Abstract
Background
Data suggesting that low physical activity levels are associated with increased mortality and exacerbations in patients with COPD have led to increasing interest in the role of physical activity in COPD. This study evaluated self-reported functional performance, a measure of physical activity impairment, according to current treatment regimen, lung function, symptoms, and Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 groups in a large sample of patients with COPD.
Methods
This multicenter, cross-sectional, observational study (study identifier: D5970R00003) included patients with COPD (≥40 years) in the USA. A self-completion questionnaire captured demographics and patient-reported outcomes, including the Functional Performance Inventory-Short Form (FPI-SF). Diagnosis and treatment history (including spirometry results) were extracted from medical charts. Multiple linear regression was used to determine the relationship between FPI-SF and FEV1 % predicted, and FPI-SF and COPD assessment test (CAT) score.
Results
Overall, 1,775 patients participated (classified as GOLD 2017 group A, 14.8%; B, 46.6%; C, 2.6%; D, 36.0%). Physical activity impairment affected patients across all treatment regimens and GOLD groups (mean FPI-SF total score: 2.1), with the greatest impairment within FPI-SF observed for domains requiring most physical exertion, “physical exercise” and “maintaining the household” (mean FPI-SF scores: 1.7 and 1.8, respectively). Patients receiving loose triple therapy and those in GOLD group D had the highest impairment (mean FPI-SF total scores: both 1.9), and the lowest FEV1 % predicted (55.5% and 54.7%, respectively). FPI-SF total score correlated with FEV1 % predicted and more strongly with CAT score (all P<0.05).
Conclusion
The stronger correlation between FPI-SF and CAT scores compared to FPI-SF and FEV1 % predicted suggests that symptoms may have a greater impact on patients’ functional performance than lung function. Further longitudinal studies are required to establish a correlation between the effect of treatment on symptoms, lung function, and physical activity.
Keywords: FPI-SF, bronchodilator, CAT score, spirometry, physical activity, functional performance inventory
Introduction
COPD is one of the leading causes of morbidity and mortality globally, and was responsible for 3.2 million deaths and 63.9 million disability-adjusted life years worldwide in 2015.1,2
Patients with COPD have significantly impaired duration, intensity, and counts of daily physical activity compared with healthy individuals,3 and a low level of physical activity has been shown to be a predictor of mortality and COPD exacerbations.4 It has been suggested that physical activity levels may also be associated with dyspnea, health-related quality of life, and exercise capacity outcomes.4 In addition, physical comorbidities that are common in patients with COPD5 may further reduce physical activity levels.6 Therefore, there is increasing interest in physical activity in patients with COPD, both as a prognostic factor and as a component of treatment.7–9
However, there is a paucity of data examining the relationship between physical activity and current COPD treatment regimen, and previous studies investigating the relationship between physical activity and GOLD group, and physical activity and lung function, were limited by relatively small patient numbers.7,8,10,11
The aim of this real-world study was to describe self-reported functional performance, a measure of how much difficulty an individual has when performing day-to-day activities, according to current treatment regimen, lung function measurements, symptoms, and Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 groups5 in a large sample of patients with COPD.
Materials and methods
Study design
This study was a multicenter, cross-sectional, observational survey (study identifier: D5970R00003) conducted in the USA, and comprised two components: a patient questionnaire and a medical chart review. Data were collected between January and June 2017.
The study protocol was submitted to a US centralized Institutional Review Board (Western IRB) for methodological approval and was granted an exemption. Due to the non-interventional nature of the study, a central approval through the Western IRB was considered appropriate for this study. This study was performed in compliance with the International Society for Pharmacoepidemiology guidelines for Good Pharmacoepidemiological Practice and in full accordance with the US Health Insurance Portability and Accountability Act 1996 and Health Information Technology for Economic and Clinical Health 2009 guidelines. The patient and physician information collected was fully anonymized. Patients must have signed an informed consent form to participate in the study.
