Abstract
PURPOSE
We aimed to evaluate the genitourinary function and quality of life (QoL) following the ablation of different prostate segments with irreversible electroporation (IRE) for localized prostate cancer (PCa).
METHODS
Sixty patients who received primary focal IRE for organ-confined PCa were recruited for this study. Patients were evaluated for genitourinary function and QoL per prostate segment treated (anterior vs. posterior, apex vs. base vs. apex-to-base, unilateral vs. bilateral). IRE system settings and patient characteristics were compared between patients with preserved vs. those with impaired erectile function and urinary continence. Data were prospectively collected at baseline, 3, 6, and 12 months using the expanded prostate cancer index composite, American Urological Association symptom score, SF-12 physical and mental component summary surveys. Difference over time within segments per questionnaire was evaluated using the Wilcoxon’s signed rank test. Outcome differences between segments were assessed using covariance models. Baseline measurements included questionnaire scores, age, and prostate volume.
RESULTS
There were no statistically significant changes over time for overall urinary (P = 0.07–0.89), bowel (P = 0.06–0.79), physical (P = 0.18–0.71) and mental (P = 0.45–0.94) QoL scores within each segment. Deterioration of sexual function scores was observed at 6 months within each segment (P = 0.001–0.16). There were no statistically significant differences in QoL scores between prostate segments (P = 0.08–0.97). Older patients or those with poor baseline sexual function at time of treatment were associated with a greater risk of developing erectile dysfunction.
CONCLUSION
IRE is a feasible modality for all prostate segments without any significantly different effect on the QoL outcomes. Older patients and those with poor sexual function need to be counseled regarding the risk of erectile dysfunction.
Focal therapy has been introduced as an alternative treatment option for patients with unifocal, organ-confined prostate cancer (PCa) (1). The nature of this therapy is selective and lesion-based to preserve genitourinary function. Important structures that are spared include the neurovascular bundles, urethra, rectal wall, urethral sphincter, and bladder neck.
The initial experience with focal therapy was derived from whole-gland cryotherapy and high-intensity focused ultrasound (HIFU) (2, 3). Over time, treatments became more lesion-based due to the increasing experience and improvements in multiparametric magnetic resonance imaging (mpMRI) (4). The feasibility of other ablative modalities were considered (e.g., radiofrequency ablation, focal brachytherapy) in light of new technologies such as photodynamic therapy, laser interstitial thermotherapy, transurethral HIFU (TULSA) and irreversible electroporation (IRE) (5). Recently, Valerio et al. (5) reported the outcomes of 73 phase 1–2 clinical trials on focal therapy. Their report established the feasibility and safety of focal therapy, demonstrating a low impact on genitourinary function and quality of life (QoL).
However, there is a lack of evidence in evaluating focal therapy against PCa treatments that are currently in the guidelines. Similarly, there is a lack of consensus and data to determine which focal therapy modality is superior. Sivaraman and Barret (6) recently proposed an alternative approach utilizing multiple ablative technologies: the “à la carte” approach. The authors advocated that rather than looking for a one-size-fits-all modality, a tailored solution to each individual patient depending on the PCa lesion localization should be used. They argue that certain ablative modalities are better suited for lesions in certain prostate segments. In their experience; to preserve genitourinary function, posterior lesions are best treated with HIFU, anterior lesions with cryotherapy, and apical lesions with focal brachytherapy. At this point, their theoretical concept has not been validated in a clinical trial. More so, there are no studies on the performance of each of the available ablative systems on the different prostate segments in terms of oncologic control, genitourinary function or QoL. Studies on the system settings used during focal therapy and the dosimetry are also lacking.
The initial feasibility trials with IRE demonstrated the safety and feasibility of this new technique with low patient morbidity and good short-term oncologic control (7–12). IRE ablates tumor tissue by delivering a direct high-voltage current between a pair of needle electrodes (13). By targeting cells with multiple consecutive electrical pulses, the cell membrane becomes irreversibly permeable, resulting in cell death (13). However, there are no studies on the performance of IRE in the different prostate segments, in terms of oncologic control, genitourinary function and QoL. An IRE ablation and resection study by Van den Bos et al. (14) showed that all the tissue within the needle configuration is ablated. Since all the prostate segments can be encompassed within the needle configurations of IRE, oncologic control should not theoretically differ between the prostate segments. Failures after IRE were recently proven to be significantly dependent on the applied safety margin and/or system errors that occurred during the treatment (12).
In this study, we evaluated the impact on the genitourinary function and QoL following the ablation of different prostate segments with IRE. In case of genitourinary functional failure, i.e., urinary incontinence requiring pads and/or erection insufficient for intercourse, patient characteristics and system settings were analyzed to assess potential risk factors.
