Abstract
Magnetic resonance imaging-guided high-intensity focused ultrasound (MRI-guided HIFU) is an effective noninvasive treatment option for symptomatic uterine fibroids. However, tissue characteristics of uterine fibroids and technical limitations can limit the patient population that can benefit from this therapy. In this article, we present our literature review focusing on the influential clinical factors that might reduce the risk of an unsuccessful MRI-guided HIFU treatment outcome of uterine fibroids.
Uterine fibroids, composed of smooth muscle cells and fibrous connective tissue derived from the myometrium, are common benign gynecological tumors that have a significant negative effect on the quality of life of a patient. Symptoms of uterine fibroids vary depending on their size and location, but the main symptoms are pelvic pain, menorrhagia or dysmenorrhea, increased frequency of urination, and reproductive dysfunction, including impaired fertility, pregnancy complications and loss (1, 2).
The choice of treatment and its rate of success are dependent on the morphology and tissue characteristics of the uterine fibroids. Hysterectomy remains the definitive treatment for uterine fibroids, but this treatment option is unsuitable for women who wish to preserve their fertility. In the last decade, patient demands have spurred the development of a noninvasive therapeutic modality for symptomatic uterine fibroids called magnetic resonance imaging-guided high-intensity focused ultrasound (MRI-guided HIFU) (3–11).
The principle of HIFU treatment of uterine fibroids is that focused ultrasonic energy causes coagulative necrosis of the target. Since its first clinical use for treating symptomatic uterine fibroids, the use of HIFU has increased worldwide because of its excellent therapeutic efficacy in relieving fibroid-related symptoms, its ability to preserve the uterus, and its high level of safety (3–7). However, as shown in previous studies (8–11), MRI-guided HIFU ablation therapy cannot be used in all patients with symptomatic fibroids because of the tissue characteristics of the uterine fibroids such as the T1 and T2 MRI signal intensity (SI), and fibroid type defined by general uterine position, fibroid size, and fibroid number. In addition, technical limitations, including the presence of scar tissue, excessive abdominal subcutaneous fat, the distance between the skin and the fibroids, the distance between the sacral bone surface and the fibroids, and the bowel in the path of sonication, play a role in whether MRI-guided HIFU therapy is appropriate.
In this article, we present a literature review of the influential clinical factors that might reduce the risk of an unsuccessful MRI-guided HIFU treatment outcome of uterine fibroids.
Tissue characteristics of uterine fibroids
T2-weighted imaging
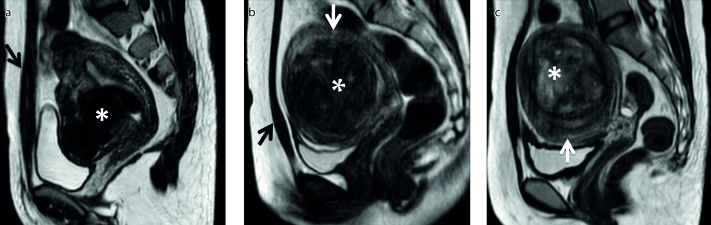
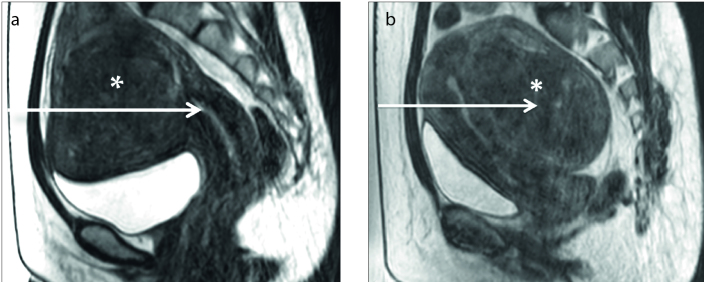
T2 SI is the primary MRI classification parameter for determining patient suitability for MRI-guided HIFU. A patient is classified as type I when the SI of the uterine fibroid is lower than that of skeletal muscle, type II when the SI is lower than that of the myometrium but higher than that of skeletal muscle, or type III when the SI is higher than that of the myometrium (Fig. 1) (4). As shown by previous studies (4–13), uterine fibroids with different SIs on T2-weighted MRI have different biological characteristics resulting in different HIFU ablation results.
Figure 1. a–c.
Uterine fibroids (asterisks) with different signal intensities (SIs) on T2-weighted images. Sagittal T2-weighted images show the fibroid classification as: (a) type I when the SI of the uterine fibroid is lower than that of skeletal muscle (black arrow); (b) type II when the SI is higher than that of skeletal muscle (black arrow) but lower than that of the myometrium (white arrow); (c) type III when the SI is higher than that of the myometrium (white arrow).
Funaki et al. (4) demonstrated that the nonperfused volume ratio in type I is higher than that in the other types, and most of the fibroids that showed significant volume reduction on follow-up were hypointense (type I). Zhao et al. (12) further found that hyperintensity on T2-weighted images of a fibroid indicates the presence of angiogenesis, richness of aqueous tissue, and cell components with less fibrous tissue, whereas hypointensity or isointensity on T2-weighted images of a fibroid indicates that angiogenesis is less common and the fibrous content is richer than that of fibroids with hyperintensity on T2-weighted images. Mikami et al. (13) reported that the uterine fibroids in the failed treatment group demonstrated heterogeneous and high SI on T2-weighted images relative to that of the myometrium, whereas the uterine fibroids in the successful treatment group demonstrated low SI relative to that of the myometrium.
Fibroids with high T2 SI, also known as type III fibroids, are generally excluded from consideration for MRI-guided HIFU treatments because of their high smooth muscle content relative to collagen fibers and their resistance to heat (4).
T1-perfusion imaging
Perfusion MRI, which is the most robust MRI technique for assessing uterine fibroid vascularity, is an alternative method for predicting HIFU treatment outcomes.
A case report by Yoon et al. (14) suggested that high T2 SI fibroids that exhibited delayed enhancement in dynamic contrast-enhanced (DCE) MRI could be treated successfully if the hyperintensity was a result of high fluid content rather than high vascularity. In another study by Mindjuk et al. (15), multivariate analysis revealed that although the nonperfused volume ratio of fibroids characterized by low SI in contrast-enhanced T1-weighted images was significantly high, T2 SI could not effectively predict the same. These two studies show that the T2 SI of a uterine fibroid cannot differentiate between vascularity and water from edema or degeneration (Figs. 2 and 3).
