Abstract
PURPOSE
In the era of multiparametric magnetic resonance imaging (mpMRI) of the prostate gland, incidental findings are occasionally discovered on imaging. We aimed to report our experience of detecting incidental bladder cancers on mpMRI of the prostate in asymptomatic patients without irritative voiding symptoms or microscopic or gross hematuria.
METHODS
A retrospective review was performed on a prospectively maintained database of all men who underwent prostate mpMRI at our institution from 2012 to 2018. Patients who were found to have incidental bladder lesions were identified and baseline demographics, imaging and histopathologic data were recorded. All patients with incidental bladder lesion detection on mpMRI, not attributable to extension of prostate cancer, underwent cystoscopy in addition to a biopsy and/or transurethral resection of bladder tumor (TURBT) if warranted on cystoscopy.
RESULTS
There were 3147 prostate mpMRIs performed during this period and 25 cases (0.8%) of incidental bladder lesions were detected. These patients did not have any presenting symptoms such as gross or microscopic hematuria to prompt bladder lesion workup. The largest diameter of incidentally discovered bladder lesions ranged from 0.4 cm to 1.7 cm. Of the 25 cases of incidental bladder lesions, five were suspected to be due to prostate cancer invasion into the bladder. Only two of these five patients underwent biopsy, which confirmed prostate adenocarcinoma in both cases. Of the 20 patients without suspected prostate cancer invasion of the bladder, four had no suspicious lesions on cystoscopy to warrant a biopsy. The remaining 16 patients had bladder lesions seen on cystoscopy and underwent a biopsy and/or TURBT. Three of these patients had benign features on pathology (urachal remnant, amyloidosis and inflammation) and the remaining 13 had stage Ta urothelial carcinoma. Seven of these patients had low-grade Ta tumors and six had high-grade Ta tumors. All patients were treated with standard management of TURBT with or without intravesical BCG. There have been no reported cases of recurrence or progression in any of the patients in our cohort at the median follow-up of 26 months (interquartile range,19–40 months).
CONCLUSION
mpMRI of the prostate may yield incidental findings, such as small bladder tumors. Awareness of the possibility of incidental bladder lesions is important as 65% of lesions reported in the bladder, not attributable to extension of prostate cancer, proved to be bladder cancer. This may allow for early intervention for asymptomatic patients with undetected bladder cancer prior to disease progression.
Among American patients in 2018, it is estimated that there will be 81 190 new cases of bladder cancer with an estimated 16 870 deaths due to this disease (1). Clinical management of bladder cancer is primarily based on distinguishing non muscle-invasive bladder cancer (NMIBC) from muscle-invasive bladder cancer (MIBC). The standard treatment for NMIBC is transurethral resection of bladder tumor (TURBT) with or without intravesical therapy, such as mitomycin C (MMC) or Bacillus Calmette-Guerin (BCG). MIBC can be treated with radical cystectomy, radiation therapy, chemotherapy or a combination of these (2). As there is no accepted screening test for bladder cancer, only a symptomatic presentation triggers a work-up, which classically consists of cross-sectional imaging, cystoscopic evaluation and histologic examination of tissue sampled during cystoscopy (3). With the increased use of multiparametric magnetic resonance imaging (mpMRI) for prostate cancer there has been recognition that early, incidental bladder cancers can be detected (4–8).
mpMRI of the prostate is now widely used to improve prostate cancer detection, localization and staging (9). Ahmed et al. (10) found that mpMRI had a greater sensitivity, up to 92%, compared with standard TRUS, up to 60%, to rule out clinically significant disease. With mpMRI becoming more common, detection of incidental bladder lesions is also more likely. mpMRI has been shown to be useful in improving bladder cancer detection and staging as well as monitoring local disease recurrence (11). One of the main advantages of mpMRI in local and nodal staging of bladder cancer is its superior soft-tissue contrast, where it appears bright on T2-weighted imaging, and may show restricted diffusion, allowing differentiation between bladder wall layers and consequently NMIBC and MIBC (11, 12). mpMRI, with a reported accuracy of up to 92% for differentiating NMIBC from MIBC, has been shown to be superior to computed tomography (CT) imaging, with a reported accuracy of up to 60% in the local staging of bladder cancer (12). Even though mpMRI can provide additional prognostic information and effectively guide treatment options, larger studies are needed to define the exact role of mpMRI in bladder cancer patients (11).