Methods
Study design and patients
Patients treated between February 2013 and August 2016 with primary IRE for localized PCa were invited to have their genitourinary function and QoL evaluated. Pretreatment template mapping biopsies and mpMRI were used to diagnose PCa lesions and to identify prostate segments requiring treatment, in accordance with the selection guidelines (15). A total of 72 patients consented for prospective evaluation, of which 60 had at least 6 months of follow-up and were included for final retrospective analysis of prospectively acquired data (Table 1). Data was collected at baseline, 3, 6, and 12 months. As outlined above, this analysis focused on the genitourinary function and QoL only but not on oncologic outcomes.
Table 1.
Patient characteristics (n=60)
| Variable | Value |
|---|---|
| Age (years), mean±SD | 68±7.0 |
|
| |
| PSA (μg/L), mean±SD | 6.0±3.3 |
|
| |
| Prostate volume (cc), mean±SD | 44±21 |
|
| |
| Clinical stage, n (%) | |
| T1c | 3 (5) |
| T2a | 40 (67) |
| T2b | 7 (12) |
| T2c | 10 (17) |
|
| |
| Gleason score, n (%) | |
| 6 | 8 (13) |
| 3+4 | 40 (67) |
| 4+3 | 10 (17) |
| 4+4 or higher | 2 (3) |
|
| |
| Ablated segments, n (%) | |
| Anterior | 18 (30) |
| Posterior | 39 (65) |
| Apex | 18 (30) |
| Base | 14 (23) |
| Apex-to-base | 26 (43) |
| Unilateral | 50 (83) |
| Bilateral | 10 (17) |
SD, standard deviation; PSA, prostate specific antigen.
Ethical approval
The institutional review board of the Human Research Ethics Committee of the St. Vincent’s Hospital (Sydney, Australia) approved prospective collection of genitourinary function and QoL data (HREC approval SVH 13/018). The data collection was executed in adherence to the declaration of Helsinki (Fortealeza, Brazil, October 2013) and written informed consent was obtained from all patients.
IRE procedure
Single-surgeon IRE was performed under general anesthesia, antibiotic prophylaxis, and deep-muscle relaxation. An indwelling catheter was placed prior to the procedure. Using the Nanoknife® system (AngioDynamics), four to six needle electrodes were placed with a transperineal approach, encircling the tumor lesion. Needle placement was guided by biplanar transrectal ultrasound (BK medical) and a floor mounted transperineal template grid. The needle locations and configuration geometry was recorded in the Nanoknife® system and operation report. The inter-electrode distance ranged from 6 to 22 mm and the active tip length from 15 to 20 mm. Ten test pulses were delivered to evaluate the obtained direct current with the standard applied voltages (~1500 V/cm). The remaining 80 treatment pulses were delivered if an adequate current was achieved (20–40 A); otherwise the applied voltage was altered until an adequate current was reached. The median minimum and maximum applied voltages were 1600 V (interquartile range [IQR], 1400–1760 V) and 2550 V (IQR, 2400–2850 V). The median minimum and maximum direct currents were 25 A (IQR, 20–28 A) and 43 A (IQR, 38–49 A). The pulse length was initially set at 70 μs (17 cases in total), but increased to 90 μs to adhere to the international treatment protocol proposed by the Clinical Research Office of the Endourological Society (CROES). The catheter was removed within 2–5 days depending on preexisting lower urinary tract symptoms.
Follow-up of genitourinary function and QoL
Genitourinary function and QoL data were prospectively evaluated using questionnaires at baseline, 3, 6, and 12 months. The expanded prostate cancer index composite (EPIC) (16), American Urological Association (AUA) symptom score (17), short form of health survey (SF-12) physical and mental component summary surveys (18) were used for genitourinary and QoL evaluation.
Segmental definitions and risk factor analysis
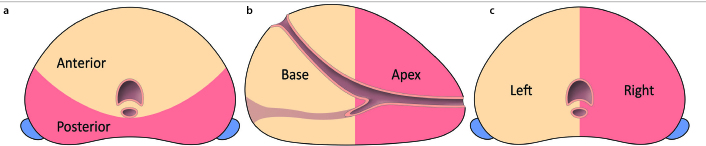
The prostate was segmentally divided into: 1) anterior (i.e., transition zone and fibromuscular stroma) vs. posterior (i.e., peripheral zone including anterior horn), 2) apex (i.e., apex or apex to mid-apex) vs. base (i.e., base or base to mid-base) vs. apex-to-base (i.e., ablation from apex to base), and 3) unilateral vs. bilateral (i.e., unifocal ablation crossing the midline or multifocal bilateral ablation). The division into the different prostate segments is illustrated in the Figure. An ablation covering at least 80% of its total ablation zone volume in one segment was deemed a segmental ablation. The ablation zone volume was determined by perioperative needle configuration and consisted of the area within the active needle configuration. For bilateral and apex-to-base ablations, 80% of the ablation zone volume covered both segments. Patients that had ablations covering multiple segments outside the specified segments were excluded from segmental analysis since potential interference was possible.