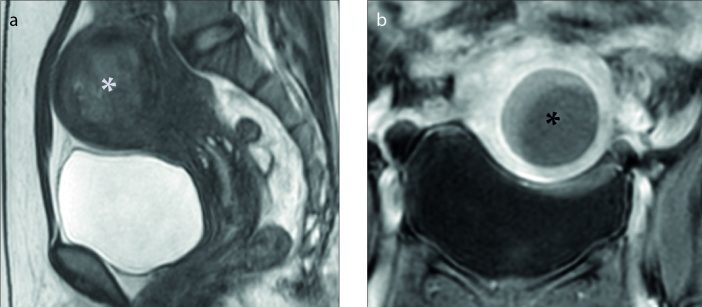
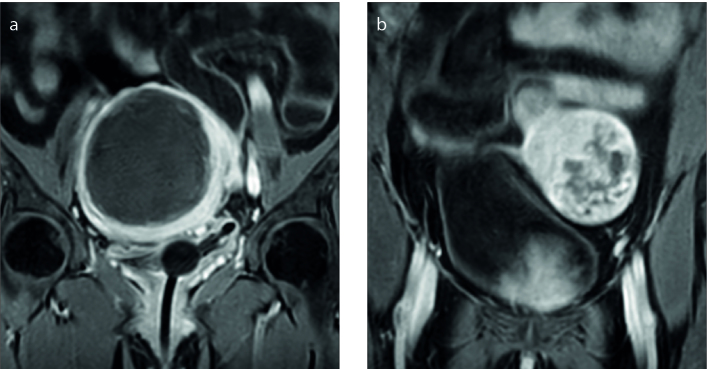
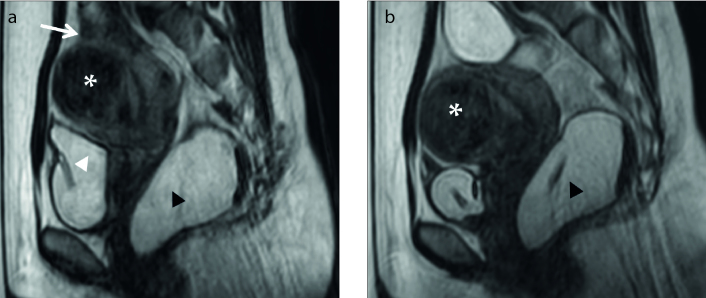
Figure 2. a, b.
Sagittal T2-weighted image (a) shows the uterine fibroid (white asterisk) with high signal intensity, categorized as type III. Coronal contrast-enhanced T1-weighted image (b) shows degenerated fibroid (black asterisk).
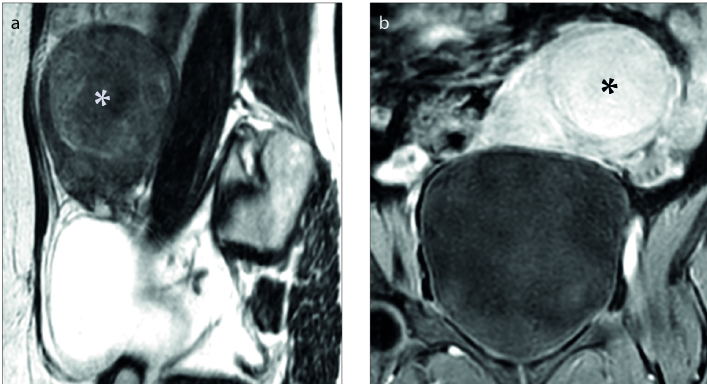
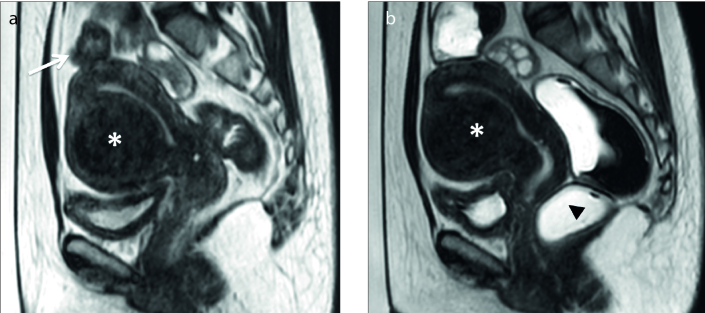
Figure 3. a, b.
Sagittal T2-weighted image (a) shows a uterine fibroid (white asterisk) with high signal intensity categorized as type III; coronal contrast-enhanced T1-weighted image (b) shows nondegenerated fibroid (black asterisk).
Kim et al. (16) investigated the ability of baseline pretreatment DCE MRI to predict the ablation efficacy of HIFU treatment of symptomatic uterine fibroids under various parameter settings and found that a high volume transfer constant (Ktrans) at baseline DCE MRI might be a significant predictor of poor treatment results. Liu et al. (17) further explored the potential clinical value of Ktrans maps at baseline, which were used to help visualize the state of perfusion inside the fibroids and locate the higher-perfusion areas. They suggested that the appropriate therapeutic acoustic power, based on initial test sonication, must be selected for different areas inside the fibroid with significantly different states of perfusion. This might greatly impact the treatment outcome.
Kim et al. (18) demonstrated that the relative peak enhancement in semiquantitative perfusion MRI was significantly associated with the efficacy of HIFU ablation of uterine fibroids and suggested a value of 220% or less as a screening guideline toward more efficient HIFU treatment. Moreover, Wei et al. (19) reported that the quantitative parameters Ktrans, blood flow, and blood volume derived from DCE MRI negatively correlated with an immediate nonperfused volume ratio of at least 70% in HIFU treatment for symptomatic uterine fibroids, indicating that higher Ktrans, blood flow, and blood volume values were associated with a poorer treatment outcome.