Study population
Primary care physicians and pulmonologists who had 5–35 years’ clinical experience, who were responsible for the treatment of patients with COPD, and who saw ≥3 patients with COPD per month were eligible for inclusion in this study. Physicians were requested to follow a consecutive approach to recruitment to avoid selection bias; each physician invited their next 1–30 consulting and eligible patients with COPD to take part in the study. Patients were recruited on a double-blind basis; the patients and study sponsor remained anonymous to one another. Patients who were ≥40 years of age with a physician-confirmed diagnosis of COPD and willing and able to complete a questionnaire by themselves were included.
Objectives
The primary objective of this study was to describe self-reported functional performance according to standard-of-care (SOC) medication class(es) received by patients with COPD. Exploratory objectives included: 1) to determine the frequency of receipt of SOC medication class(es) among GOLD 2017 groups; 2) to determine self-reported functional performance among GOLD 2017 groups; 3) to evaluate spirometric measures of lung function according to SOC medication class(es) received; and 4) to examine the relationship between self-reported functional performance and lung function among patients with COPD. The relationship between self-reported functional performance and patient symptom burden (COPD assessment test [CAT] score) was also examined.
Patient self-completion questionnaire (PSC)
Patients who were identified by their physician completed the paper-based PSC post-consultation, which took a maximum of 10 minutes. The PSC captured patient demographics and incorporated several patient-reported outcome tools, including the Functional Performance Inventory-Short Form (FPI-SF) and the CAT. The completed PSC was then given to the site staff.
The FPI-SF comprises 32 items and assesses the level of difficulty patients had with physical activities across six domains, including: body care (five items), maintaining the household (eight items), physical exercise (five items), recreation (five items), spiritual activities (four items), and social interaction (five items). Patients responses were scored as 1= much difficulty, 2= some difficulty, or 3= no difficulty, and if patients did not perform an activity, they could select either “don’t do for health reasons” or “choose not to” (both rated as 0). The FPI-SF has been validated as a self-completed assessment in patients with COPD12 and was used as a patient proxy measurement of physical activity impairment.
The CAT has been developed to quantify the impact of COPD on patient health in routine practice across the following eight items: energy, sleep, confidence, activities, breathlessness, chest tightness, phlegm, and cough. Patients rated items on a scale of 0 to 5, and total scores ranged from 0 to 40, with higher scores representing worse health.13 CAT scores contributed toward the derivation of GOLD 2017 groups in this study.5
Medical chart review
Patient medical charts were reviewed and data extracted by the physician onto an electronic case report form, which captured diagnosis, and clinical and treatment history of patients, including their most recent results of spirometric lung function tests. In addition to current medications, if the patient had been receiving their current medication for less than 12 months, any other medications from the last 12 months were also included. Data were only collected once the physician had received the PSC. Not all information was available for each patient due to patients having incomplete records or not having had a specific test.
Statistical analyses
Classification into GOLD 2017 groups was derived from the information provided by the physician following the medical chart review (number of exacerbations and hospitalization and/or emergency room admission) and each patient’s CAT score.5 For the analysis, treatment regimens were classified into six groups: 1) “short-acting bronchodilator” included treatment with a short-acting muscarinic antagonist (SAMA) or short-acting β2-agonist (SABA); 2) “mono long-acting bronchodilator” included treatment with a long-acting muscarinic antagonist (LAMA) or long-acting β2-agonist (LABA); 3) “dual long-acting” bronchodilator included treatment with a LAMA and a LABA; 4) “ICS/long-acting bronchodilator” included treatment with inhaled corticosteroids (ICSs) and a LAMA or LABA; 5) “triple therapy” included treatment with ICS, a LAMA, and a LABA; and 6) “other treatments” included, but were not limited to ICS, short-acting bronchodilators in combination with other treatments, and phosphodiesterase-4 inhibitors or oral corticosteroids alone or in combination with other treatments. Combination therapies could have been administered via separate inhalers or as fixed-dose combinations (FDCs), although no FDC triple therapies were approved during the study period.