Figure.
Schematic overview of the different prostate segments. (a) The anterior segment (beige) consists of the fibromuscular stroma with the transition zone. The posterior segment (red) consists of the peripheral zone, including the anterior horn of the transition zone. (b) The apical segment (red) includes any ablation performed within the mid to upper apex. The base segment (beige) is considered as any ablation in the area from the mid to the distal base. The apex-to-base segment included both the apex and base for more than 80% of the ablation zone volume. (c) A bilateral ablation was divided as an ablation that had more than 80% of the ablation zone volume in both the left and right hemi-prostate. Unilateral ablation was performed when more than 80% of the ablation occurred in either the left (beige) or right (red) hemi-prostate.
For each individual segment, the 6-month questionnaire was used to assess changes on genitourinary function and QoL over time compared to baseline. Summary score differences from baseline and 6 months were used to assess the performance of IRE per prostate segment, correcting for baseline age and prostate volume. In patients with genitourinary functional failure following IRE treatment (i.e., urinary incontinence requiring pads and/or erections insufficient for intercourse), the standard system settings (interelectrode distance, voltage, amperage, pulse length) and patient characteristics (age, prostate volume, baseline urinary/sexual function) were compared with patients without genitourinary functional failure. As 6 patients did not answer the question (Q32) regarding erections sufficient for intercourse in the 6-month questionnaire, despite completing the questionnaire, their answer to that question in their latest questionnaire (3 or 12 months) was used as a substitute.
Statistical analysis
The operational hypothesis of differences in genitourinary function and QoL between segments was tested by the analysis of covariance (ANCOVA) model. In this model, the dependent variable was the measured value at month 6, the independent variable was the treatment group, and the covariate was the measured baseline value. All data were log-transformed prior to the analysis. This ANCOVA model was preferred to simpler methods such as the paired t-test, because by including baseline values in the modeling, it removes the potential “regression toward the mean” effect. Post-hoc comparison between groups was conducted with the Tukey’s honest significant difference test within the R statistical environment (R Development Core Team, 2011) (19). The level of significance was set at P < 0.05.
Results
The study included 60 patients (median age, 67 years; IQR, 62–73 years) who underwent IRE treatment. The median prostate specific antigen level was 5.9 μg/L (IQR, 3.6–7.6 μg/L). In three patients, the ablation covered both the anterior and posterior segment and these patients were excluded for anterior vs. posterior segmental analysis. Likewise, two patients received multiple ablations interfering with apex vs. base vs. apex-to-base segmental analysis. Table 1 summarizes the patient characteristics, including the number of patients per segmental ablation. None of the patients started androgen deprivation therapy in the course of follow-up.
By the 6th month, no statistically significant deteriorations on the AUA symptom score (P = 0.17–0.89), EPIC urinary (P = 0.07–0.88), EPIC bowel (P = 0.06–0.79) and both SF-12 physical (P = 0.18–0.71) and mental (P = 0.45–0.94) scores were observed from baseline for each individually treated segment. For each segment, a significant decline in the EPIC summary sexual score was found (P = 0.001–0.046), except for the bilateral segment (P = 0.16) despite a decline within that segment (median decline from 83 to 63). This decline on the EPIC sexual score would have been significant at 12 months (P = 0.28) compared with baseline. None of the segments were significantly associated with better-preserved or deteriorated genitourinary function or QoL when the outcomes were compared with the opposite group (P = 0.08–0.97). In Tables 2–4 the median summary scores per segment treated over time are presented.
Table 2.