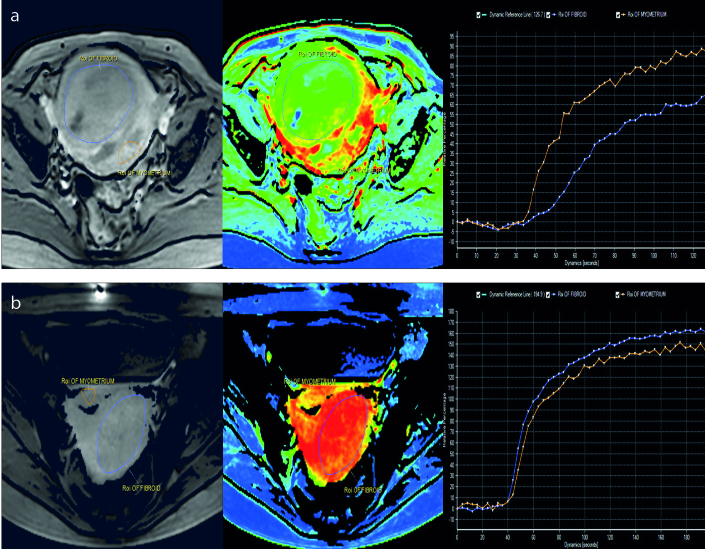
Recently, a new classification method based on MRI T1 perfusion-based time–SI curves for fibroid tissue compared with those for the myometrium in screening MRI was introduced by Keserci et al. (20) for predicting the treatment outcome of HIFU ablation. The fibroids were classified into group A if the time–SI curve was lower than that for the myometrium (Fig. 4a) and into group B if the time–SI curve was equal to or higher than that for the myometrium (Fig. 4b). Bivariate analysis revealed a very strong correlation between the T1 perfusion-based classification and the immediate nonperfused volume ratio (Fig. 5). The preliminary findings at the 6-month follow-up after HIFU treatment showed a significant correlation between the immediate nonperfused volume ratio and the transformed symptom severity score improvement ratio, and the fibroid volume reduction ratio. In another study, Keserci et al. (21) showed through multivariate analysis of semiquantitative perfusion MRI parameters that peak enhancement of the fibroid, time-to-peak of the fibroid, and the ratio of the area under the curve for the fibroid to that for the myometrium were the significant parameters for predicting the treatment outcome of HIFU ablation when the nonperfused volume ratio was at least 90%. This suggested that these factors should be considered in screening MRI images.
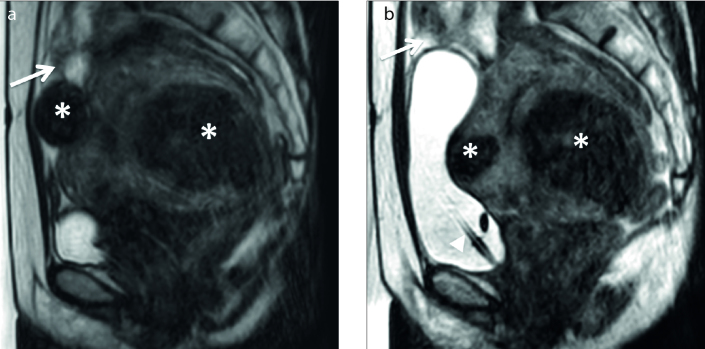
Figure 4. a, b.
Region of interest within the area of the fibroid and the myometrium on perfusion MRI (left panel). The perfusion map (middle panel). The time–SI curve of the fibroid (right panel). Group A fibroids have a lower time–SI curve than the myometrium (a), while group B fibroids have a higher time–SI curve than the myometrium (b).
Figure 5. a, b.
The nonperfused volume ratio immediately after treatment was 100% in (a) and 38% in (b).
According to the Pennes’ bioheat transfer equation (22), blood perfusion may be the main parameter that affects the temperature increase used for predicting the outcome of HIFU treatment, since heat capacity is related to primarily the blood perfusion rate and the heat conduction coefficient, which are relatively fixed in similar fibroids.
Fibroid types
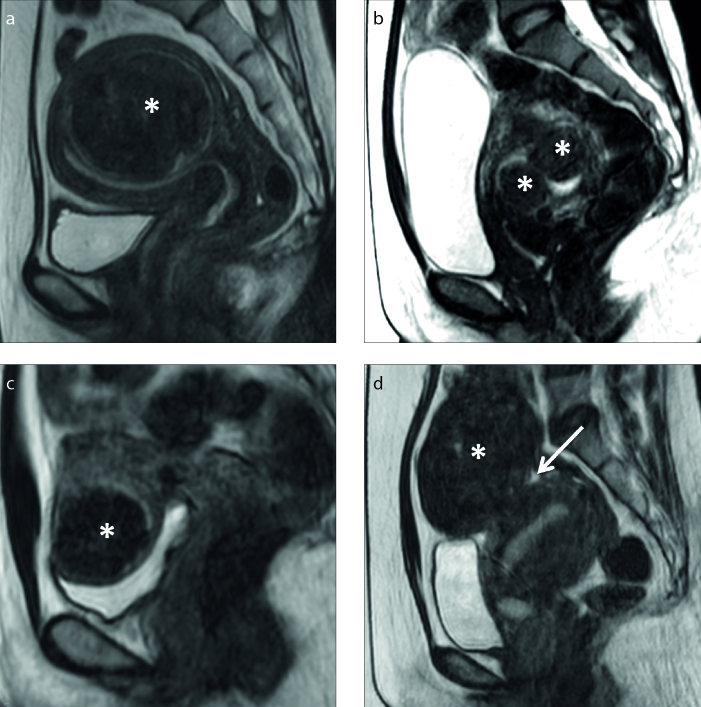
On the basis of their location in the uterus, uterine fibroids are often described as intramural (IM), submucosal (SM), or subserosal (SS). IM fibroids lie within the wall of the uterus, separated from the surrounding myometrium by a layer of connective tissue. SM fibroids tend to protrude into the endometrial cavity and distort the endometrium. SS fibroids are on the surface of the uterus and tend to grow outward (Fig. 6).
Figure 6. a–d.
Sagittal T2-weighted images show the different types of uterine fibroids (asterisks) as: (a) intramural, (b) submucosal, (c) subserosal, and (d) subserosal with a small stalk (arrow).
Patients with pedunculated SS fibroids on a narrow stalk were not considered for uterine artery embolization (UAE) and HIFU, as ablation of these fibroids can lead to stalk necrosis and detachment of the fibroid from the uterus and expulsion into the abdominal cavity (8, 23–25). However, Park et al. (26) suggested that MRI-guided HIFU might be a safe and effective treatment for pedunculated SS fibroids. In their study, of the 135 women with symptomatic uterine fibroids, 9 had a single pedunculated SS fibroid. Throughout the treatment and post-HIFU follow-up period, there were no serious or unexpected adverse events related to HIFU treatment. In addition, there were no cases of disconnected stalks or infectious complications; the fibroid was selectively ablated while the stalk was preserved by keeping it out of the beam pathway.
Because ablation of SM fibroids may cause severe endometrial impairment, Filipowska et al. (27) suggested that such fibroids be treated up to the endometrial surface in women who do not wish to preserve their fertility. Endometrium consists of two layers: a basal layer which is permanent, and a functional layer which is shed at the time of menses. If a large area of basal layer is destroyed, then complete regeneration from surrounding basal layer tissue might not be possible, leading to localized scarring in the endometrium. This potentially reduces possibilities of future pregnancy and/or may increase pregnancy complications and losses. However, a recent study by Kim et al. (28) reported that in most cases the endometrium was preserved intact or minimally impaired after HIFU ablation of SM fibroids, suggesting that an impaired endometrium, which is more common after treating endometrial protruded fibroids, may recover spontaneously.
