Abstract
This article outlines new minimum standards for reporting adult cochlear implant outcomes. These standards have been endorsed by the Implantable Hearing Devices Committee and the Hearing Committee of the American Academy of Otolaryngology—Head and Neck Surgery. The lack of a standardized method for reporting outcomes following cochlear implantation in clinical trials has hampered the ability of investigators to draw comparisons across studies. Variability in data reported in articles and presentation formats inhibits meta-analyses, making it impossible to accumulate the large patient cohorts needed for statistically significant inference. While investigators remain unrestricted in publishing their adult cochlear implant outcome data in additional formats that they believe to be valuable, they should include the presently proposed minimal data set to facilitate interstudy comparability and consistency of reporting.
Keywords: scientific reporting, hearing preservation, cochlear implantation, adult, outcomes
Rationale
A standardized method for reporting outcomes after adult cochlear implantation does not currently exist. This deficiency negatively affects the cochlear implant literature because studies and clinical trials cannot be effectively compared and appropriate meta-analyses remain challenging. This deficiency became especially apparent with the advent of hearing preservation cochlear implantation and the subsequent development of bimodal electric-acoustic stimulation, also referred to as A+E, hybrid, or EAS. Many reports described hearing preservation outcomes at audiometric levels that are not functionally useful to the individual. The members of the Implantable Hearing Devices Committee of the American Academy of Otolaryngology—Head and Neck Surgery identified a variety of clinical factors and reporting guidelines that will allow for more consistent outcome reporting following adult cochlear implantation. This reporting standard will serve as a minimum template for investigators to report results from their scientific work with the ultimate goal to improve patient outcomes.
Design Considerations
Cochlear implantation is a treatment for hearing loss that has a profound effect on an individual’s communication abilities and quality of life. For the past 40 years, the literature has been inundated with studies that investigated the benefits of cochlear implantation in people with postlingual severe to profound hearing loss. When cochlear implants were first introduced in the 1980s, insertion of the electrode was thought to damage any remaining hearing in these recipients, who were provided with electric-only stimulation through the cochlear implant. With improved technology and the increased application of hearing preservation electrodes, patients with greater degrees of residual acoustic hearing are now being considered candidates. Thus, patients today may have significant residual acoustic hearing prior to and following surgery. As such, these patients may use electric (cochlear implant) and acoustic stimulation, with or without amplification, in one or both ears.1,2
Due to this expansion in cochlear implant candidacy, much research within the past 10 to 15 years has focused on studying the outcomes of individuals with severe to profound high-frequency sensorineural hearing loss and normal to moderate low-frequency hearing who were implanted with a hearing preservation electrode. Literature has shown that residual acoustic hearing is advantageous for hearing in quiet and noisy backgrounds,3–5 localization of sound,6–8 and improving quality of sound and music.9,10
While hearing preservation procedures have been demonstrated to enhance cochlear implant outcomes,11–13 they have also introduced a new level of complexity, especially with regard to outcomes reporting. Through various reviews of the published data that lack consistency when describing hearing preservation outcomes and benefits of low-frequency residual acoustic hearing, it became apparent that a standardization of the practices for reporting cochlear implant outcomes was necessary.
A variety of hearing reporting measures are needed to convey the effects of this intervention on hearing: (1) information about the implanted device; (2) subject demographics and hearing loss history (age, sex, duration of hearing loss, etiology, and history of hearing aid use); (3) individual ear unaided acoustic thresholds and aided speech perception prior to surgery; and (4) postactivation unaided acoustic thresholds and speech perception in various stimulation conditions in the ear (or ears) that was implanted and, if applicable, in combination with the contralateral ear.
Reporting Parameters
Device Nomenclature and Number of Active Electrodes
Proper reporting of each ear should include exact terminology of the device (or devices) placed. As there are currently no generic names for the various cochlear implant systems, the actual name of the product should be utilized. This will allow for precise identification of the device and will avoid future confusion.