Anterior vs. posterior genitourinary function and quality of life
| Baseline | 3 months | 6 months | 12 months | Segment difference Baseline/6 months |
Different treatment impact Anterior vs. Posterior |
|
|---|---|---|---|---|---|---|
| Anterior | (n=18) | (n=17) | (n=17) | (n=4) | ||
| Posterior | (n=39) | (n=39) | (n=35) | (n=20) | ||
| AUA | ||||||
| Anterior | 6 (3–14) | 6 (3–11) | 4 (3–10) | 4 (2–5) | No (P = 0.55) | No (P = 0.97, |
| Posterior | 6 (3–12) | 7 (3–10) | 5 (2–11) | 4 (2–11) | No (P = 0.19) | E= −0.05, CI ±2.5) |
|
| ||||||
| EPIC urinary | ||||||
| Anterior | 93 (72–98) | 89 (69–96) | 94 (79–98) | 92 (82–97) | No (P = 0.68) | No (P = 0.83, |
| Posterior | 89 (81–98) | 92 (81–98) | 92 (83–98) | 94 (85–98) | No (P = 0.24) | E= −0.71, CI ±6.6) |
|
| ||||||
| EPIC sexual | ||||||
| Anterior | 60 (25–82) | 52 (29–71) | 46 (14–79) | 27 (2–79) | Yes (P = 0.03) | No (P = 0.41, |
| Posterior | 67 (48–81) | 47 (31–74) | 49 (29–69) | 42 (19–76) | Yes (P = 0.008) | E= −4.1, CI ±9.6) |
|
| ||||||
| EPIC bowel | ||||||
| Anterior | 96 (92–100) | 96 (93–98) | 96 (91–99) | 93 (87–99) | No (P = 0.79) | No (P = 0.80, |
| Posterior | 96 (93–98) | 96 (89–100) | 96 (89–100) | 97 (92–100) | No (P = 0.70) | E= 0.51, CI ±3.9) |
|
| ||||||
| SF-12 physical | ||||||
| Anterior | 55 (44–56) | 55 (48–56) | 55 (40–57) | 57 (43–58) | No (P = 0.64) | No (P = 0.74, |
| Posterior | 56 (52–56) | 55 (52–57) | 55 (52–57) | 55 (52–57) | No (P = 0.35) | E= −0.71, CI ±4.1) |
|
| ||||||
| SF-12 mental | ||||||
| Anterior | 56 (39–58) | 56 (50–58) | 56 (40–60) | 53 (48–60) | No (P = 0.80) | No (P = 0.64, |
| Posterior | 56 (50–58) | 57 (53–59) | 56 (48–58) | 57 (56–59) | No (P = 0.45) | E= 1.1, CI ±4.4) |
Data are presented as median (interquartile range).
AUA, American Urological Association; E, the effect size of anterior vs. posterior; CI, confidence interval (represents 47.5% deviation from the mean); EPIC, expanded prostate cancer index composite; SF-12, short form of health survey.
Table 3.
Apex vs. Base vs. Apex-to-Base genitourinary function and quality of life
| Segment difference Baseline/6 months |
Different treatment impact | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Baseline | 3 months | 6 months | 12 months | Apex vs. Base | Apex vs. Apex-to-Base | Base vs. Apex-to-Base | ||
| Apex | (n=18) | (n=17) | (n=17) | (n=10) | ||||
| Base | (n=14) | (n=14) | (n=13) | (n=4) | ||||
| Apex-to-Base | (n=26) | (n=26) | (n=24) | (n=11) | ||||
| AUA | ||||||||
| Apex | 3 (2–16) | 7 (3–10) | 4 (2–12) | 4 (2–8) | No (P = 0.86) | No (P = 0.79, | No (P = 0.28, | No (P = 0.41, |
| Base | 10 (4–12) | 10 (4–13) | 7 (4–14) | 8 (2–23) | No (P = 0.89) | E= 0.43, | E= −1.5, | E= 1.9, |
| Apex-to-Base | 6 (4–14) | 6 (3–11) | 5 (3–10) | 4 (3–5) | No (P = 0.19) | CI ±3.1) | CI ±2.7) | CI ±3.0) |
|
| ||||||||
| EPIC urinary | ||||||||
| Apex | 96 (81–98) | 94 (78–99) | 96 (77–98) | 94 (90–96) | No (P = 0.88) | No (P = 0.64, | No (P = 0.34, | No (P = 0.93, |
| Base | 87 (78–94) | 89 (74–96) | 90 (84–97) | 85 (70–98) | No (P = 0.33) | E= 2.0, | (E= 3.4, | E= −1.5, |
| Apex-to-Base | 92 (77–98) | 89 (72–98) | 93 (84–98) | 95 (89–98) | No (P = 0.23) | CI ±8.2) | CI ±7.0) | CI ±7.8) |
|
| ||||||||
| EPIC sexual | ||||||||
| Apex | 67 (55–90) | 54 (39–75) | 53 (41–76) | 48 (26–87) | Yes (P = 0.008) | No (P = 0.53, | No (P = 0.91, | No (P = 0.72, |
| Base | 62 (49–76) | 51 (36–74) | 54 (23–73) | 50 (8–72) | Yes (P = 0.046) | E= −3.7, | E= 0.60, | E= −4.3, |