Prevention of progression is a key goal in the treatment of patients with NMIBC (Ta/T1/carcinoma in situ). Morris et al. (13) found that up to 47% of bladder cancer related deaths were potentially avoidable, emphasizing the importance of early detection of bladder cancer. With the increased use of mpMRI for prostate cancer management, detection of incidental bladder lesions on prostate mpMRI may allow for early detection and treatment of asymptomatic bladder cancer. However, there are no previous studies comprehensively evaluating the detection of incidental bladder pathology found on prostate mpMRI. Here, we report the prevalence and clinical significance of incidental bladder findings found during routine use of mpMRIs for prostate cancer management.
Methods
All patients were enrolled on an institutional review board approved protocol (05-CC-0091) and provided informed consent. A retrospective review was performed on a prospectively maintained database of all men who underwent prostate imaging at our center from February 2012 to January 2018. Each patient underwent 3 Tesla mpMRI (Achieva; Philips Healthcare) with endorectal coil (BPX-30; Medrad) or 16-channel cardiac surface coil (SENSE; Philips Healthcare), as described previously (14). Two radiologists (P.L.C. and B.T.) prospectively identified and scored suspicious prostatic lesions using a previously validated in-house scoring system and, after its implementation in 2015, the Prostate Imaging Reporting and Data System version 2 (PI-RADS v2) scoring system using the T2-weighted, diffusion-weighted, and dynamic contrast-enhanced (DCE) sequences. Incidental bladder lesions, which are detected on all prostate mpMRI sequences, were prospectively noted in the prostate mpMRI reports, which were then retrospectively queried for incidental bladder lesions. Even though incidental bladder lesions were detected on all three prostate mpMRI sequences, the T2-weighted sequence was the most reliable as it was the only sequence to cover the entire bladder in all prostate mpMRI scans, including in the axial, sagittal, and coronal planes. The diffusion-weighted and DCE sequences did not necessarily cover the entire bladder in all the prostate mpMRI scans due to technical limitations. Patients with incidental bladder lesions were identified and baseline demographic, imaging and histopathologic data were recorded, including age, race, tumor size, number of lesions on mpMRI as well as tumor stage, grade and treatment received. All patients with incidental bladder lesion detection on prostate mpMRI, not attributable to extension of prostate cancer, underwent cystoscopy in addition to a biopsy and/or TURBT if warranted on cystoscopy.
Results
There were 3147 prostate mpMRIs performed during this period and 25 cases of incidental bladder lesions were detected (0.8%). None of these patients had any presenting symptoms to prompt a bladder lesion workup, including gross or microscopic hematuria. Baseline demographics and prostate biopsy results are shown in Table 1. The mean age of these patients was 69.2±8.0 years. The majority of patients were Caucasian (84%) and nonsmokers (80%). The diameter of incidentally discovered bladder lesions ranged from 0.4 cm to 1.7 cm. In total, 17 patients (68%) had Gleason score 6 or less prostate cancer. Three patients (12%) were found to have Gleason score 7 prostate cancer and five (20%) had Gleason score 8–10 prostate cancer.
Table 1.
Characteristics of patients found to have an incidental bladder lesion on mpMRI
| Demographics | |
|---|---|
| Age (years), mean±SD | 69.2±8.0 |
|
| |
| Race, n (%) | |
| Caucasian | 21 (84) |
| African American | 4 (16) |
|
| |
| Smoking history, n (%) | |
| Yes | 5 (20) |
| No | 20 (80) |
|
| |
| MRI-fusion biopsy Gleason score, n (%) | |
| ≤6 | 17 (68) |
| 7 | 3 (12) |
| 8 | 1 (4) |
| 9 | 3 (12) |
| 10 | 1 (4) |
mpMRI, multiparametric magnetic resonance imaging; SD, standard deviation.
Of the 25 patients with an incidental bladder lesion detected, five were suspected to be due to prostate cancer invasion into the bladder. Only two of these five patients underwent biopsy, which confirmed prostate adenocarcinoma in both cases. All five patients with suspected prostatic invasion into the bladder had high-risk prostate cancer (Gleason score ≥8; mean prostate-specific antigen, 61.5±97.7) and went on to receive androgen deprivation therapy and/or radiation therapy. Of the 20 patients without suspected prostate cancer invasion of the bladder, four had no suspicious lesions on cystoscopy to warrant a biopsy. The remaining 16 patients had bladder lesions seen on cystoscopy and underwent a biopsy and/or TURBT. Three of these patients had benign features on pathology (urachal remnant, amyloidosis, and inflammation) and the remaining 13 (0.4%) had stage Ta urothelial carcinoma (Table 2). Seven of these patients had low-grade Ta tumors and the remaining six had high-grade Ta tumors. Of the patients without mpMRI suspicion of prostate cancer invasion into the bladder, 65% of bladder lesions were found to be bladder cancer. All patients were treated with standard management of TURBT with or without intravesical BCG. No recurrences have been seen with a median follow-up of 26 months (interquartile range,19–40 months). The mpMRI findings for three of these patients are shown in the Fig.
