI (S.K.V.). Imaging features including size, number, location, echogenicity, attenuation, density, signal intensity, enhancement pattern and associated morphologic features of cirrhotic morphology, fatty liver, portal hypertension were noted.
We also performed a comprehensive literature review for imaging features of BDA since 2000 to provide additional context for our relatively small series. A systematic literature review was conducted using a PubMed search with keywords “bile duct adenoma, peribiliary hamartoma, or biliary adenoma”. Date range of this literature review was from January 1, 2000 through August 31, 2016 to account for availability of advanced CT and MRI techniques and histologic evaluation. Given the confusion with BDA nomenclature in the published literature, particularly with biliary hamartomas (Von Meyenberg Complexes), articles were included if the described histologic description of BDA was specified (i.e., numerous and small ducts interspersed with fibrous stroma) (8). Our search identified 10 studies with 13 cases in total (7, 10–18). These results are summarized in the Table, including imaging findings from the present study.
Table.
Imaging findings of bile duct adenoma from current and prior studies
| No | Reference | Sex | Age (yrs) | Sizea (cm) | US | CT | MRI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| NC | Art | Portal/Delayed | T1W | T2W | DWI | Art | Portal/Delayed | Eovist | ||||||
| 1 | Maeda, 2006 | F | 78 | 2 | ↑ | ↑ | ↓ | ↓ | ||||||
|
| ||||||||||||||
| 2 | Kim YS, 2010 | F | 59 | 1.7 | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ | ↓ | ↓ | ||
|
| ||||||||||||||
| 3 | Kim YC, 2010 | M | 53 | ↑ | ↑ | ↑ | ||||||||
|
| ||||||||||||||
| 4 | Koga, 2012 | M | 70 | 9 | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ | ↓ | ↓ | ||
|
| ||||||||||||||
| 5 | An, 2013 | M | 64 | 1.4 | ↓ | ↑ | = | ↓ | ↑ | ↑ | = | ↓ | ||
|
| ||||||||||||||
| 6 | Takumi, 2013 | M | 65 | 0.8 | ↓ | ↓ | ↑ | ↑ | ↓ | = | ↑ | ↑ | ↓ | ↓ |
|
| ||||||||||||||
| 7 | Chen, 2014 | M | 51 | 1.5 | ↓ | ↓ | ↓ | |||||||
|
| ||||||||||||||
| 8 | Liang, 2015 | F | 51 | 0.7 | ↓ | ↑ | ↑ | ↑ | ↑ | |||||
|
| ||||||||||||||
| 9 | Liang, 2015 | F | 21 | 1.2 | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ||||
|
| ||||||||||||||
| 10 | Liang, 2015 | M | 55 | 1.7 | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ||||
|
| ||||||||||||||
| 11 | Liang, 2015 | F | 38 | 1.6 | ↓ | ↑ | ↑ | ↑ | ↑ | |||||
|
| ||||||||||||||
| 12 | Wei, 2015 | F | 63 | 3.3 | ↓ | ↑ | ↑ | ↑ | ||||||
|
| ||||||||||||||
| 13 | Ahn, 2015 | M | 51 | 1 | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | = | ||
|
| ||||||||||||||
| 14 | Present, 2017 | M | 64 | 2.1 | = to ↑b | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | |||
|
| ||||||||||||||
| 15 | Present, 2017 | F | 26 | 1.2 | ↓ | ↓ | ↓ | |||||||
|
| ||||||||||||||
| 16 | Present, 2017 | M | 55 | 1.6 | ↓ | ↑ | ↑ | |||||||
|
| ||||||||||||||
| 17 | Present, 2017 | M | 76 | 0.1 | ↑ | |||||||||
|
| ||||||||||||||
| 18 | Present, 2017 | F | 49 | 1.2 | ↑ | ↑ | ||||||||
|
| ||||||||||||||
| 19 | Present, 2017 | F | 53 | 0.8 | ↓ | ↑ | ↑ | ↑ | ↑ | |||||
|
| ||||||||||||||
| 20 | Present, 2017 | M | 57 | 1 | ↓ | ↑ | ↑ | ↑ | ||||||
F, female; M, male; CT, computed tomography; MRI, magnetic resonance imaging; NC, noncontrast; Art, arterial phase; DWI, diffusion-weighted imaging; ↑, hyperintense; ↓, hypointense; =, isointense.
Maximum diameter;
Incomplete halo.