| Apex-to-Base | 60 (27–85) | 42 (18–73) | 41 (21–69) | 35 (6–77) | Yes (P = 0.001) | CI ±11.6) | CI ±10.1) | CI ±11.0) |
|
| ||||||||
| EPIC bowel | ||||||||
| Apex | 96 (91–98) | 96 (94–100) | 98 (96–100) | 97 (94–100) | No (P = 0.055) | No (P = 0.08, | No (P = 0.11, | No (P = 0.93, |
| Base | 97 (91–100) | 93 (84–100) | 93 (85–100) | 86 (71–100) | No (P = 0.44) | E= −4.3, | E= −3.5, | E= −0.87, |
| Apex-to-Base | 96 (91–100) | 96 (91–99) | 96 (89–98) | 96 (91–100) | No (P = 0.44) | CI ±4.7) | CI ±4.1) | CI ±4.6) |
|
| ||||||||
| SF-12 physical | ||||||||
| Apex | 56 (53–56) | 55 (53–56) | 56 (53–57) | 55 (54–57) | No (P = 0.53) | No (P = 0.26, | No (P = 0.63, | No (P = 0.73, |
| Base | 56 (52–58) | 56 (47–57) | 52 (40–57) | 47 (44–56) | No (P = 0.18) | E= −2.9, | E= −1.1, | E= −1.9, |
| Apex-to-Base | 54 (45–57) | 55 (46–57) | 56 (42–58) | 56 (53–58) | No (P = 0.71) | CI ±5.0) | CI ±4.3) | CI ±4.8) |
|
| ||||||||
| SF-12 mental | ||||||||
| Apex | 56 (52–58) | 7 (54–58) | 57 (54–58) | 58 (57–59) | No (P = 0.94) | No (P = 0.94, | No (P = 0.77, | No (P = 0.94, |
| Base | 57 (48–58) | 56 (44–58) | 56 (41–57) | 48 (42–55) | No (P = 0.66) | E= −0.23, | E= 0.73, | E= −0.96, |
| Apex-to-Base | 57 (44–59) | 556 (50–59) | 54 (45–59) | 56 (49–60) | No (P = 0.62) | CI ±5.6) | CI ±4.9) | CI ±5.4) |
Data are presented as median (interquartile range).
AUA, American Urological Association; E, effect size; CI, confidence interval; EPIC, expanded prostate cancer index composite; SF-12, short form of health survey.
Table 4.
Bilateral vs. unilateral genitourinary function and quality of life
| Baseline | 3 months | 6 months | 12 months | Segment difference baseline/6 months |
Different treatment impact Bilateral vs. Unilateral |
|
|---|---|---|---|---|---|---|
| Unilateral | (n=50) | (n=49) | (n=47) | (n=21) | ||
| Bilateral | (n=10) | (n=10) | (n=8) | (n=6) | ||
| AUA | ||||||
| Unilateral | 6 (3–13) | 7 (3–11) | 6 (2–11) | 4 (2–9) | No (P = 0.17) | No (P = 0.75, |
| Bilateral | 11 (4–13) | 5 (2–12) | 4 (3–14) | 5 (4–13) | No (P = 0.25) | E= −0.71, CI ±6.6) |
|
| ||||||
| EPIC urinary | ||||||
| Unilateral | 92 (80–98) | 91 (77–98) | 93 (81–98) | 94 (92–98) | No (P = 0.46) | No (P = 0.084, |
| Bilateral | 84 (76–95) | 88 (70–94) | 95 (90–99) | 88 (79–94) | No (P = 0.068) | E= 7.4, CI ±8.3) |
|
| ||||||
| EPIC sexual | ||||||
| Unilateral | 62 (45–79) | 47 (31–72) | 43 (26–69) | 38 (15–77) | Yes (P < 0.001) | No (P = 0.54, |
| Bilateral | 83 (63–90) | 41 (21–76) | 63 (37–84) | 59 (28–77) | No (P = 0.16) | E= 3.8, CI ±12.0) |
|
| ||||||
| EPIC bowel | ||||||
| Unilateral | 96 (93–98) | 96 (91–100) | 96 (91–100) | 98 (93–100) | No (P = 0.67) | No (P = 0.62, |
| Bilateral | 95 (89–96) | 96 (90–98) | 93 (86–96) | 93 (82–97) | No (P = 0.31) | E= −1.3, CI ±5.1) |
|
| ||||||
| SF-12 physical | ||||||
| Unilateral | 56 (45–57) | 55 (50–57) | 56 (51–57) | 56 (53–57) | No (P = 0.63) | No (P = 0.31, |
| Bilateral | 55 (48–56) | 55 (49–57) | 54 (49–57) | 51 (44–56) | No (P = 0.40) | E= 2.6, CI ±4.9) |
|
| ||||||
| SF-12 mental | ||||||
| Unilateral | 57 (49–58) | 57 (51–58) | 56 (48–58) | 57 (55–59) | No (P = 0.46) | No (P = 0.94, |
| Bilateral | 58 (43–60) | 56 (46–59) | 56 (49–60) | 58 (49–61) | No (P = 0.61) | E= 0.21, CI ±5.5) |
Data are presented as median (interquartile range).
AUA, American Urological Association; E, the effect size of bilateral vs. unilateral; CI, confidence interval (47.5% deviation from the mean); EPIC, expanded prostate cancer index composite; SF-12, short form of health survey.