Given the ability of MRI-guided HIFU to deliver high energy to targeted tissues precisely, it can be used as a nonsurgical option for the treatment of pedunculated SS and SM fibroids. However, prospective studies with large patient populations and a longer follow-up period are needed to confirm the suitability of HIFU to treat these types of fibroids.
Uterine fibroid size and number
The risk of deep venous thrombosis (DVT) resulting from the relatively lengthy procedure time of MRI-guided HIFU ablation compared with that of surgery is an obstacle to the more widespread adoption of the procedure (Fig. 7). Evidence-based analysis from several institutions using the MRI-guided HIFU system showed that the maximum uterine fibroid diameter is 10 cm for a successful treatment outcome (11). To overcome this limitation of diameter size, several new techniques, including volumetric ablation, one-layer strategy in volumetric ablation, variable length sonication in a point-by-point approach, oxytocin pretreatment, and gonadotropin-releasing hormone agonists, have been introduced to enable MRI-guided HIFU treatment of larger fibroids (7, 23, 29–34).
Figure 7.
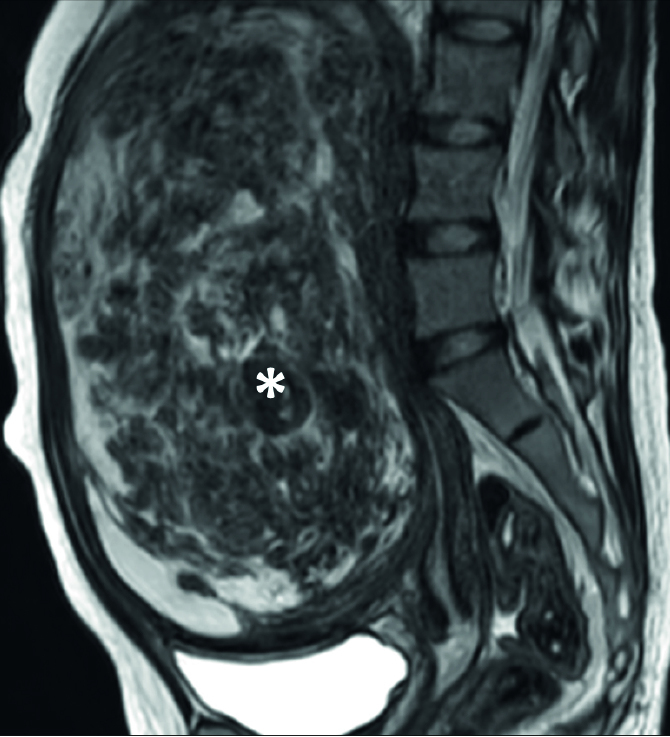
Sagittal T2-weighted image shows an excessively large uterine fibroid (asterisk) that conquered all of pelvic cavity and abdominal cavity.
A patient can have a single uterine fibroid or two or more fibroids. Currently, patients with more than five fibroids are not good candidates for MRI-guided HIFU ablation. Since the length of the procedure is the limiting factor for its use, the size of each fibroid associated with symptoms determines how many fibroids can be treated within the allowed treatment time (Fig. 8) (7, 8, 11, 23, 29).
Figure 8.
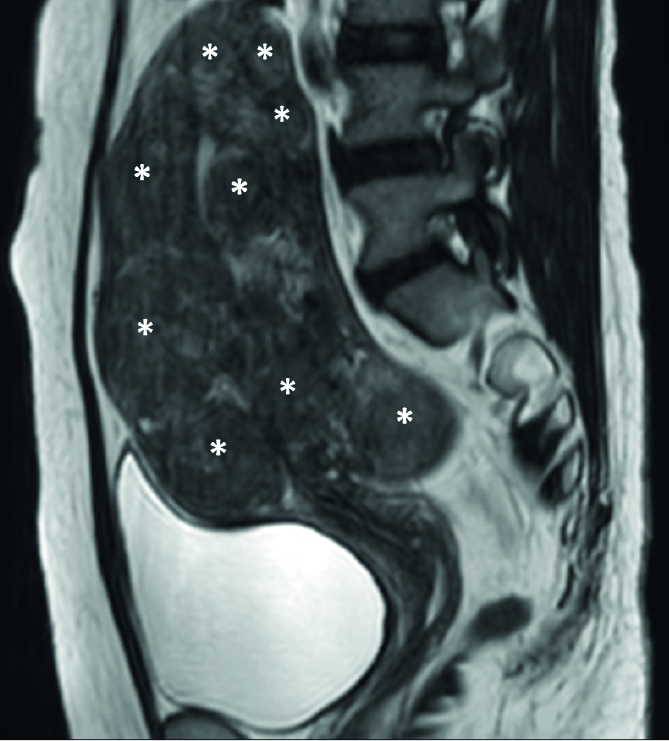
Sagittal T2-weighted image shows multiple uterine fibroids (asterisks), affecting most of the uterus region.
Technical limitations
Presence of scar tissue
Because scar tissue is less vascular and more fibrotic than normal tissue, the presence of abdominal scars may limit access to the target area and lead to higher temperature increases in the near field of the ultrasound beam path. Uterine fibroid patients with transverse scars might be managed by angling the transducer to increase the protected area; the beam-shaping feature, which reduces the intensity of the ultrasound field in the selected region by shutting off some sonication elements; the urinary bladder-filling technique to avoid the scar; or a combination of these approaches (8, 10, 23, 35, 36). However, longitudinal scars are more problematic because they are usually at the midline where the ultrasonic energy has to penetrate other intermediate tissue layers.
To overcome these drawbacks, acoustic patches placed on the scar to reflect the ultrasound energy from the scar have been introduced (37). Yoon et al. (38) reported that the scar patch is an effective option for patients with abdominal scars located in the beam path and who were previously excluded from HIFU treatment because of the increased risk of skin burns. The safety of a scar patch in HIFU treatment of hypovascular fibroid patients with transverse and longitudinal scars was recently investigated using a volumetric technique; the scar patch did not affect the clinical efficacy of the treatment (39).