Data were analyzed using descriptive statistics; FPI-SF and post-bronchodilator FEV1 % predicted were stratified by treatment regimens and GOLD 2017 groups. In addition, data for treatment regimens among GOLD 2017 groups were tabulated. Multiple linear regression analysis was used to determine the relationship between FPI-SF and FEV1, and FPI-SF and its domains were used as dependent variables in separate regressions, with FEV1 as an independent variable. Multiple linear regression was also used to determine the relationship between FPI-SF and CAT score, with CAT score as an independent variable. The r2 values and P-values (from a Wald test) associated with FEV1 and CAT score were used to determine their relationship with FPI-SF scores.
Standard errors in regressions were adjusted to allow for intragroup correlation within reporting physician, relaxing the usual requirement that the observations be independent (patients were independent across physicians but not necessarily within physician). Age, gender, body mass index (BMI), socioeconomic status, medication, smoking status, and comorbidities (using Charlson Comorbidity Index) confounding variables were included as covariates. Time since diagnosis was listed as a covariate but was not included, and the number of pack-years was substituted with smoking status as it limited the sample size. A proxy for severity was not included since lung function may be correlated with severity and, therefore, this risked controlling for the effect being measured. No adjustments for multiplicity were made in this study. All analyses were conducted by the Adelphi Real World statistical department using Stata 14.1 or later (Stata Statistical Software: Release 14; StataCorp LP, College Station, TX, USA).
As the majority of planned analyses were descriptive, the sample size only affected the precision of the point estimates. A sample size of 62 was needed to generate a 95% CI that was within 0.25 SDs of the mean. For the regression analysis, complete data for all outcomes and covariates were required to be included in the model.
Results
Study population and clinical characteristics
A total of 206 physicians agreed to participate, and 148 recruited patients to the study. Of those, 101 (68.2%) were primary care physicians and 47 (31.8%) were pulmonologists, who saw an average of 77 and 168 patients with COPD per month, respectively. A total of 1,775 patients with physician-confirmed COPD, who had a mean age of 65.2 years and a mean BMI of 27.5 kg/m2 participated in this study (Table 1). The majority of patients were Caucasian (71.9%) and current or former-smokers (87.1%), and just over half of patients were male (55.1%) and had experienced at least one exacerbation in the last 12 months (55.4%). According to GOLD 2017, 14.8% of patients were classified in group A, 46.6% in group B, 2.6% in group C, and 36.0% in group D. Around half of patients (46.6%) had comorbidities, with hypertension being the most common (41.3%; Table 1). A full overview of patient comorbidities is provided in Table S1. Fewer than half of the patients had data available for the spirometry lung function tests; only a very small proportion of patients (<6.1%) had data available for maximal voluntary ventilation and inspiratory capacity. Furthermore, 65.2% (666 of 1,021) of patients had FEV1 tests performed by pulmonologists, whereas only 22.8% (233 of 1,021) of patients had FEV1 tests performed by primary care physicians. Demographic information and clinical characteristics are summarized in Table 1.
Table 1.