At baseline, 40 men (66%) had erections sufficient for intercourse, of whom 27 men (68%) maintained their erectile ability to have intercourse during the course of this study. Of these 27 men, the use of medicinal aids increased from 7.4% (2/27) at baseline to 18.5% (5/27) during the course of this study. Thirteen patients experienced an erectile function insufficient for intercourse following IRE. This was significantly associated with an older age at time of treatment (P = 0.001) and a lower baseline sexual summary score on the EPIC questionnaire (P = 0.002). None of the system settings were significantly associated with an increased risk for genitourinary functional failure. Table 5 displays the patient characteristics, ablated segments and system settings for patients with and without erectile dysfunction following IRE.
Table 5.
Patient characteristics and system parameters of patients with and without erections sufficient for intercourse after IRE
| Patient factors | Erection sufficient for intercourse (n=27) | Erections insufficient for intercourse (n=13) | P |
|---|---|---|---|
| Age | 63 (59–67) | 73 (66–78) | 0.001 |
| Prostate volume | 35 (30–47) | 38 (29–62) | 0.55 |
| Baseline EPIC sexual | 85 (71–90) | 65 (55–77) | 0.002 |
| Segment ablated | |||
| Apex | 10 | 3 (23) | |
| Base | 6 | 4 (40) | |
| Apex-to-Base | 11 | 4 (27)a | |
| Anterior | 8 | 3 (27) | |
| Posterior | 18b | 9 (33)b | |
| Unilateral | 21 | 10 (32) | |
| Bilateral | 6 | 3 (33) | |
Two patients received multiple ablations;
One patient was treated in both anterior and posterior segments.
| System settings | |||
|---|---|---|---|
| Minimum interelectrode distance (mm) | 9 (8–10) | 9 (8–10) | 0.63 |
| Maximum interelectrode distance (mm) | 18 (17–21) | 18 (16–20) | 0.23 |
| Minimum voltage (V) | 1620 (1200–1760) | 1600 (1440–1710) | 0.13 |
| Maximum voltage (V) | 2550 (2400–2850) | 2550 (2400–2775) | 0.53 |
| Minimum amperage (A) | 25 (20–28) | 26 (21–31) | 0.44 |
| Maximum amperage (A) | 41 (37–45) | 44 (38–50) | 0.16 |
| Pulse length (μs) | 90 (70–90) | 90 (70–90) | 0.09 |
| Average length (μs) | 79.6 | 85.4 | |
Data are presented as median (interquartile range) or n (%).
EPIC, expanded prostate cancer index composite.
Of the 58 men that were pad-free continent at baseline (97%, 58/60), only one patient needed one pad per day for urinary leakage at 6 months. The patient characteristics or system parameters could not be statistically assessed due to the limited events of urinary incontinence. This patient was treated with a unilateral anterior ablation, including the basal quadrant and did not differ from pad-free continent patients in terms of baseline age, prostate volume and EPIC urinary summary score. The urinary continence of this patient improved at 12 months. Therefore pad-free continence was preserved in all men at their latest QoL evaluation.
Discussion
This study showed that primary focal IRE could be safely performed on all prostate segments. There were no statistically significant differences between prostate segments in terms of genitourinary function and QoL, indicating that this ablative modality is a feasible modality for any prostate segment. Apex-targeted IRE demonstrated improved bowel function on the EPIC questionnaire at 6 months compared with a decline in bowel function for base-targeted IRE. The clinical significance is negligible since both apex and base directed IRE treatments have remarkably high summary bowel scores at 6 months, and no clinical symptoms were described (e.g., rectal pain, bleeding or fistula). There were no statistically significant QoL deteriorations at 6 months within the individual segments treated. However, sexual function was impaired for all segments at 6 months. This deterioration was not statistically significant with bilateral IRE, which is most likely due to the limited number of patients in that group. A decline was observed on the EPIC sexual summary score over time that would have been significantly decreased at 12 months compared with baseline. Moreover, there was some heterogeneity within the bilateral group that may have affected the outcomes. Most bilateral cases were single-ablative IRE, targeting anterior midline lesions. However, multi-ablative bilateral ablations performed in three patients resulted in two having erectile dysfunction. The more extensive ablative procedures in most bilateral cases may have led to the improved urinary function scores at 6 months in this group due to post-treatment prostate volume reduction (20).
Promising rates of preserved pad-free urinary continence were found. It was demonstrated that older patients at time of treatment and those with poor baseline sexual function were significantly at risk of developing erections insufficient for intercourse. This may be unexpected since animal studies suggested that in pigs the endoneural architecture was preserved following IRE and showed signs of regeneration (21). In a preclinical study, it was also shown in rat sciatic nerves that the number of myelinated axons and the thickness of myelin sheath were preserved at 10 weeks following IRE exposure (22). None of the system settings used could be identified to be a significant risk factor for erectile dysfunction. A prolonged pulse length showed a trend of significance, which could be explained by increased Joule-heating. It was shown in a polyacrylamide gel that applying longer pulses is associated with more Joule-heating and potentially more thermal damage due to higher energy delivery (23). Surprisingly, erectile dysfunction also occurred in ablations that were far away from the neurovascular bundle, suggesting another mechanism behind the observed decrease in sexual function that may be elucidated in larger datasets. Future in vivo studies must provide the ideal system settings to successfully ablate prostate tissue while minimizing Joule-heating.