Table 2.
Clinical characteristics of patients with cancerous lesions on biopsy
| Largest lesion diameter on mpMRI (cm) | Histopathology | Management | |
|---|---|---|---|
| Stage | Grade | ||
| 0.9 | Ta | Low | TURBT |
| 0.4 | Ta | Low | TURBT |
| 0.9 | Ta | Low | TURBT |
| 1.8 | Ta | High | TURBT+BCG |
| 1.7 | Ta | High | TURBT+BCG |
| 1.4 | Ta | Low | TURBT |
| 1.1 | Ta | High | TURBT+BCG |
| 1.4 | Ta | High | TURBT+BCG |
| 1.4 | Ta | Low | TURBT |
| 0.8 | Ta | High | TURBT+BCG |
| 0.8 | Ta | High | TURBT+BCG |
| 0.8 | Ta | Low | TURBT |
| 0.4 | Ta | Low | TURBT |
TURBT, transuretheral resection of bladder tumor; BCG, Bacillus Calmette-Guerin.
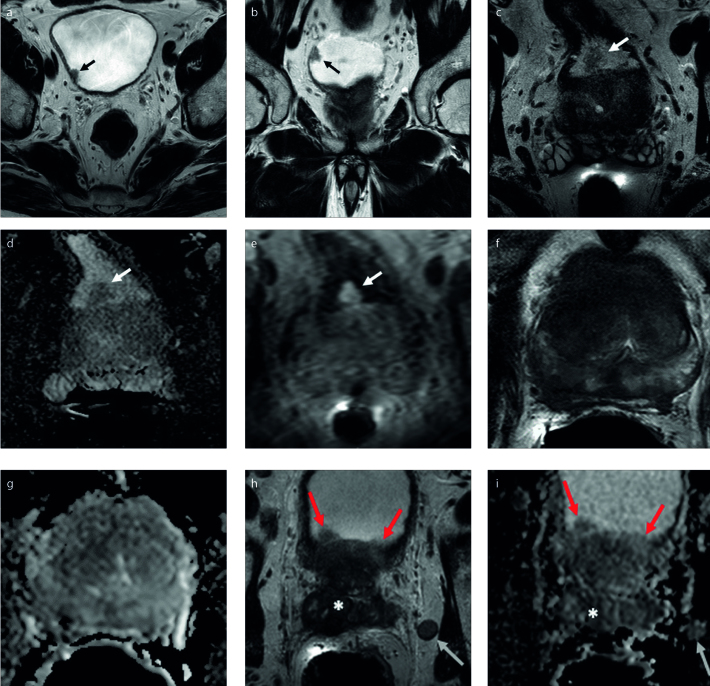
Figure. a–i.
A 75-year-old man with a serum PSA of 7.75 ng/mL and Gleason 3+3 prostate cancer (a, b). Axial (a) and coronal (b) T2-weighted images show an incidental papillary lesion in the left posterolateral bladder wall (arrows). A 76-year-old man with a serum PSA of 8.0 ng/mL and Gleason 3+3 prostate cancer (c–e). Axial T2-weighted image (c) shows a papillary lesion (arrow) in the posterior bladder wall at proximity of the intravesical prostate lobe with mild diffusion restriction on ADC map of DWI (d, arrow). DCE MRI (e) shows diffuse and early enhancement within this papillary lesion (arrow). Both of these patients were found to have low-grade Ta bladder cancer on pathology. In contrast to the prior images (a–e), the ensuing images (f–i) are of a 66-year-old male with a serum PSA of 48.90 ng/mL, Gleason 4+5 prostate cancer and prostate cancer invasion into the bladder. The lesion appears as a diffuse hypointense signal abnormality on T2-weighted image (f) and ADC map (g). The lesion involves almost the entire prostate gland and shows bladder wall (red arrows), seminal vesicle (asterisk) involvement on axial T2-weighted image (h) and ADC map (i). Additionally noted is a metastatic lymph node adjacent to the left seminal vesicles (h, i, white arrow). PSA, prostate-specific antigen; MRI, magnetic resonance imaging; ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; DCE, dynamic contrast enhanced.