Patient demographics and clinical characteristics
| All (N=1,775) | |
|---|---|
| Mean age, years | 65.2 |
| Male, n (%) | 978 (55.1) |
| Ethnicity, n (%) | |
| White/Caucasian | 1,277 (71.9) |
| African American | 284 (16.0) |
| Hispanic/Latino | 97 (5.5) |
| Asian | 71 (4.0) |
| Other | 46 (2.6) |
| Mean body mass index, kg/m2 | 27.5 |
| Smoking status,a n (%) | |
| Former | 896 (51.1) |
| Current | 632 (36.0) |
| Never | 227 (12.9) |
| Mean number of pack-years smokedb | 32.7 |
| Mean Charlson Comorbidity Index (post-treatment) | 1.6 |
| Most common comorbidities,c n (%) | |
| None | 947 (53.4) |
| Hypertension | 733 (41.3) |
| Elevated cholesterol/hyperlipidemia | 399 (22.5) |
| Diabetes mellitus | 255 (14.4) |
| Obesity | 255 (14.4) |
| Coronary heart disease | 240 (13.5) |
| Gastroesophageal reflux disease | 205 (11.5) |
| Depression | 184 (10.4) |
| Anxiety | 164 (9.2) |
| Obstructive sleep apnea | 157 (8.8) |
| Congestive heart failure | 154 (8.7) |
| Previous asthma diagnosis, n (%) | 224 (12.6) |
| Mean time since COPD diagnosis, years | 2.1 |
| Patients with exacerbations in the last 12 months, n (%) | |
| 0 exacerbations | 792 (44.6) |
| 1 exacerbation | 234 (13.2) |
| ≥2 exacerbations | 749 (42.2) |
| Mean number of exacerbations | 1.0 |
| Physician-reported COPD severity, n (%) | |
| Mild | 517 (29.1) |
| Moderate | 809 (45.6) |
| Severe | 333 (18.8) |
| Very severe | 107 (6.0) |
| Do not know | 9 (0.5) |
| GOLD 2017 groups,d n (%) | |
| GOLD A | 257 (14.8) |
| GOLD B | 812 (46.6) |
| GOLD C | 45 (2.6) |
| GOLD D | 627 (36.0) |
| Mean post-bronchodilator FEV1,e % predicted (SD) | 61.2 (18.3) |
| Mean MVV,f L/min (SD) | 87.9 (32.4) |
| Mean IC,g L (SD) | 2.2 (0.7) |
Notes:
N=1,755;
N=850;
comorbidities occurring in >150 patients;
N=1,741;
N=758;
N=96;
N=108.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; IC, inspiratory capacity; MVV, maximal voluntary ventilation.
Current treatment classes prescribed
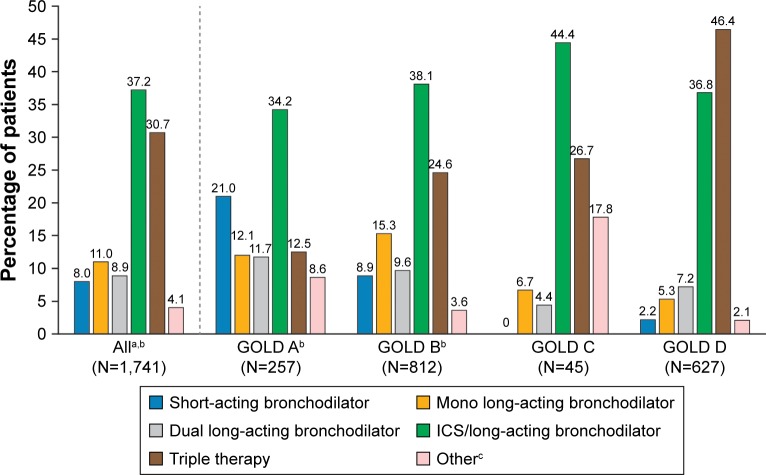
Overall, 67.9% of patients were receiving an ICS in combination with a long-acting bronchodilator (LAMA or LABA; 37.2%) or as part of a triple therapy (LAMA+ LABA; 30.7%; Figure 1). In the dual bronchodilation group, 64% were receiving LAMA/LABA FDCs. When stratified by GOLD 2017 groups, ICS/long-acting bronchodilator was the most commonly prescribed treatment in group A (34.2%), group B (38.1%), and group C (44.4%), whereas in group D triple therapy was the most frequent treatment (46.4%; Figure 1).
Figure 1.
Current treatment classes prescribed to COPD patients, overall and by GOLD 2017 group.
Notes: aNumber of patients with current treatment regimen identified who were classified by GOLD 2017. bPercentages for all patients, and GOLD groups A and B do not add up to 100% due to rounding. cOther treatments included, but were not limited to ICS, short-acting bronchodilators in combination with other treatments, and phosphodiesterase-4 inhibitors or oral corticosteroids alone or in combination with other treatments.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid.
