Abstract
We aimed to present a case of effective and successful endovascular treatment of acute lower limb thromboembolism with Clearlumen-II, a new aspiration thrombectomy device. Also, we emphasize the superiority of endovascular treatment compared with surgery, especially together with acute and chronic occlusive diseases, as in our case.
Acute lower extremity ischemia is a clinical emergency resulting from altered arterial blood flow to the extremities and is potentially harmful to the viability of the limb, or even life threatening (1). The most common cause is arterial thrombosis and consequent embolic occlusion. Immediate intervention is critical for the prognosis (1). Treatment options include endovascular or surgical revascularization (2); the use of the former approach is increasingly preferable due to the insufficiency and higher complication rates of surgery in many cases (3). Endovascular approach is indicated as a first-line treatment or after unsuccessful surgical attempt. Catheter-mediated thrombolysis in which a thrombolytic agent is directly applied in the vessel lumen, percutaneous aspiration thrombectomy or percutaneous mechanic thrombectomy are the methods used in endovascular treatment. One of the first identified percutaneous treatment methods is manual aspiration thrombectomy (4). Automated aspiration devices are also being implemented with the availability of more sophisticated technology. This report presents a case of acute lower extremity ischemia with an unsuccessful surgical thrombectomy followed by the first successful peripheral arterial endovascular use of ClearLumen-II, a novel automated aspiration with pulse spray thrombectomy device.
Technique
A 73-year-old female presented to our emergency department with an excruciating left extremity pain. She stated that the pain started 10 days ago and reached an intolerable point within the last 24 hours. Physical examination revealed cold, pale extremity with discoloration, decreased strength in dorsal and pedal muscles as well as infrapopliteal anesthesia/dysesthesia. Thromboses were identified in total left superficial femoral artery and popliteal artery using B-mode ultrasonography (US), and Doppler US demonstrated lack of blood flow in these arteries along with plantar and dorsalis pedis arteries. Intracardiac thrombus was excluded by echocardiography, and no intraabdominal emboli were seen by computed tomography angiography. Rutherford stage 2b acute lower extremity ischemia was diagnosed. The patient underwent surgical thrombectomy treatment in the cardiovascular surgery unit using a Fogarty catheter. Fogarty catheter failed to proceed beyond popliteal artery, therefore thrombectomy was limited only to suprapopliteal segment, but not to infrapopliteal segment. Symptoms were not relieved postsurgically and the patient was referred for percutaneous thrombectomy and intervention on possible chronic atherosclerotic occlusions.
Preprocedure US showed recurrent acute thrombus at the superficial femoral artery starting from the proximal segments. After receiving the informed consent, the patient was taken to the angiography suite. After sterilization and local anesthesia, US-guided antegrade 8 F sheath was placed from left main femoral artery towards superficial artery. Preoperative angiography evaluation showed occlusion due to the total thrombus at superficial femoral artery starting from proximal segment (Fig. 1). A 5 F vertebral guide catheter and a 0.035-inch guidewire were used to pass through the occluded segment and to reach popliteal artery. Resistance was encountered at the popliteal artery segment and we failed to reach further segments. A 0.018-inch guidewire was placed and ClearLumen-II catheter (Clearlumen-II, Groupates) was used to aspirate thrombus to the resistant level at popliteal artery. After total thrombus aspiration, we passed the resistant level with the vertebral catheter and a 0.018-inch wire and reached the proximal segment of peroneal artery. Angioplasties were performed on popliteal artery and peroneal artery, using two balloons of 5×50 mm and 2.5×200 mm (Simpass, Simeks), respectively. After angioplasty, blood flow was recovered at superficial femoral artery, popliteal artery, and peroneal artery; there was a focal residual stenosis at popliteal artery. A 5×60 mm self-expandable stent (Protege, Medtronic) was placed and postdilatation was performed using a 5 mm balloon. Occluded anterior tibial artery was passed using the vertebral catheter and a 0.018-inch guidewire and true lumen at dorsalis pedis artery was reached. Angioplasty was performed at the anterior tibial artery using a 3×200 mm balloon (Simpass, Simeks). Angiography demonstrated successful patency in anterior tibial artery, despite filling defect and focal residual stenosis at proximal segment of peroneal artery, and a 3×20 mm balloon expandable stent (Simflex, Simeks) was placed there. Patency was postoperatively demonstrated with digital subtraction angiography (DSA) in the superficial femoral artery, popliteal artery, peroneal artery, anterior tibial artery, and dorsalis pedis artery (Fig. 2). The sheath was removed and a 8 F angioseal was used for hemostasis. Heparin 7500 IU bolus was administered during the procedure.