Adequate device terminology includes
Device manufacturer
Receiver/stimulator model
Electrode name (per the manufacturer)
Insertion length—classified as “full” or “partial” (with partial insertions, the number of electrode contacts left outside the cochlea should be documented)
Number of active channels in the map at device activation
Surgical Factors
Several reports suggested the influence of certain surgical variables on speech perception. Specifically, the position of the electrode array within the cochlea was shown to influence speech outcomes.14–16 Also, the choice of the surgical approach, insertion depth, and number of active electrodes might have an effect on performance.17,18 Therefore, the following factors should be recorded for each surgery:
Electrode type (straight/not preformed, preformed)
Electrode length (mm)
Insertion depth (mm/electrodes left outside, linear or angular)
Cochlear opening (separate cochleostomy, round window only, enlarged round window cochleostomy)
Complications (eg, a perilymph gusher)
Intratympanic/systemic steroids
Anatomy (labyrinthine anomalies)
Laterality (right, left)
Timing of Auditory Testing
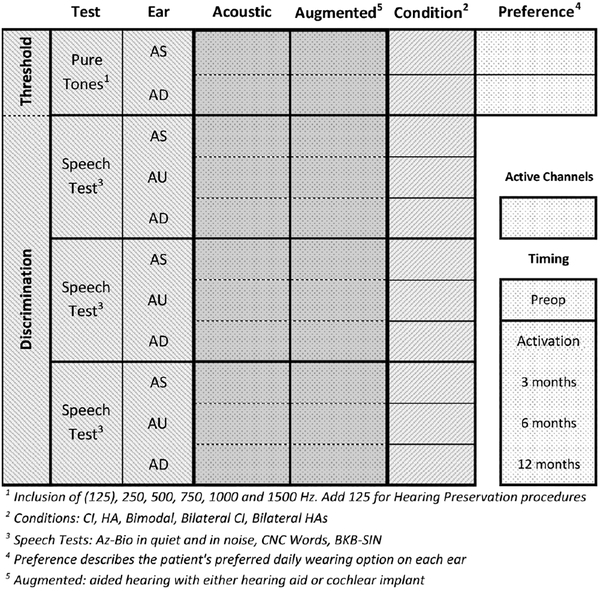
In accordance with the Minimum Speech Test Battery (1996, 2011), the pre- and postoperative testing should be timed per the following intervals (Figure 1):
Figure 1.
Test matrix. This matrix should be used for the preoperative evaluation as well as for testing during all postoperative intervals. “Augmented” denotes hearing with either a hearing aid or a cochlear implant. AD, right ear; AS, left ear; AU, both ears or binaural condition; BKB-SIN, Bamford-Kowal-Bamford Speech-in-Noise; CI, cochlear implant; CNC, consonant-nucleus-consonant; HA, hearing aid.
Preoperative (within 8 weeks prior to surgery)
2–4 weeks (time of device activation)
3 months
6 months
12 months
Auditory Thresholds
Preoperative pure tone thresholds should be documented on every patient for each ear.
Air conduction thresholds at 125, 250, 500, 1000, 1500, 2000, 4000, and 8000 Hz
Bone conduction thresholds at 250, 500, 1000, 1500, 2000, and 4000 Hz
If no response can be obtained (at the limit of the audiometer), the frequency should be marked as 120 dB HL.
Preservation >of acoustic hearing should be determined preoperatively based on the presence of functionally relevant unaided low-frequency pure tone thresholds. Functional hearing is defined with a pure tone average (PTA) <80 dB HL19–22 at 125, 250, and 500 Hz. Guidance for documentation of postoperative auditory thresholds is outlined here.
Tiered Approach Based on Residual Hearing.
To remove unnecessary complexity, postoperative documentation of acoustic hearing in patients with a preoperative unaided low-frequency PTA ≥80 dB HL (at 125, 250, 500 Hz) is not required: a patient with this degree of preoperative hearing will seldom have functional hearing preservation at device activation. Thus, postoperative acoustic hearing reporting guidelines should be specific to the presence or lack of residual functional hearing based on preoperative reports with this tiered approach:
If…
Functionally nonrelevant preoperative low-frequency PTA (125, 250, 500 Hz) is ≥80 dB HL, then documentation of postoperative residual hearing is not required.
Functionally relevant preoperative low-frequency PTA (125, 250, 500 Hz) is <80 dB HL, then documentation of postoperative residual hearing is required.
Each frequency should be reported individually as opposed to a PTA. Thresholds to be tested should include
Air conduction thresholds at 125, 250, 500, 1000, 1500, 2000, 4000, and 8000 Hz
Bone conduction thresholds at 250, 500, 1000, 1500, 2000, and 4000 Hz
If a patient’s residual acoustic hearing, as defined by the low-frequency unaided PTA at 125, 250, and 500 Hz, has become poorer than 80 dB HL, either in the immediate postoperative period or in a delayed fashion, it is no longer necessary to perform acoustic measures on that particular ear after that visit.