Functional performance
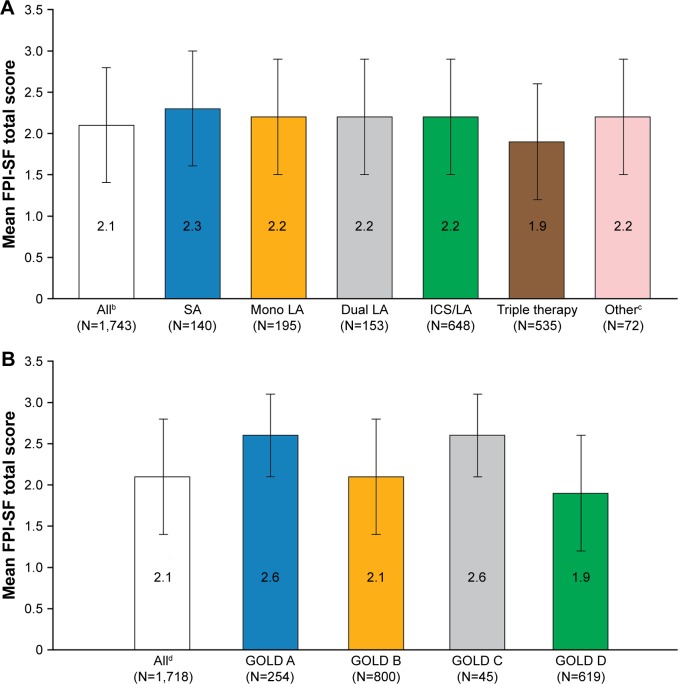
Patients reported difficulty with physical activity based on FPI-SF total scores across all treatment regimens and GOLD 2017 groups (mean total score overall: 2.1; Figure 2). Patients who were receiving triple therapy at the time of observation had the most impairment, with a mean FPI-SF total score of 1.9 (Figure 2). Patients classified within GOLD groups A and C reported similar functional performance (2.6), followed by GOLD group B (2.1); GOLD group D (1.9) reported the lowest FPI-SF scores (Figure 2). The lowest FPI-SF scores were reported within the FPI-SF domains “physical exercise” and “maintaining the household” (mean scores of 1.7 and 1.8 for all patients, respectively). This trend was observed consistently, regardless of treatment regimen (with the exception of patients treated with short-acting bronchodilators who had a lower mean FPI-SF score for “spiritual activity” than “maintaining the household” [Table 2]), or GOLD 2017 group (Table 3).
Figure 2.
Patient-reported functional performance (FPI-SF)a by current treatment regimen (A) and GOLD 2017 group (B).
Notes: Error bars represent SD. aHigher scores indicate less difficulty with physical activity.12 bNumber of patients with completed FPI-SF who were classified by treatment regimen. cOther treatments included, but were not limited to ICS, short-acting bronchodilators in combination with other treatments, and phosphodiesterase-4 inhibitors or oral corticosteroids alone or in combination with other treatments. dNumber of patients with completed FPI-SF who were classified by GOLD 2017.
Abbreviations: FPI-SF, Functional Performance Inventory-Short Form; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; LA, long-acting bronchodilator; SA, short-acting bronchodilator.
Table 2.