Discussion
With the increasing use of mpMRI in the setting of prostate cancer, incidental findings are likely. While our rate of bladder cancer (0.4%) was low, this disease has considerable mortality if progression occurs and early diagnosis is key. Additionally, there is no accepted screening test for bladder cancer and diagnosis is usually made after a symptomatic presentation which triggers an evaluation (3). Up to 92% of bladder cancer patients present with painless hematuria, making this the most common presenting symptom (3, 15, 16). Other presenting symptoms, like bone pain or flank pain, can occur and typically present with more advanced disease. However, none of the patients in our cohort had any presenting symptoms, including gross or microscopic hematuria.
The 13 patients with biopsy-confirmed bladder cancer in our cohort all had noninvasive Ta urothelial carcinoma (7 low-grade, 6 high-grade). At the time of diagnosis, approximately 75% of bladder cancer is NMIBC (17). NMIBC is still susceptible to intravesical therapy, potentially sparing the patient from more aggressive treatments. However, up to 50% of NMIBC may still progress to invasive disease (17). A long-term follow-up study of 152 patients with initial low-grade Ta tumors noted stage progression in 5.3% of subjects and grade progression in 15% (18). Long-term follow-up studies of patients with high-grade Ta tumors have shown that lamina propria invasion occurs in as many as 40% of these patients and progression to MIBC occurs in up to 25% (19). Disease progression is one of the most relevant clinical outcomes for patients with NMIBC and the prevention of progression is a key goal in the treatment of these patients. When comparing the mean intervals to tumor recurrence, progression and death for MIBC vs. high-grade NMIBC, overall survival is significantly worse in MIBC (4.3 vs. 7.0 years, 8.4 vs. 12.5 years, and 4.6 vs. 8.2 years, respectively) (20). Not only is early detection of NMIBC associated with lower rates of recurrence, progression, and death but it can often avoid the morbidity associated with invasive, radical surgery that may be required for MIBC (2). With a median follow-up time of 26 months, there have been no reported cases of recurrence or progression in any of the patients in our cohort after treatment with TURBT with or without intravesical BCG.
Evidence has shown that bladder cancer is significantly more lethal than prostate cancer, with 5-year survival rates of 77.3% vs. 98.6%, respectively (21, 22). The increased mortality associated with bladder cancer compared with prostate cancer is even more striking when comparing the 5-year-survival rates by initial stage at diagnosis. Prostate cancer reports higher 5-year-survival than bladder cancer when diagnosed with localized (100% vs. 70.1%), regional (100% vs. 35.3%), and distant disease (29.8% vs. 5%) at initial presentation (21, 22). Awareness of the possibility of small bladder cancers is important as 65% of lesions reported in the bladder in our cohort, not attributable to extension of prostate cancer, proved to be small bladder cancers. As our results suggest, the increased use of mpMRI in the diagnosis and treatment of prostate cancer presents physicians with a unique opportunity to detect asymptomatic bladder cancer, which tends to be much more lethal than prostate cancer, and potentially treat it before disease progression. The routine use of mpMRI as a method of screening is likely unfeasible due to the cost and low incidence. However, already performed prostate mpMRIs should be carefully evaluated by both radiologists and urologists to carefully look at the bladder and identify any suspicious bladder lesions, especially in those with preexisting risk factors.
Our study has several limitations as our results are only applicable to men. Furthermore, the study is retrospective in nature and not all patients were ultimately treated for bladder cancer at our facility. However, the current study includes a uniform review of prostate mpMRI and complete pathology results on all patients with bladder cancer.
In conclusion, with mpMRI becoming more common in the setting of prostate cancer diagnosis and management, physicians should be aware of incidental findings to provide early intervention for asymptomatic patients with undetected bladder cancer prior to disease progression. This may be of clinical importance as lower stage bladder cancer is associated with significantly lower rates of progression, recurrence, and mortality.
Main points.
In our series, prostate mpMRI detected incidental bladder lesions at a rate of 0.8%.
Of incidental bladder lesions, 52% proved to be noninvasive Ta urothelial carcinoma (7 low-grade, 6 high-grade), 20% were suspected and/or confirmed high-grade prostate cancer invading into the bladder, 12% were benign on pathology, and 16% were false positive findings on mpMRI with no lesions seen on cystoscopy.
Detection of incidental bladder cancer on prostate mpMRI may be of clinical importance as lower stage bladder cancer is associated with significantly lower rates of progression, recurrence, and mortality.
Prostate mpMRIs should be carefully evaluated by both radiologists and urologists to carefully look at the bladder and identify any suspicious bladder lesions, especially in those with preexisting risk factors.
Footnotes
Financial disclosure
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. This research was also made possible in part through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, the Howard Hughes Medical Institute, the American Association for Dental Research, the Colgate-Palmolive Company, and other private donors.