Speech Perception Test Materials and Test Conditions
A committee composed of representatives from the American Academy of Audiology, the American Academy of Otolaryngology—Head and Neck Surgery, and cochlear implant manufacturers identified a battery of speech perception tests to be used clinically and in research studies to assess the speech perception performance of adult patients with cochlear implants. Application of this test protocol, the Minimum Speech Test Battery,14,15 to assess the patient’s speech perception abilities is critical and will subsequently be endorsed in this article. Furthermore, it is recommended that guidelines for proper room setup and equipment calibration be utilized to provide reliable results.23,24 The following test battery, administered at 60 dBA, and test conditions are recommended.25,26
Preoperatively (left ear, right each, and both ears/binaural condition):
Consonant-nucleus-consonant (in quiet)
AzBio (in quiet and noise) OR Bamford-Kowal-Bamford Speech-in-Noise; speech and noise presented at 0° to azimuth (Minimum Speech Test Battery)
Postoperatively (tested in the implanted ear, contralateral ear, and both ears/binaural condition):
Consonant-nucleus-consonant (in quiet)
AzBio (in quiet and noise) OR Bamford-Kowal-Bamford Speech-in-Noise; speech and noise presented at 0° to azimuth (Minimum Speech Test Battery)
Daily Listening Condition
The patient’s daily listening condition should be documented at each test interval. Due to the multitude of device-wearing options as a result of varying amounts of residual hearing on each ear, preferred daily listening conditions should be reported for each ear.
Daily listening conditions (left ear and right ear):
No amplification
Conventional hearing aid
Cochlear implant speech processor only
Combined processor (speech processor combining cochlear implant and hearing aid)
The manufacturer and model of the speech processor and hearing aid should be included in the report.
Revision Procedures
As the number of recipients of cochlear implant increases, there is an higher chance that some patients will need to undergo revision surgery for either device malfunction or medical reasons. As with the initial cochlear implant procedure, reporting outcomes pre- and postrevision are important metrics. The committee recommends following the guidelines in the same manner described in this document.
Timeline for Guideline Reevaluation
Historically, the clinical application of cochlear implantation has evolved at a rapid pace. Thus, it is anticipated that the field will continue to evolve in the near future. As such, the Implantable Hearing Devices Committee of the American Academy of Otolaryngology—Head and Neck Surgery recommends a formal reevaluation of these guidelines in 5 years from the date of implementation. This reevaluation should include a thorough review of the literature at that time and revision of the present minimum reporting standard as needed.
Conclusions
To enable a more precise and comprehensive representation of the complexity of adult cochlear implant outcome data, the present document will serve as a minimum reporting standard for scientific purposes. The goal is that it will allow for a more comprehensive assessment of the benefits of adult cochlear implantation in various scenarios. Specifically, by clearly defining the minimum requirements for reporting, meaningful comparisons across study sites, specific studies, and patient populations will be enhanced. Furthermore, it will provide the scientific community with a tool for more effective communication. This seems especially important given the likely expansion of candidacy criteria and the more controlled utilization of residual hearing in the near future. Naturally, adoption of this standard by scientific journals will be critical and is a potential limitation of this work.
Disclosures
Competing interests: Oliver F. Adunka, advisor—MED-EL Corporation and Advanced Bionics; ownership—Advanced Cochlear Diagnostics. Bruce J. Gantz, advisor—Cochlear Corporation. Camille Dunn, advisor—MED-EL Corporation, Earlens Corporation; grant funding—National Institute on Deafness and Other Communication Disorders, Department of Defense. Richard K. Gurgel, advisor—MED-EL Corporation; research funding—Advanced Bionics Corporation, Cochlear Corporation. Craig A. Buchman, consultant, research support—Cochlear Corporation; ownership—Advanced Cochlear Diagnostics; consultant, patient licensing fees—Advanced Bionics Corporation; consultant—Envoy.
Sponsorships: None.
Funding source: None.