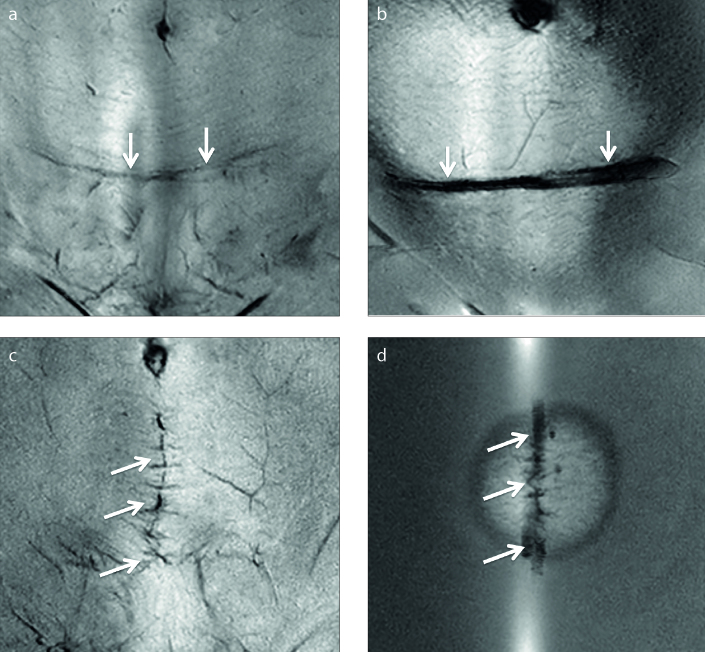
A recent clinical study by Keserci et al. (40) assessed the success of HIFU treatment, defined as an immediate nonperfused volume ratio of 80%; the therapeutic efficacy of the treatment, defined as fibroid volume reduction; the improvement in the symptom severity score (SSS) at the 6-month follow-up; and the safety of the treatment in terms of adverse effects. The parameters of patients with and without abdominal scars were compared. The scar tissues were covered with water-resistant polyethylene foam scar patches that are visible on MRI (Fig. 9). Among the 63 patients who were available to provide 6-month follow-up data, the degrees of fibroid volume reduction and symptom improvement were not statistically significantly different between the patients with and without abdominal scars. In addition, no serious adverse effects were reported (40).
Figure 9. a–d.
Coronal images show (a) transverse scar (arrows), (b) acoustic patch placed on the transverse scar (arrows), (c) longitudinal scar (arrows), and (d) acoustic patch placed on the longitudinal scar (arrows).
Thickness of abdominal subcutaneous fat
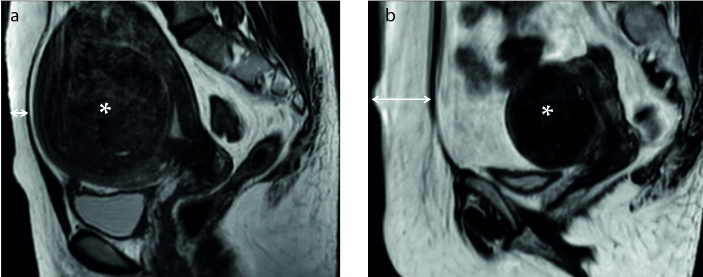
The increase in the energy deposited in the near field of the ultrasound beam path causes an increase in temperature within the skin, mainly in the subcutaneous tissue because of its lower specific heat capacity, insulating properties, and lower blood supply than those of other tissues in the abdominal wall (Fig. 10) (41–44).
Figure 10. a, b.
Sagittal T2-weighted images show uterine fibroids (asterisks) and subcutaneous fat thickness (two-headed arrows): (a) thin subcutaneous fat thickness, (b) large subcutaneous fat thickness.
A study by Mikami et al. (13) suggested that thick subcutaneous or visceral fat tissue attenuates the energy from exposure to sonication which causes an inadequate increase in temperature at the target area. Kim et al. (18) proved that the thickness of the subcutaneous fat has a significant effect on the HIFU ablation of uterine fibroids. In a recent study by Keserci et al. (21), multivariate analysis of baseline parameters also suggested that the thickness of the subcutaneous fat layer in the anterior abdominal wall was a valuable predictor of the outcome of HIFU ablation when the nonperfused volume ratio was at least 90%.
Therefore, the results of our literature review show that excessively thick subcutaneous fat in the abdominal wall causes distortion or absorption of acoustic energy that leads to the accumulation of heat in the near field of the ultrasonic beam path, which increases the risk of fat burn and affects the beam focus quality. A decrease in beam focus quality limits the degree of tissue ablation in MRI-guided HIFU treatment of uterine fibroids.
Distance between the skin and uterine fibroids
The location of the uterine fibroid should be evaluated in consideration of the limit in treatable depth, which is up to 12 cm from the skin in the current commercially available MRI-guided HIFU systems (e.g., ExAblate O.R./ExAblate One, InSightec and R3.5 Profound Medical Inc.) depending on the maximal focal length of the transducer (Fig. 11) (5, 45). Therefore, it should be assured that the therapeutic ultrasound can reach to the deepest layer of the uterine fibroid with a safety margin.
Figure 11. a, b.
Sagittal T2-weighted images show uterine fibroids (asterisks) and available treatment depth (arrows): (a) tumor located in the abdominal wall and within the available treatment depth, (b) tumor is close to the sacral bone and outside the available treatment depth.
Tissues in the acoustic pathway will absorb, reflect, and scatter ultrasonic waves when focused ultrasound is applied in ablation of deep target, and consequently result in ultrasonic energy attenuation with more tissue in front of the focus. Peng et al. (46) has shown that the energy efficiency factor (EEF), which is the amount of energy required for tissue ablation per unit volume, has a positive correlation with the depth of focus. Furthermore, Liu et al. (47) demonstrated that the distance from the surface of the anterior side of a fibroid to the skin correlated well with the EEF, while the distance from the surface of the posterior side of the fibroid to the skin correlated well with sonication time.
Distance between sacral bone surface and uterine fibroids
In general, HIFU treatment of uterine fibroids located on the posterior uterine wall, close to the surface of the sacral bone and the adjacent lumbosacral nerves, should be done with care to prevent sciatic nerve damage (Fig. 12).
Figure 12.
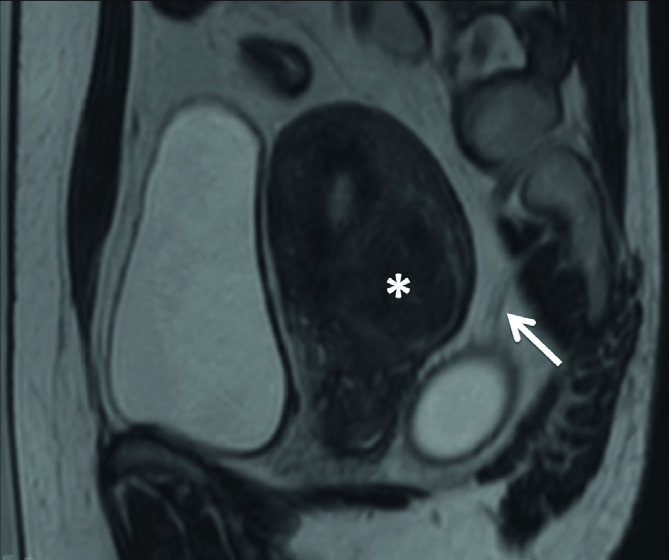
Sagittal T2-weighted image shows the uterine fibroid located on the posterior wall of the uterus (asterisk) and the sciatic nerves in close proximity to the tumor (arrow).