Results
The final group of seven patients included four males and three females ranging in age from 22 to 66 years (mean, 52.9 years). Of these seven BDAs, four were detected on imaging and targeted for ultrasound-guided biopsy. The remaining three cases underwent surgical resection/biopsy and were retrospectively correlated with imaging using landmarks. Five lesions were in the right hepatic lobe and two lesions in the left hepatic lobe. All seven patients had no prior history of trauma, biliary disease, infectious disease, or liver abscess.
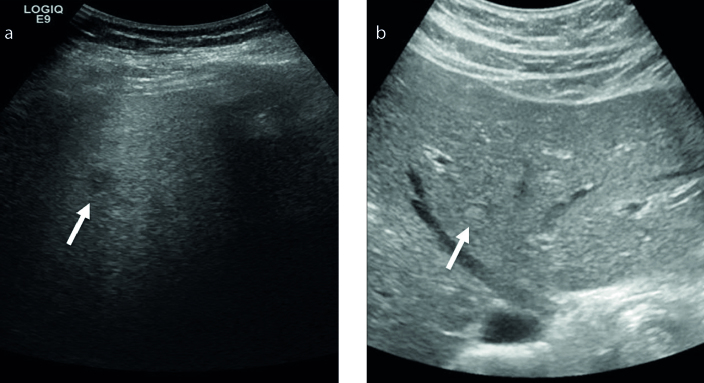
The imaging findings are summarized in the Table. US images were available in three patients. Although four of our seven cases were targeted for ultrasound-guided biopsy, only US studies of three cases were thought to be of diagnostic quality for appropriate lesion characterization. Two BDAs were hypoechoic with one having internal geographic echogenic areas. The second hypoechoic lesion had ill-defined borders, which may be the result of overall poor sonographic penetration secondary to background hepatic steatosis (Fig. 1). The third BDA was iso- to slightly hyperechoic with an incomplete hypoechoic halo (Fig. 1).
Figure 1. a, b.
US images in two different patients showing variable appearance of bile duct adenoma (BDA). The more common appearance is a hypoechoic lesion (arrow, a), but can also appear iso- to hyperechoic to liver parenchyma (arrow, b).
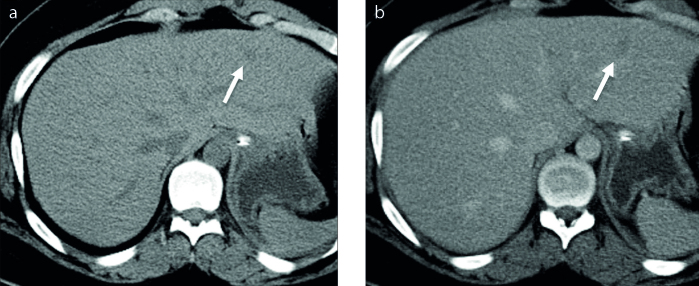
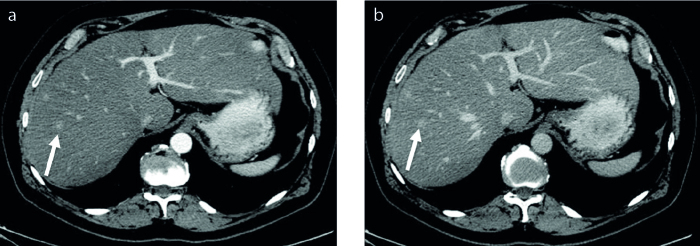
Unenhanced CT images of BDA was available in one patient. This BDA was hypodense relative to hepatic parenchyma (Fig. 2). Contrast-enhanced CT images were available in two cases in our series. Arterial phase hyperenhancement was seen in both cases (Fig. 3). Portal venous and/or delayed phase images were available in five cases. Four cases demonstrated portal venous (Fig. 3) or delayed phase hyperenhancement and one was hypodense on delayed phase images (Fig. 2).
Figure 2. a, b.
Unenhanced (a) and delayed phase (b) axial CT images of a BDA show a hypodense lesion (arrow) relative to background hepatic parenchyma.
Figure 3. a, b.
Contrast-enhanced CT images of BDA (arrow) show arterial phase hyperenhancement (a) that persists through the delayed phase (b).