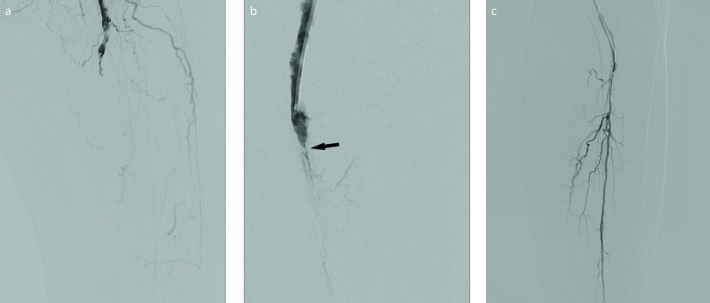
Figure 1. a–c.
Preoperative digital subtraction angiograms (DSA). DSA (a) of the proximal thigh shows occlusion of the superficial femoral artery. Filling defects starting at the proximal part which is consistent with an acute thrombus. Common femoral artery, which was normal, is not shown. DSA (b) shows popliteal artery also occluded by acute thrombi. Thrombus is narrowed at this level (arrow), suggesting chronic occlusive disease. Panel (c) shows that after pass through occlusive segment, only peroneal artery was patent.
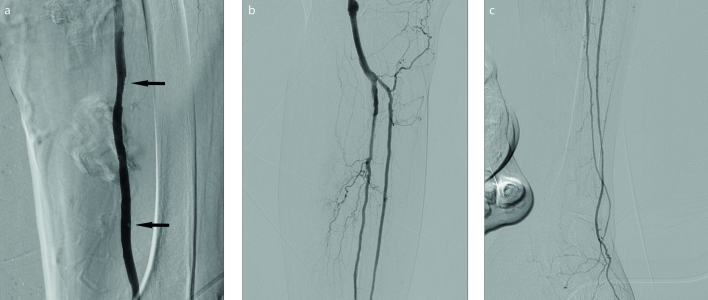
Figure 2. a–c.
Postoperative DSAs. After aspiration by using Clearlumen-II device (a), superficial femoral artery is almost completely patent, except minimal thrombi (arrows). DSA (b) after the aspiration, shows angioplasties performed on popliteal artery, peroneal artery and anterior tibial artery that had chronic lesions. Stents were placed because of focal residual stenosis and dissections on popliteal and peroneal arteries. DSA (c) of the calf shows that patency of anterior tibial artery and peroneal artery was granted at the end of treatment.
Clexane 0.6 mg (BID for ten days), acetyl-salicylic acid 100 mg (once a day for life) and clopidogrel 75 mg (4×75 mg loading dose first day, followed by once a day for 6 months) were prescribed. Physical examination showed improved popliteal artery and dorsalis pedis artery pulses, and resolved paleness, coldness, and discoloration, and the patient was discharged on the third day of hospitalization.
Discussion
If left untreated, acute arterial lower extremity ischemia is harmful to the extremity and may even threaten life. TransAtlantic Inter-society Consensus report shows that Rutherford stage 1 and 2a ALI may be treated with thrombolysis or embolectomy, whereas stage 2b should promptly undergo embolectomy. Currently, surgery is the treatment of choice. Traditional surgical method is bypass; however, due to presence of serious complications including high rates of major amputations, Fogarty catheter thrombectomy has been replacing the surgical bypass approach (5). Albeit its popularity, Fogarty catheter thrombectomy poses risks of vascular damage and distal embolus. Moreover, background chronic atherosclerotic disease—as also seen in our case—or chronic thrombus reduce the success rates. This method has variable success and complication rates in the literature (6).
Endovascular methods are increasingly preferable due to their high success rate and their availability for patients not eligible for surgery. Endovascular approaches include catheter mediated thrombolysis, aspiration thrombectomy, and percutaneous mechanic thrombectomy. Another advantage of these methods is the therapeutic intervention on chronic thrombus or underlying atherosclerotic disease. In our patient, Fogarty catheter failed to pass beyond the proximally popliteal artery segment due to a possible chronic atherosclerotic occlusion that may have also caused thrombus. In this situation, artery lumen was initially opened with the novel ClearLumen-II and after reaching beyond this segment, the underlying pathology was simultaneously treated; this is the most prominent superiority of endovascular treatment over surgery. Finally, adverse effects caused by anesthesia is much lower with the endovascular method; local anesthesia was sufficient in our procedure.