Clinical studies (7, 20, 39, 40, 45) suggested that the cells to be treated must be located on the T2-weighted planning images, and the safety margin or distance from the border of the cells to be treated to critical organs such as the sacral bone should be taken into consideration (e.g., 4 cm), since heat accumulation in the bone can subsequently be transferred to the perineural fat and adjacent nerves. The adverse effects encountered in these studies were partly related to far-field heat absorption by distant bony structures (e.g., sciatic nerve symptoms). Therefore, a patient whose fibroids are close to the lumbosacral plexus or to the surface of another bone should be considered carefully before the patient is deemed suitable for MRI-guided HIFU treatment.
Bowel interposition in the sonication path
The interposition of the bowel in the sonication path is considered a contraindication for HIFU treatment, since gas bubbles and hard particles in the bowel tend to reflect the ultrasound beam, resulting in reduced treatment efficacy and potentially leading to thermal damage and even bowel perforation (10, 23). Therefore, it is very important to verify that the bowel is not in the path of the ultrasound beam while establishing a safe acoustic window before each sonication during HIFU treatment.
Depending on the anatomy of the patient, several ways to displace interposing bowel loops have been suggested: (i) filling the urinary bladder with saline, (ii) filling the rectum with ultrasound gel, or (iii) using a convex gel pad (48, 49). The major drawback of these approaches is the possibility of increasing the distance of the fibroid from the skin, which could prevent the ablation of a deep fibroid.
Park et al. (50) introduced the BRB (bladder-rectum-bladder) bowel-manipulation technique, which consists of filling the urinary bladder, filling the rectum, and then emptying the urinary bladder with the patient in the prone position (Fig. 13). This technique seems to be effective for displacing the interposing bowel loop away from the sonication path in the HIFU treatment of fibroids in the anteverted uterus, which is defined as the condition in which the uterus is tilted towards the abdomen. However, bowel may still pose a problem with the BRB technique, when it is located anterior to the uterus or remains at the edge of the beam path.
Figure 13. a, b.
Sagittal T2-weighted images show the uterine fibroid (asterisk), bowel, bladder and rectum. In panel (a), the bladder filled with 300 mL normal saline (white arrowhead) and rectum filled with 200 mL ultrasound gel (black arrowhead) pushes the uterus upward and forward. The bowel loops are still above the tumor (white arrow). Meanwhile in panel (b), the bladder is emptied with the pressure of the filled rectum. The uterus moves forward and downward and the bowel loops are pushed out of treatment window.
Kim et al. (51) used the BRB technique initially in patients with an anteverted uterus (Fig. 14) and then in patients with a retroverted uterus, which is defined as the condition in which the uterus is tipped backwards so that it aims towards the rectum (Fig. 15). The HIFU procedure was a technical success in 42 of 49 patients with an anteverted uterus and in 7 of 11 patients with a retroverted uterus. The HIFU procedure was successfully completed using through-the-bladder sonication in 5 of 49 patients with an anteverted uterus and in 3 of 11 patients with a retroverted uterus. However, sonication could not be initiated in two anteverted uterus cases and one retroverted uterus case. In addition, their multivariate analysis revealed that a small uterus on treatment day was the only independent risk factor for BRB failure.
Figure 14. a, b.
Sagittal T2-weighted images show the uterine fibroid on the anterior wall of anteverted uterus (asterisk). In panel (a), the bowel loops are inside of the treatment window (white arrow). In panel (b), the rectum filled with 200 mL ultrasound gel (black arrowhead) pushes the uterus upward and forward, making the space between the tumor and abdominal wall smaller and pushing the bowel loops outside of the treatment window.
Figure 15. a, b.
Sagittal T2-weighted images show uterine fibroids (asterisks) that affect the anterior and posterior walls of a retroverted uterus. In panel (a), the bowel loops are inside the treatment window (arrow). In panel (b), the bladder filled with 500 mL normal saline (arrowhead) pushes bowel loops outside of the treatment window (arrow).
In a recent study, Jeong et al. (52) proposed a “modified BRB technique” in which downward traction on the uterus is induced to change its location so the beam path avoids the bowel and other structures. Of the 29 cases in their study, the group that underwent induced downward traction of the uterus needed significantly lower acoustic sonication power compared with the group without induced downward traction of the uterus. With this new approach, it was possible to reinforce the acoustic sonication power by moving the fibroids from the posterior side to the center of the treatment window.
The BRB and modified BRB bowel displacement techniques greatly decrease the screening failure rate of MRI-guided HIFU treatment of uterine fibroids. With these techniques, bowel interposition should no longer exclude a patient from undergoing this therapeutic procedure.
Conclusion
Because patient selection is a significant factor in reducing the risk of an unsuccessful outcome of MRI-guided HIFU treatment of uterine fibroids, the gynecologist and interventional radiologist must consider the fibroid tissue characteristics and the technical limitations addressed in this study carefully in the screening phase.
Main points.
Magnetic resonance imaging-guided high-intensity focused ultrasound (MRI-guided HIFU) offers another interventional radiology option for treating uterine fibroids.
Tissue characteristics of the uterine fibroids are the T1 and T2 signal intensity (SI), and fibroid type defined by general uterine position, fibroid size and number.
Technical limitations in MRI-guided HIFU treatment are presence of scar tissue, excessive abdominal subcutaneous fat, distance between the skin and the fibroids, distance between the sacral bone surface and the fibroids, and bowel in the path of sonication.