References
- 1.von Ilberg C, Kiefer J, Tillein J, et al. Electric-acoustic stimulation of the auditory system: new technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec 1999;61:334–340. [DOI] [PubMed] [Google Scholar]
- 2.Gantz BJ, Turner CW. Combining acoustic and electrical hearing. Laryngoscope. 2003;113:1726–1730. [DOI] [PubMed] [Google Scholar]
- 3.Gantz BJ, Dunn C, Oleson J, Hansen M, Parkinson A, Turner C. Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: final outcomes. Laryngoscope. 2016;126:962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. [DOI] [PubMed] [Google Scholar]
- 5.Roland JT Jr, Gantz BJ, Waltzman SB, Parkinson AJ; Multicenter Clinical Trial Group. United States multicenter clinical trial of the cochlear nucleus hybrid implant system. Laryngoscope. 2016;126:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorman MF, Loiselle LH, Cook SJ, Yost WA, Gifford RH. Sound source localization by normal-hearing listeners, hearing-impaired listeners and cochlear implant listeners. Audiol Neurootol. 2016;21: 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn CC, Perreau A, Gantz B, Tyler RS. Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. J Am Acad Audiol 2010;21:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gifford RH, Grantham DW, Sheffield SW, Davis TJ, Dwyer R, Dorman MF. Localization and interaural time difference (ITD) thresholds for cochlear implant recipients with preserved acoustic hearing in the implanted ear. Hear Res. 2014;312:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boex C, Baud L, Cosendai G, Sigrist A, Kos MI, Pelizzone M. Acoustic to electric pitch comparisons in cochlear implant subjects with residual hearing. J Assoc Res Otolaryngol 2006;7: 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gfeller KE, Olszewski C, Turner C, Gantz B, Oleson J. Music perception with cochlear implants and residual hearing. Audiol Neurootol. 2006;11(suppl 1):12–15. [DOI] [PubMed] [Google Scholar]
- 11.Gantz BJ, Hansen MR, Turner CW, Oleson JJ, Reiss LA, Parkinson AJ. Hybrid 10 clinical trial: preliminary results. Audiol Neurootol. 2009;14(suppl 1):32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gstoettner WK, van de Heyning P, O’Connor AF, et al. Electric acoustic stimulation of the auditory system: results of a multi-centre investigation. Acta Otolaryngol. 2008;128:968–975. [DOI] [PubMed] [Google Scholar]
- 13.Skarzynski H, Lorens A, Piotrowska A, Anderson I. Partial deafness cochlear implantation provides benefit to a new population of individuals with hearing loss. Acta Otolaryngol. 2006;126:934–940. [DOI] [PubMed] [Google Scholar]
- 14.Skinner MW, Holden TA, Whiting BR, et al. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol Suppl. 2007;197:2–24. [PubMed] [Google Scholar]
- 15.Skinner MW, Ketten DR, Holden LK, et al. CT-derived estimation of cochlear morphology and electrode array position in relation to word recognition in Nucleus-22 recipients. J Assoc Res Otolaryngol. 2002;3:332–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aschendorff A, Kromeier J, Klenzner T, Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007;28:75S–79S. [DOI] [PubMed] [Google Scholar]
- 17.Buchman CA, Dillon MT, King ER, Adunka MC, Adunka OF, Pillsbury HC. Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol. 2014;35:1773–1779. [DOI] [PubMed] [Google Scholar]
- 18.Hamzavi J, Arnoldner C. Effect of deep insertion of the cochlear implant electrode array on pitch estimation and speech perception. Acta Otolaryngol. 2006;126:1182–1187. [DOI] [PubMed] [Google Scholar]
- 19.Hogan CA, Turner CW. High-frequency audibility: benefits for hearing-impaired listeners. J Acoust Soc Am. 1998;104: 432–441. [DOI] [PubMed] [Google Scholar]
- 20.Hornsby BW, Ricketts TA.The effects of hearing loss on the contribution of high- and low-frequency speech information to speech understanding: II. Sloping hearing loss. J Acoust Soc Am. 2006;119:1752–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Summers V Do tests for cochlear dead regions provide important information for fitting hearing aids? J Acoust Soc Am. 2004;115:1420–1423. [DOI] [PubMed] [Google Scholar]
- 22.Vickers DA, Moore BC, Baer T. Effects of low-pass filtering on the intelligibility of speech in quiet for people with and without dead regions at high frequencies. J Acoust Soc Am. 2001;110:1164–1175. [DOI] [PubMed] [Google Scholar]
- 23.Uhler K, Gifford RH. Current trends in pediatric cochlear implant candidate selection and postoperative follow up. Am J Audiol. 2014;23:309–325. [DOI] [PubMed] [Google Scholar]
- 24.Spahr AJ, Dorman MF, Litvak LM, et al. Development and validation of the AzBio sentence lists. Ear Hear. 2012;33:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luxford WM; Ad Hoc Subcommittee of the Committee on Hearing and Equilibrium of the American Academy of Otolaryngology—Head and Neck Surgery. Minimum speech test battery for postlingually deafened adult cochlear implant patients. Otolaryngol Head Neck Surg. 2001;124:125–126. [DOI] [PubMed] [Google Scholar]
- 26.Advanced Bionics LLC, Cochlear Americas, MED-EL Corporation. Minimum speech test battery for adult cochlear implant users, 2011. http://www.auditorypotential.com/MSTBfiles/MSTBManual2011-06-20%20.pdf. Published June 2011. Accessed February 2018.