Aspiration thrombectomy is an earlier method than mechanical thrombectomy. Manual aspiration of the thrombus was described by Snidermann et al. (4) in 1984. Aspiration thrombectomy, which has been widely used in deep venous thrombosis, dialysis fistulas, renal vein thrombosis and pulmonary thromboembolism, was not common in lower extremity embolisms. On the other hand, recent studies have shown that aspiration thrombectomy is highly effective in acute lower extremity thromboembolism (7). In these studies, low complication rates have been shown along with high success rates. This procedure, in which the thrombus is removed quickly, easily and effectively, was more commonly used as manual aspiration. With the developing technology, pulse spray thrombolysis method has been developed to allow the thrombus to break down.
Acute emboli can be treated with various thrombectomy devices produced by numerous companies, both in the peripheral vascular bed and in patients with acute ischemic stroke. A new device, the 8 F sheath compatible ClearLumen-II system, is one of them. Unlike conventional vacuum-based automatic aspiration devices, simultaneous aspiration and thrombus hydrolysis performed with high-pressure jet saline flow (pulse spray thrombolysis) is used in this system. This system is aimed to facilitate the aspiration of the thrombus, to reduce catheter occlusion and at the same time to prevent distal embolization by applying constant negative pressure to the thrombus from the catheter tip. The device can work over a 0.014–0.035 inch wire, with a wider lumen left for aspiration when the wire diameter is thin. In patients who have underlying chronic atherosclerotic disease, the stiffness of wire might be necessary to carry the device. Thus, in this case, we preferred to use the 0.018-inch wire. The ClearLumen system was first used in 2016 by Luigi Biasco et al. (8) in coronary emboli, and as far as we know, it is the only report in English literature (8): aspiration of thrombus was successfully performed in 19 of 20 patients (95%), requiring balloon dilation aid in only one patient. In addition, only one independent device-related complication was seen in 20 patients (side branch occlusion). To our knowledge, there are no published articles so far on peripheral artery embolectomy treatment. In our case, the acute thrombus in the superficial femoral artery was successfully treated with ClearLumen-II device, allowing the underlying chronic occlusion to be visualized.
In conclusion, new ClearLumen-II automated pulse spray thrombolysis aspiration thrombectomy device in acute lower limb ischemia successfully removed the thrombus without complication and enabled the diagnosis and treatment of chronic atherosclerotic stenosis. The number of clinical trials with this device is quite limited and further studies are required for reliability.
Main points.
Endovascular treatment is increasingly preferred, as acute lower limb ischemia and chronic thrombus or underlying atherosclerotic disease can be treated at the same time.
Endovascular treatment is available for patients not eligible for surgery or patients unsuccessfully treated by surgery.
ClearLumen-II is a novel automated aspiration with pulse spray thrombectomy device.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Walker TG. Acute limb ischemia. Tech Vasc Interv Radiol. 2009;12:117–129. doi: 10.1053/j.tvir.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA Focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery. J Vasc Surg. 2011;54:e32–e58. doi: 10.1016/j.jvs.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Vorwerk D. Mechanical thrombectomy is an alternative way to go: the European experience commentary on: quality improvement guidelines for percutaneous management of acute limb ischemia. Cardiovasc Interv Radiol. 2006;29:7–10. doi: 10.1007/s00270-005-8888-7. [DOI] [PubMed] [Google Scholar]
- 4.Sniderman K, Bodner L, Saddekni S, Srur M, Sos T. Percutaneous embolectomy by transcatheter aspiration. Work in progress. Radiology. 1984;150:357–361. doi: 10.1148/radiology.150.2.6228952. [DOI] [PubMed] [Google Scholar]
- 5.Antusevas A, Aleksynas N. The surgical treatment of acute ischemia of the lower limb. Medicina (Kaunas) 2002;39:646–653. [PubMed] [Google Scholar]
- 6.Blaisdell F, Steele M, Allen R. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978;84:822–834. [PubMed] [Google Scholar]
- 7.Oguzkurt L, Özkan U, Gümüs B, Coskun I, Koca N, Gülcan Ö. Percutaneous aspiration thrombectomy in the treatment of lower extremity thromboembolic occlusions. Diagn Interv Radiol. 2010;16:79. doi: 10.4261/1305-3825.DIR.2654-09.1. [DOI] [PubMed] [Google Scholar]
- 8.Biasco L, Götberg M, Harnek J, et al. First in-man experience with the ClearLumen thrombectomy system as an adjunctive therapy in primary percutaneous coronary interventions. J Interv Cardiol. 2016;29:155–161. doi: 10.1111/joic.12285. [DOI] [PubMed] [Google Scholar]