Evaluation of tissue characteristics of the uterine fibroids and technical limitations in the screening phase might reduce the risk of an unsuccessful MRI-guided HIFU treatment outcome of uterine fibroids.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91:1215–1223. doi: 10.1016/j.fertnstert.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 3.Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: Safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189:48–54. doi: 10.1067/mob.2003.345. [DOI] [PubMed] [Google Scholar]
- 4.Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2 weighted magnetic resonance images. Am J Obstet Gynecol. 2007;196:184.e1–6. doi: 10.1016/j.ajog.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Stewart EA, Gostout B, Rabinovici J, Kim HS, Regan L, Tempany CM. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110:279–287. doi: 10.1097/01.AOG.0000289083.50506.1a. [DOI] [PubMed] [Google Scholar]
- 6.Lenard ZM, McDannold NJ, Fennessy FM, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery-imaging predictors of success. Radiology. 2008;249:187–194. doi: 10.1148/radiol.2491071600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YS, Kim JH, Rhim H, et al. Volumetric MR-guided high-intensity focused ultrasound ablation with a one-layer strategy to treat large uterine fibroids: initial clinical outcomes. Radiology. 2012;263:600–609. doi: 10.1148/radiol.12111707. [DOI] [PubMed] [Google Scholar]
- 8.Yoon SW, Lee C, Kim KA, et al. Patient selection guidelines in MR-guided high-intensity focused ultrasound surgery of uterine fibroids: a pictorial guide to relevant findings in screening pelvic MRI. Eur Radiol. 2008;18:2997–3006. doi: 10.1007/s00330-008-1086-7. [DOI] [PubMed] [Google Scholar]
- 9.Behera MA, Leong M, Johnson L, Brown H. Eligibility and accessibility of magnetic resonance-guided focused ultrasound (MRgFUS) for the treatment of uterine leiomyomas. Fertil Steril. 2010;94:1864–1868. doi: 10.1016/j.fertnstert.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 10.Arleo EK, Khilnani NM, Ng A, Min RJ. Features influencing patient selection for fibroid treatment with magnetic resonance-guided focused ultrasound. J Vasc Interv Radiol. 2007;18:681–685. doi: 10.1016/j.jvir.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Pron G. Magnetic Resonance-Guided High-Intensity Focused Ultrasound (MRgHIFU) Treatment of Symptomatic Uterine Fibroids: An Evidence-Based Analysis. Ont Health Technol Assess Ser. 2015;15:1–86. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao WP, Chen JY, Zhang L, et al. Feasibility of ultrasound-guided high intensity focused ultrasound ablating uterine fibroids with hyperintense on T2-weighted MR imaging. Eur J Radiol. 2013;82:e43–49. doi: 10.1016/j.ejrad.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Mikami K, Murakami T, Okada A, Osuga K, Tomoda K, Nakamura H. Magnetic resonance imaging-guided focused ultrasound ablation of uterine fibroids: early clinical experience. Radiat Med. 2008;26:198–205. doi: 10.1007/s11604-007-0215-6. [DOI] [PubMed] [Google Scholar]
- 14.Yoon SW, Lee C, Kim KA, Kim SH. Contrast enhanced dynamic MR imaging of uterine fibroids as a potential predictor of patient eligibility for MR guided focused ultrasound (MRgFUS) treatment for symptomatic uterine fibroids. Obstet Gynecol Int. 2010:1–4. doi: 10.1155/2010/834275. 834275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mindjuk I, Trumm CG, Herzog P, Stahl R, Matzko M. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317–1328. doi: 10.1007/s00330-014-3538-6. [DOI] [PubMed] [Google Scholar]
- 16.Kim YS, Lim HK, Kim JH, et al. Dynamic contrast-enhanced magnetic resonance imaging predicts immediate therapeutic response of magnetic resonance–guided high-intensity focused ultrasound ablation of symptomatic uterine fibroids. Invest Radiol. 2011;46:639–647. doi: 10.1097/RLI.0b013e318220785c. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Keserci B, Yang X, et al. Volume transfer constant (Ktrans) maps from dynamic contrast enhanced MRI as potential guidance for MR-guided high intensity focused ultrasound treatment of hypervascular uterine fibroids. Magn Reson Imaging. 2014;32:1156–1161. doi: 10.1016/j.mri.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Kim YS, Kim BG, Rhim H, et al. Uterine fibroids: semiquantitative perfusion MR imaging parameters associated with the intra-procedural and immediate postprocedural treatment efficiencies of MR imaging-guided high-intensity focused ultrasound ablation. Radiology. 2014;273:462–471. doi: 10.1148/radiol.14132719. [DOI] [PubMed] [Google Scholar]
- 19.Wei C, Fang X, Wang CB, Chen Y, Xu X, Dong JN. The predictive value of quantitative DCE metrics for immediate therapeutic response of high intensity focused ultrasound ablation (HIFU) of symptomatic uterine fibroids. Abdom Radiol. 2018;43:2169–2175. doi: 10.1007/s00261-017-1426-7. [DOI] [PubMed] [Google Scholar]
- 20.Keserci B, Nguyen D. The role of T1 perfusion-based classification in magnetic resonance-guided high-intensity focused ultrasound ablation of uterine fibroids. Eur Radiol. 2017;27:5299–5308. doi: 10.1007/s00330-017-4885-x. [DOI] [PubMed] [Google Scholar]
- 21.Keserci B, Nguyen D. Magnetic resonance imaging parameters in predicting the treatment outcome of high-intensity focused ultrasound ablation of uterine fibroids with an immediate nonperfused volume ratio of at least 90% Acad Radiol. 2018 doi: 10.1016/j.acra.2018.01.022. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1948;1:93–122. doi: 10.1152/jappl.1948.1.2.93. [DOI] [PubMed] [Google Scholar]
- 23.Zaher S, Gedroyc WM, Regan L. Patient suitability for magnetic resonance guided focused ultrasound surgery of uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2009;143:98–102. doi: 10.1016/j.ejogrb.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Katsumori T, Akazawa K, Mihara T. Uterine artery embolization for pedunculated subserosal fibroids. AJR Am J Roentgenol. 2005;184:399–402. doi: 10.2214/ajr.184.2.01840399. [DOI] [PubMed] [Google Scholar]
- 25.Smeets AJ, Nijenhuis RJ, Boekkooi PF, et al. Safety and effectiveness of uterine artery embolization in patients with pedunculated fibroids. J Vasc Interv Radiol. 2009;20:1172–1175. doi: 10.1016/j.jvir.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Park H, Yoon SW, Kim KA, Jung KD, Jung SG. Magnetic resonance imaging–guided focused ultrasound treatment of pedunculated subserosal uterine fibroids: a preliminary report. J Vasc Interv Radiol. 2012;23:1589–1593. doi: 10.1016/j.jvir.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Filipowska J, Łoziński T. Magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU) in treatment of symptomatic uterine myomas. Pol J Radiol. 2014;79:439–443. doi: 10.12659/PJR.890606. https://doi.org/10.12659/PJR.890606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YS, Kim TJ, Lim HK, et al. Preservation of the endometrial enhancement after magnetic resonance imaging-guided high-intensity focused ultrasound ablation of submucosal uterine fibroids. Eur Radiol. 2017;27:3956–3965. doi: 10.1007/s00330-017-4765-4. [DOI] [PubMed] [Google Scholar]