Our study is limited we did not include any data on oncologic performance. We choose to exclude the oncologic control per prostate segment based on previous results from an ablation and resection study (14). Moreover, infield oncologic failures following IRE were proven to be significantly dependent on the applied safety margin and/or system errors occurring during IRE (12). In this cohort, 7 patients had infield residual disease, including all different prostate segments (anterior, posterior, apex, mid, base), justifying our exclusion of oncologic analysis. Other limitations are the small cohort size and the short follow-up time. Murray et al. (8) showed that (marginal) improvement of erectile function was seen between 6–12 months, which may be applicable to our results. Furthermore, division of ablations into true segmental ablations can be arbitrary. An ablation was deemed segmental if an estimated 80% of its ablation volume (volume between electrodes) occurred in one segment. It has been shown that the histopathologic ablation zone extends the needle configuration by 2.5–2.9 times (two-dimensionally) (24). Moreover, in our institution, a T2-weighted MRI was performed 1 week after IRE to confirm if the predefined treatment region was covered by IRE. The coverage often extended the needle configuration, potentially invading other segments. The ablation zone volume on T2-weighted MRI has been shown to closely correlate with the volume on histopathology (25). However, this correlation was performed at 4 weeks after IRE, and the swelling and edema seen on T2-weighted MRI at 1 week can easily obscure the histopathologic effect. We aimed to reduce the extended ablation effect by using the exact needle locations/geometry registered in the Nanoknife® system and surgical report. Although the ablation zone dimensions may invade into other segments, we showed that wherever the electrodes were placed, good genitourinary function and QoL could be obtained. Interestingly, some ablations included the urethra or extended the capsule, without causing significant side effects seen with other ablative modalities (e.g., urethral sloughing with cryotherapy) (26).
In conclusion, IRE can be safely performed in each prostate segment without significantly different effects on genitourinary function and QoL outcomes, establishing this technique as a feasible focal ablative modality with good functional outcomes for all prostate segments treated. Patients who were older at time of treatment, or those with a poor baseline sexual function, had higher risk to develop erectile dysfunction. Future comparative trials need to elucidate whether the trifecta outcomes of focal therapy supersedes those of current radical treatments.
Main points.
Focal irreversible electroporation for localized prostate cancer can be performed in all prostate segments without jeopardizing different quality of life outcomes.
Older patients and those with poor baseline sexual function need to be counseled regarding the risk for erectile dysfunction.
All men that were pad-free continent at baseline remained pad-free continent at their latest quality of life evaluation.
Acknowledgements
We thank Quoc Nguyen and Anne-Maree Haynes from the Australian Prostate Cancer Research Centre-NSW (APCRC-NSW), IT Applications Group and CANSTO Database at Garvan Institute. We thank Jayne Matthews for clinical support.
Footnotes
This work was presented at the annual meeting of the western section of the AUA 2017.
Financial disclosure
The Australian Prostate Cancer Research Centre-NSW and the St. Vincent’s Prostate Cancer Centre funded this research project.
Conflict of interest disclosure
Scheltema received a PhD grant from the Cure for Cancer Foundation. Scheltema, Chang and Gielchinsky received a fellowship grant from the St Vincent’s Prostate Cancer Centre and the Australian Prostate Cancer Research Centre-NSW (APCRC-NSW). De la Rosette is paid consultant to AngioDynamics. AngioDynamics had no role throughout the study. All other authors have nothing to disclose.