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (Stages Ta, T1, and Tis): 2007 Update. J Urol. 2007;178:2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Shephard EA, Stapley S, Neal RD, Rose P, Walter FM, Hamilton WT. Clinical features of bladder cancer in primary care. Br J Gen Pract. 2012;62:598–604. doi: 10.3399/bjgp12X654560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEvoy SH, Lavelle LP, Purcell YM, et al. Should abdominal sequences be included in prostate cancer MR staging studies? Eur J Radiol. 2015;84:1019–1022. doi: 10.1016/j.ejrad.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Sherrer RL, Lai WS, Thomas JV, Nix JW, Rais-Bahrami S. Incidental findings on multiparametric MRI performed for evaluation of prostate cancer. Abdom Radiol (NY) 2018;43:696–701. doi: 10.1007/s00261-017-1237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sardari A, Thomas JV, Nix JW, et al. Incidental bladder cancer detected on multiparametric magnetic resonance imaging of the prostate gland. Case Rep Urol. 2015;2015:1–4. doi: 10.1155/2015/503154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmi A, Tabatabaei S, Talab SS, Hedgire SS, Cao K, Harisinghani M. Incidental findings at initial imaging workup of patients with prostate cancer: clinical significance and outcomes. AJR Am J Roentgenol. 2012;199:1305–1311. doi: 10.2214/AJR.11.8417. [DOI] [PubMed] [Google Scholar]
- 8.Lee B, Harvey S. Incidental lung cancer found on screening breast MRI with eventual lymphatic metastasis to the breast. Breast Dis. 2015;35:207–210. doi: 10.3233/BD-150406. [DOI] [PubMed] [Google Scholar]
- 9.Quintana L, Ward A, Gerrin SJ, et al. Gleason misclassification rate is independent of number of biopsy cores in systematic biopsy. Urology. 2016;91:143–149. doi: 10.1016/j.urology.2015.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–822. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- 11.de Haas RJ, Steyvers MJ, Fütterer JJ. Multiparametric MRI of the bladder: ready for clinical routine? AJR Am J Roentgenol. 2014;202:1187–1195. doi: 10.2214/AJR.13.12294. [DOI] [PubMed] [Google Scholar]
- 12.Verma S, Rajesh A, Prasad SR, et al. Urinary bladder cancer: role of MR imaging. Radiographics. 2012;32:371–387. doi: 10.1148/rg.322115125. [DOI] [PubMed] [Google Scholar]
- 13.Morris DS, Weizer AZ, Ye Z, Dunn RL, Montie JE, Hollenbeck BK. Understanding bladder cancer death. Cancer. 2009;115:1011–1020. doi: 10.1002/cncr.24136. [DOI] [PubMed] [Google Scholar]
- 14.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: Histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186:1818–1824. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pashos CL, Botteman MF, Laskin BL, Redaelli A. Bladder cancer: epidemiology, diagnosis, and management. Cancer Pract. 2002;10:311–322. doi: 10.1046/j.1523-5394.2002.106011.x. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez D, Gupta A, Canter D, et al. Microscopic haematuria at time of diagnosis is associated with lower disease stage in patients with newly diagnosed bladder cancer. BJU Int. 2016;117:783–786. doi: 10.1111/bju.13345. [DOI] [PubMed] [Google Scholar]
- 17.Chang SS, Bochner BH, Chou R, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol. 2017;198:552–559. doi: 10.1016/j.juro.2017.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leblanc B, Duclos AJ, Bénard F, et al. Long-term follow-up of initial Ta grade 1 transitional cell carcinoma of the bladder. J Urol. 1999;162:1946–1950. doi: 10.1016/S0022-5347(05)68075-5. [DOI] [PubMed] [Google Scholar]
- 19.Lamm D, Persad R, Brausi M, et al. Defining progression in nonmuscle invasive bladder cancer: it is time for a new, standard definition. J Urol. 2014;191:20–27. doi: 10.1016/j.juro.2013.07.102. [DOI] [PubMed] [Google Scholar]
- 20.Cao D, Vollmer RT, Luly J, et al. Comparison of 2004 and 1973 World Health Organization grading systems and their relationship to pathologic staging for predicting long-term prognosis in patients with urothelial carcinoma. Urology. 2010;76:593–599. doi: 10.1016/j.urology.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 21.Cancer Stat Facts: Bladder Cancer Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute [Internet] 2017. Cited February 2018. Available from: https://seer.cancer.gov/statfacts/html/urinb.html.
- 22.Cancer Stat Facts: Prostate Cancer Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute [Internet] 2017. Cited February 2018. Available from: https://seer.cancer.gov/statfacts/html/prost.html.