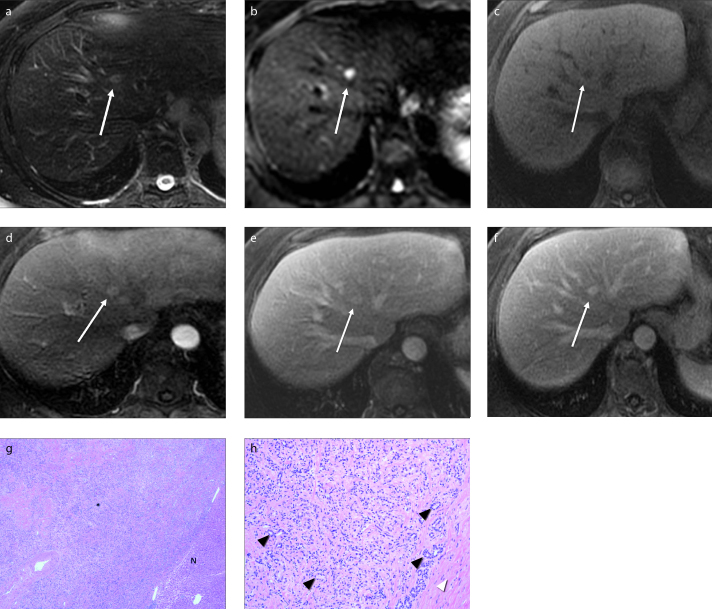
MRI was available in three cases in our series. All three lesions were T1-hypointense and T2-hyperintense. The two lesions, where diffusion-weighted imaging (DWI) was performed, demonstrated hyperintense signal intensity. On the ADC maps, the lesions appeared mildly hypointense to surrounding parenchyma with ADC values ranging from 1.16–1.23 × 10−3/mm2. There was no signal loss on the in-phase or opposed-phase images and lesions remained hypointense. On dynamic contrast-enhanced images, all three lesions demonstrated arterial phase hyperenhancement that persisted into the portal venous and delayed phases (Fig. 4).
Figure 4. a–h.
MRI of BDA. BDA (white arrow) appears hyperintense on T2-weighted (a) and diffusion-weighted (b) images, and hypointense on T1-weighted (c) image. On contrast-enhanced arterial phase (d), the lesion shows hyperenhancement that persists through portal (e) and delayed (f) phases. Histology of lesion with low power (g, 40× original magnification, H-E stain) shows the neoplasm (asterisk) on left and normal liver parenchyma (N) in right lower corner. Higher magnification (h, 100× original magnification) shows multiple undilated bile ductules (black arrowheads) surrounded by variable bands of fibrous tissue (white arrowhead).
Follow-up CT imaging was available in three patients over a period of 1 to 6 years. The biopsied lesions were stable on follow-up.
Discussion
We report on a series of seven patients with pathology-proven BDA (Fig. 4) and present their imaging findings. To the best of our knowledge, this is probably the largest single case series in English literature to describe the imaging features of BDA.
On US imaging, four cases were previously reported and described as hypoechoic to liver parenchyma (10–12) (Table 1). Of the three cases with US imaging in our series, there was a varied appearance, with one hyperechoic and two hypoechoic lesions. The hyperechoic lesion in our series had an incomplete peripheral halo. Interestingly, despite arterial phase enhancement seen in two cases on both CT and MRI, color Doppler flow was not identified at US. The cause for the variable appearance on US is unknown and this may relate to the composition of the lesion or the echogenicity of the background hepatic parenchyma.
BDA was hypodense in one case in our series with unenhanced CT imaging. Of the six cases reported in literature with unenhanced imaging, five were hypodense (7, 10, 13, 15, 16) and one was hyperdense with calcifications (14). Although BDA can have calcifications that can account for hyperdensity on unenhanced CT, majority of cases are likely to be hypodense.
Arterial phase hyperenhancement was seen in six of seven cases reported in literature (7, 10, 12–15). One case showed hypoenhancement relative to hepatic parenchyma (16). Two cases in our series, where arterial phase enhanced images had been obtained, also showed arterial phase hyperenhancement. Of the six cases in the literature where portal venous phase or delayed phase contrast images had been obtained, four demonstrated hyperenhancement (7, 10, 12, 13), one was isodense (15), and one was hypodense (16). Similar findings were also seen in our series with four of five cases demonstrating hyperenhancement on portal venous phase or delayed phase images. Overall, the most common BDA features on contrast-enhanced CT images included arterial phase hyperenhancement that persisted on portal venous and/or delayed phases. The persistence of hyperenhancement is probably related to the fibrous stroma, which is a characteristic component of this lesion.