Refecences
- 1.Donaldson I, Alonzi R, Barratt D, et al. Focal therapy: patients, interventions, and outcomes-a report from a consensus meeting. Eur Urol. 2015;67:771–777. doi: 10.1016/j.eururo.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed HU, Zacharakis E, Dudderidge T, et al. High-intensity-focused ultrasound in the treatment of primary prostate cancer: the first UK series. Br J Cancer. 2009;101:19–26. doi: 10.1038/sj.bjc.6605454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones JS, Rewcastle JC, Donnelly BJ, Lugnani FM, Pisters LL, Katz AE. Whole gland primary prostate cryoablation: initial results from the cryo on-line data registry. J Urol. 2008;180:554–558. doi: 10.1016/j.juro.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 4.Matsuoka Y, Numao N, Saito K, et al. Candidate selection for quadrant-based focal ablation through a combination of diffusion-weighted magnetic resonance imaging and prostate biopsy. BJU Int. 2016;117:94–101. doi: 10.1111/bju.12901. [DOI] [PubMed] [Google Scholar]
- 5.Valerio M, Cerantola Y, Eggener SE, et al. New and established technology in focal ablation of the prostate: a systematic review. Eur Urol. 2017;44:17–34. doi: 10.1016/j.eururo.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 6.Sivaraman A, Barret E. Focal therapy for prostate cancer: an “À la Carte” approach. Eur Urol. 2016;69:2015–2017. doi: 10.1016/j.eururo.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Ting F, Tran M, Böhm M, et al. Focal irreversible electroporation for prostate cancer: functional outcomes and short-term oncological control. Prostate Cancer Prostatic Dis. 2016;1:46–52. doi: 10.1038/pcan.2015.47. [DOI] [PubMed] [Google Scholar]
- 8.Murray KS, Ehdaie B, Musser J, et al. Pilot study to assess safety and clinical outcomes of irreversible electroporation for partial gland ablation in men with prostate cancer. J Urol. 2016;196:883–890. doi: 10.1016/j.juro.2016.02.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valerio M, Stricker PD, Ahmed HU, et al. Initial assessment of safety and clinical feasibility of irreversible electroporation in the focal treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2014;17:343–347. doi: 10.1038/pcan.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valerio M, Dickinson L, Ali A, et al. Nanoknife electroporation ablation trial (NEAT): a prospective development study investigating focal irreversible electroporation in men with localised prostate cancer. J Urol. 2017;3:647–654. doi: 10.1016/j.juro.2016.09.091. [DOI] [PubMed] [Google Scholar]
- 11.van den Bos W, De Bruin D, Veelo D, et al. Quality of life and safety outcomes following irreversible electroporation treatment for prostate cancer: results from a phase I-Ii study. J Cancer Sci Ther. 2015;7:312–321. [Google Scholar]
- 12.van den Bos W, Scheltema MJ, Siriwardana AR, et al. Focal irreversible electroporation as primary treatment for localized prostate cancer. BJU Int. 2018;121:716–724. doi: 10.1111/bju.13983. [DOI] [PubMed] [Google Scholar]
- 13.Davalos RV, Mir LM, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 14.van den Bos W, Jurhill RR, de Bruin DM, et al. Histopathological outcomes after irreversible electroporation for prostate cancer: results of an ablate and resect study. J Urol. 2016;196:552–559. doi: 10.1016/j.juro.2016.02.2977. [DOI] [PubMed] [Google Scholar]
- 15.Scheltema MJ, Tay KJ, Postema AW, et al. Utilization of multiparametric prostate magnetic resonance imaging in clinical practice and focal therapy: report from a Delphi consensus project. World J Urol. 2017;35:695–701. doi: 10.1007/s00345-016-1932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/S0090-4295(00)00858-X. [DOI] [PubMed] [Google Scholar]
- 17.Barry MJ, Fowler FJ, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992;148:1549–1557. doi: 10.1016/S0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 18.Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: Results from the IQOLA project. J Clin Epidemiol. 1998;51:1171–1178. doi: 10.1016/S0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 19.Core Team R. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. [Google Scholar]
- 20.Scheltema MJ, Postema AW, de Bruin DM, et al. Irreversible electroporation for the treatment of localized prostate cancer: a summary of imaging findings and treatment feedback. Diagnostic Interv Radiol. 2017;23:365–370. doi: 10.5152/dir.2017.16608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoellnast H, Monette S, Ezell PC, et al. The delayed effects of irreversible electroporation ablation on nerves. Eur Radiol. 2013;23:375–380. doi: 10.1007/s00330-012-2610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Fan Q, Ji Z, Qiu X, Li Z. The effects of irreversible electroporation (IRE) on nerves. PLoS One. 2011;6:e18831. doi: 10.1371/journal.pone.0018831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Den Bos W, Scheffer HJ, Vogel JA, et al. Thermal energy during irreversible electroporation and the influence of different ablation parameters. J Vasc Interv Radiol. 2016;27:433–443. doi: 10.1016/j.jvir.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 24.van den Bos W, de Bruin DM, Jurhill RR, et al. The correlation between the electrode configuration and histopathology of irreversible electroporation ablations in prostate cancer patients. World J Urol. 2016;34:657–664. doi: 10.1007/s00345-015-1661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Bos W, de Bruin DM, van Randen A, et al. MRI and contrast-enhanced ultrasound imaging for evaluation of focal irreversible electroporation treatment: results from a phase I–II study in patients undergoing IRE followed by radical prostatectomy. Eur Radiol. 2016;26:2252–2260. doi: 10.1007/s00330-015-4042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez SA, Arias Fúnez F, Bueno Bravo C, et al. Cryotherapy for primary treatment of prostate cancer: intermediate term results of a prospective study from a single institution. Prostate Cancer. 2014;2014 doi: 10.1155/2014/571576. 571576. [DOI] [PMC free article] [PubMed] [Google Scholar]