- 29.Froling V, Kroncke TJ, Schreiter NF, et al. Technical eligibility for treatment of magnetic resonance-guided focused ultrasound surgery. Cardiovasc Intervent Radiol. 2014;37:445–450. doi: 10.1007/s00270-013-0678-z. [DOI] [PubMed] [Google Scholar]
- 30.Köhler MO, Mougenot C, Quesson B, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys. 2009;36:3521–3535. doi: 10.1118/1.3152112. [DOI] [PubMed] [Google Scholar]
- 31.Kim YS, Keserci B, Partanen A, et al. Volumetric MR-HIFU ablation of uterine fibroids: role of treatment cell size in the improvement of energy efficiency. Eur J Radiol. 2012;81:3652–3659. doi: 10.1016/j.ejrad.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Smart OC, Hindley JT, Regan L, Gedroyc WM. Magnetic resonance guided focused ultrasound surgery of uterine fibroids: the tissue effects of GnRH agonist pre-treatment. Eur Radiol. 2006;59:163–167. doi: 10.1016/j.ejrad.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Trumm CG, Stahl R, Clevert DA, et al. Magnetic resonance imaging-guided focused ultrasound treatment of symptomatic uterine fibroids: impact of technology advancement on ablation volumes in 115 patients. Invest Radiol. 2013;48:359–365. doi: 10.1097/RLI.0b013e3182806904. [DOI] [PubMed] [Google Scholar]
- 34.Huang X, He M, Liu YJ, Zhang L, Wang ZB. Effect of oxytocin on uterine fibroids treated by ultrasound ablation (in Chinese) Zhonghua Fu Chan Ke Za Zhi. 2011;46:412–415. [PubMed] [Google Scholar]
- 35.Kim YS, Bae DS, Park MJ, et al. Techniques to expand patient selection for MRI-guided high-intensity focused ultrasound ablation of uterine fibroids. AJR Am J Roentgenol. 2014;202:443–451. doi: 10.2214/AJR.13.10753. [DOI] [PubMed] [Google Scholar]
- 36.Zaher S, Gedroyc W, Lyons D, Regan L. A novel method to aid in the visualization and treatment of uterine fibroids with MRgFUS in patients with abdominal scars. Eur J Radiol. 2010;76:269–273. doi: 10.1016/j.ejrad.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Gorny KR, Chen S, Hangiandreou NJ, et al. Initial evaluation of acoustic reflectors for the preservation of sensitive abdominal skin areas during MRgFUS treatment. Phys Med Biol. 2009;54:N125–133. doi: 10.1088/0031-9155/54/8/N02. [DOI] [PubMed] [Google Scholar]
- 38.Yoon SW, Seong SJ, Jung SG, Lee SY, Jun HS, Lee JT. Mitigation of abdominal scars during MR-guided high-intensity focused ultrasound treatment of uterine leiomyomas with the use of an energy-blocking scar patch. J Vasc Interv Radiol. 2011;22:1747–1750. doi: 10.1016/j.jvir.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Zhu Y, Keserci B, Viitala A, Wei J, Yang X, Wang X. Volumetric MR-guided high-intensity focused ultrasound ablation to treat uterine fibroids through the abdominal scars using scar patch: a case report. J Ther Ultrasound. 2016;4:20. doi: 10.1186/s40349-016-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keserci B, Nguyen D. Volumetric magnetic resonance-guided high-intensity focused ultrasound ablation of uterine fibroids through abdominal scars: the impact of a scar patch on therapeutic efficacy and adverse effects. J Ther Ultrasound. 2017;5:22. doi: 10.1186/s40349-017-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henriques FC, Moritz AR. Studies of thermal injury in the conduction of heat to and through skin and the temperatures attained therein: A theoretical and experimental investigation. Am J Pathol. 1947;23:531–549. [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson GS. Human morphology and temperature regulation. Int J Biometeorol. 1999;43:99–109. doi: 10.1007/s004840050123. [DOI] [PubMed] [Google Scholar]
- 43.Cohen ML. Measurement of the thermal properties of human skin. A review. J Invest Dermatol. 1977;69:333–338. doi: 10.1111/1523-1747.ep12507965. [DOI] [PubMed] [Google Scholar]
- 44.Heinonen I, Kemppainen J, Kaskinoro K, Knuuti J, Boushel R, Kalliokoski KK. Capacity and hypoxic response of subcutaneous adipose tissue blood flow in humans. Circ J. 2014;78:1501–1506. doi: 10.1253/circj.CJ-13-1273. [DOI] [PubMed] [Google Scholar]
- 45.Chen R, Keserci B, Bi H, et al. The safety and effectiveness of volumetric magnetic resonance-guided high intensity focused ultrasound treatment of symptomatic uterine fibroids: Early clinical experience in China. J Ther Ultrasound. 2016;4:27. doi: 10.1186/s40349-016-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng S, Zhang L, Hu L, et al. Factors influencing the dosimetry for high-intensity focused ultrasound ablation of uterine fibroids: a retrospective study. Medicine. 2015;94:1–10. doi: 10.1097/MD.0000000000000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z, Gong C, Liu Y, Zhang L. Establishment of a scoring system for predicting the difficulty level of high-intensity focused ultrasound ablation of uterine fibroids. Int J Hyperthermia. 2018;34:77–86. doi: 10.1080/02656736.2017.1325015. [DOI] [PubMed] [Google Scholar]
- 48.Hesley GK, Felmlee JP, Gebhart JB, et al. Noninvasive treatment of uterine fibroids: early Mayo Clinic experience with magnetic resonance imaging-guided focused ultrasound. Mayo Clin Proc. 2006;81:936–942. doi: 10.4065/81.7.936. [DOI] [PubMed] [Google Scholar]
- 49.Nyapathy V, Polina L. MRgFUS treatment of uterine fibroid in a nulliparous woman with acute retention of urine. J Radiol Case Rep. 2012;6:1–8. doi: 10.3941/jrcr.v6i2.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park MJ, Kim YS, Rhim H, Lim HK. Technique to displace bowel loops in MRI-guided high-intensity focused ultrasound ablation of fibroids in the anteverted or anteflexed uterus. AJR Am J Roentgenol. 2013;201:W761–764. doi: 10.2214/AJR.12.10081. [DOI] [PubMed] [Google Scholar]
- 51.Kim YS, Lim HK, Rhim H. Magnetic resonance imaging-guided high-intensity focused ultrasound ablation of uterine fibroids: effect of bowel interposition on procedure feasibility and a unique bowel displacement technique. PloS ONE. 2016;11:1–14. doi: 10.1371/journal.pone.0155670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeong JH, Hong GP, Kim YR, Ha JE, Lee KS. Usefulness of modified BRB technique in treatment to ablate uterine fibroids with magnetic resonance image-guided high intensity focused ultrasound. Obstet Gynecol Sci. 2017;60:92–99. doi: 10.5468/ogs.2017.60.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]