On MRI, BDAs appear as hypointense relative to liver on T1-weighted images, hyperintense on T2-weighted images, and hyperintense on DWI. More specifically, all eleven lesions reported in the literature appeared hypointense on T1-weighted images (7, 10–15, 18) and 10 of 12 cases hyperintense on T2-weighted images (7, 11–15, 17, 18). Of the remaining two cases, one demonstrated hypointense signal (14) and the other lesion was isointense (10) on T2-weighted images. Of the seven cases in the literature where DWI had been obtained, all seven lesions were hyperintense on DWI (10, 11, 13). MRI appearances of the lesions in our series are in keeping with the literature with all three cases demonstrating hypointense T1-weighted signal intensity and hyperintense T2-signal intensity. Additionally, hyperintense signal intensity was seen in the two lesions in our series with DWI.
BDA also demonstrated consistent features on dynamic enhanced MRI. All eleven published cases demonstrated arterial phase enhancement (7, 10–13, 15, 17, 18), six continued to show hyperenhancement in portal venous and delayed phases (11, 17, 18), whereas three were hypointense (7, 10, 13) and two were isointense (12, 15). These findings were overall consistent with our case series with all three cases demonstrating arterial phase hyperenhancement that persisted on portal venous or delayed phase imaging. Hepatobiliary specific contrast agents were not used in our series; however, BDAs were hypointense to liver parenchyma in all four cases in the literature that used a hepatobiliary specific contrast agent (7, 10, 13, 15).
Clinically significant alternative diagnostic considerations when considering BDA include hepatocellular carcinoma (HCC), metastatic disease, and cholangiocarcinoma. The presence of persistent enhancement on portal venous or delayed phase images seen in cases of BDA would argue against HCC and metastatic disease. Imaging features of BDA may overlap with cholangiocarcinoma; however, the lack of additional biliary ductal abnormalities (e.g., biliary dilation or strictures) may help in differentiating the two entities. Tumor markers such as serum alpha-fetoprotein (HCC) and serum CA 19-9 (cholangiocarcinoma) levels may also assist in arriving at a correct diagnosis.
Benign entities that should be considered in differential diagnosis include small focal hepatic lesions such as a small hemangioma or focal nodular hyperplasia (FNH). However, differentiation of these lesions on portal venous and delayed phase imaging is critical for accurate diagnosis. More specifically, a hemangioma should follow blood pool in portal venous and delayed phase images and an FNH may appear isodense or isointense on portal venous and delayed phase images. This is in contrast to BDA, which shows hyperenhancement on arterial, portal venous and delayed phase imaging.
The “sonographic halo sign”, a hyperechoic lesion with hypoechoic rim, has been described in the literature as a feature that favors malignant isoechoic or hyperechoic tumors over benign lesions (19). In our case series we observed one lesion with this characteristic appearance. This is an important finding, as BDA may provide a benign differential consideration for a previous finding suggestive of malignancy. In general, a hypoechoic liver lesion will be indeterminate and prompt further imaging with CT or MRI.
This study has limitations. It is a retrospective series of a small study population. It is possible that many of these lesions may have been missed in routine clinical practice due to their small size. Additionally, imaging with CT, MRI, and US were not available in all patients.
In conclusion, BDAs tend to have imaging characteristics that are variable on US, hypodense on unenhanced CT with arterial phase hyperenhancement that persists into portal and/or delayed phase on contrast-enhanced CT. BDAs show T1 hypointensity, T2 hyperintensity and DWI hyperintensity with arterial phase hyperenhancement that persists into the portal venous and delayed phases. These imaging findings may be useful for characterization of BDAs and their differentiation from other lesions, particularly metastases.
Main points.
Bile duct adenoma (BDA) is a rare indolent benign neoplasm, and its differentiation from malignant or other benign lesions on imaging or histopathology can be challenging.
BDAs can show variable imaging features on US.
On CT, BDA appears hypodense on unenhanced phase, with arterial phase hyperenhancement that persists into portal and/or delayed phase.
BDAs have characteristic MRI features including T1 hypointensity, T2 hyperintensity, and DWI hyperintensity, with arterial phase hyperenhancement that persists into the portal venous and delayed phases.
MRI may be useful for characterization of BDAs and its differentiation from other lesions, particularly metastases.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Tsui WM. How many types of biliary hamartomas and adenomas are there? Adv Anat Pathol. 1998;5:16–20. doi: 10.1097/00125480-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Allaire GS, Rabin L, Ishak KG, Sesterhenn IA. Bile duct adenoma. A study of 152 cases. Am J Surg Pathol. 1988;12:708–715. doi: 10.1097/00000478-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Goodman ZD, Terracciano LM, Wee A. Tumours and tumour-like lesions of the liver. In: Burt A, Portman B, Ferrell L, editors. MacSween’s pathology of the liver. 6th ed. Edinburgh: Churchill Livingstone Elsevier; 2012. pp. 799–800. [DOI] [Google Scholar]
- 4.Lee K-B. Histopathology of a benign bile duct lesion in the liver: Morphologic mimicker or precursor of intrahepatic cholangiocarcinoma. Clin Mol Hepatol. 2016;22:400–405. doi: 10.3350/cmh.2016.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhathal PS, Hughes NR, Goodman ZD. The so-called bile duct adenoma is a peribiliary gland hamartoma. Am J Surg Pathol. 1996;20:858–864. doi: 10.1097/00000478-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Yantiss RK. Intraoperative evaluation of hepatic biliary lesions. In: Yantiss RK, editor. Frozen section library: liver, extrahepatic biliary tree and gallbladder. New York: Springer; 2011. pp. 21–44. [DOI] [Google Scholar]
- 7.Koga F, Tanaka H, Takamatsu S, et al. A case of very large intrahepatic bile duct adenoma followed for 7 years. World J Clin Oncol. 2012;3:63–66. doi: 10.5306/wjco.v3.i4.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pujals A, Amaddeo G, Castain C, et al. BRAF V600E mutations in bile duct adenomas. Hepatology. 2012;61:403–405. doi: 10.1002/hep.27133. [DOI] [PubMed] [Google Scholar]
- 9.Yang G. Liver tumors. In: Bibbo M, Wilbur D, editors. Comprehensive cytopathology. 4th ed. Philadelphia: Saunders/Elsevier; 2015. pp. 744–745. [Google Scholar]
- 10.Takumi K, Fukukura Y, Nagasato K, Nakajo M, Natsugoe S, Higashi M. Intrahepatic bile duct adenoma mimicking hepatic metastasis: case report and review of the literature. Magn Reson Med Sci. 2013;12:141–145. doi: 10.2463/mrms.2012-0078. [DOI] [PubMed] [Google Scholar]
- 11.Liang W, Xu S. Magnetic resonance imaging of intrahepatic bile duct adenoma. J Comput Assist Tomogr. 2015;39:747–751. doi: 10.1097/RCT.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 12.Ahn JM, Paik Y-H, Lee JH, et al. Intrahepatic bile duct adenoma in a patient with chronic hepatitis B accompanied by elevation of alpha-fetoprotein. Clin Mol Hepatol. 2015;21:393–397. doi: 10.3350/cmh.2015.21.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YS, Rha SE, Oh SN, et al. Imaging finding of intrahepatic bile duct adenoma (peribiliary gland hamartoma): a case report and literature review. Korean J Radiol. 2010;11:560–565. doi: 10.3348/kjr.2010.11.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda E, Uozumi K, Kato N, et al. Magnetic resonance findings of bile duct adenoma with calcification. Radiat Med. 2006;24:459–462. doi: 10.1007/s11604-006-0044-z. [DOI] [PubMed] [Google Scholar]
- 15.An C, Park S, Choi YJ. Diffusion-weighted MRI in intrahepatic bile duct adenoma arising from the cirrhotic liver. Korean J Radiol. 2013;14:769–775. doi: 10.3348/kjr.2013.14.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Xu MY, Chen F. Bile duct adenoma: a case report and literature review. World J Surg Oncol. 2014;12:125. doi: 10.1186/1477-7819-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YC, Park M-S, Chung YE, et al. MRI findings of uncommon non-hepatocyte origin primary liver tumours with pathological correlation. Br J Radiol. 2010;83:1080–1086. doi: 10.1259/bjr/61140265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei J, Zhang D, Yang J, Xu C. Intrahepatic bile duct adenoma (peribiliary gland hamartoma): a case report and review of literature. Int J Clin Exp Pathol. 2015;8:5908–5913. [PMC free article] [PubMed] [Google Scholar]
- 19.Wernecke K, Vassallo P, Bick U, Diederich S, Peters PE. The distinction between benign and malignant liver tumors on sonography: value of a hypoechoic halo. AJR Am J Roentgenol. 1992;159:1005–1009. doi: 10.2214/ajr.159.5.1329454. [DOI] [PubMed] [Google Scholar]