Patient-reported functional performance (FPI-SF)a by current treatment regimen, mean (SD)
| FPI-SF total | Body care | Maintaining the household | Physical exercise | Recreation: activities for personal pleasure | Spiritual activities | Social interaction; family and friends | |
|---|---|---|---|---|---|---|---|
| Allb (N=1,743) | 2.1 (0.7) | 2.5 (0.7) | 1.8 (0.8) | 1.7 (0.8) | 2.4 (0.8) | 2.1 (1.2) | 2.1 (0.9) |
| Short-acting bronchodilator (N=140) | 2.3 (0.7) | 2.7 (0.5) | 2.2 (0.8) | 1.9 (0.8) | 2.6 (0.7) | 2.1 (1.2) | 2.2 (0.9) |
| Mono long-acting bronchodilator (N=195) | 2.2 (0.7) | 2.6 (0.7) | 2.0 (0.8) | 1.8 (0.8) | 2.5 (0.8) | 2.1 (1.2) | 2.2 (0.9) |
| Dual long-acting bronchodilator (N=153) | 2.2 (0.7) | 2.6 (0.7) | 1.9 (0.8) | 1.8 (0.8) | 2.6 (0.7) | 2.0 (1.2) | 2.2 (0.8) |
| ICS/long-acting bronchodilator (N=648) | 2.2 (0.7) | 2.6 (0.6) | 1.9 (0.8) | 1.7 (0.8) | 2.5 (0.8) | 2.2 (1.1) | 2.2 (0.9) |
| Triple therapy (N=535) | 1.9 (0.7) | 2.4 (0.7) | 1.5 (0.8) | 1.4 (0.7) | 2.3 (0.8) | 2.0 (1.2) | 1.9 (0.9) |
| Otherc (N=72) | 2.2 (0.7) | 2.6 (0.6) | 2.0 (0.9) | 1.8 (0.9) | 2.6 (0.7) | 2.1 (1.2) | 2.2 (0.9) |
Notes:
Patients rated how difficult each activity was for them to perform and their responses were scored on a three-point scale: 1= much difficulty, 2= some difficulty, 3= no difficulty. If patients did not perform an activity (either for health reasons or by choice), the item received a score of 0.12
Number of patients with completed FPI-SF who were classified by current treatment regimen.
Other treatments included, but were not limited to: ICS, short-acting bronchodilators in combination with other treatments, and phosphodiesterase-4 inhibitors or oral corticosteroids alone or in combination with other treatments.
Abbreviations: FPI-SF, Functional Performance Inventory-Short Form; ICS, inhaled corticosteroid.
Table 3.
Patient-reported functional performance (FPI-SF)a by GOLD 2017 group, mean (SD)
| FPI-SF total | Body care | Maintaining the household | Physical exercise | Recreation: activities for personal pleasure | Spiritual activities | Social interaction; family and friends | |
|---|---|---|---|---|---|---|---|
| Allb (N=1,718) | 2.1 (0.7) | 2.5 (0.7) | 1.8 (0.8) | 1.7 (0.8) | 2.4 (0.8) | 2.1 (0.9) | 2.1 (0.9) |
| GOLD A (N=254) | 2.6 (0.5) | 2.9 (0.4) | 2.5 (0.6) | 2.4 (0.6) | 2.9 (0.4) | 2.7 (0.6) | 2.7 (0.6) |
| GOLD B (N=800) | 2.1 (0.7) | 2.6 (0.7) | 1.8 (0.8) | 1.6 (0.8) | 2.4 (0.8) | 2.1 (0.9) | 2.1 (0.9) |
| GOLD C (N=45) | 2.6 (0.5) | 2.8 (0.4) | 2.5 (0.6) | 2.4 (0.5) | 2.8 (0.6) | 2.6 (0.7) | 2.6 (0.7) |
| GOLD D (N=619) | 1.9 (0.7) | 2.3 (0.7) | 1.5 (0.8) | 1.4 (0.7) | 2.2 (0.8) | 1.8 (0.9) | 1.8 (0.9) |
Notes:
Patients rated how difficult each activity was for them to perform and their responses were scored on a three-point scale: 1= much difficulty, 2= some difficulty, 3= no difficulty. If patients did not perform an activity (either for health reasons or by choice), the item received a score of 0.12
Number of patients with completed FPI-SF who were classified by GOLD 2017.
Abbreviations: FPI-SF, Functional Performance Inventory-Short Form; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Lung function
The highest mean post-bronchodilator FEV1 % predicted value was reported for patients receiving treatment with short-acting bronchodilators only (76.2%), and the lowest value was reported for patients receiving triple therapy (55.5%; Figure 3A). The post-bronchodilator FEV1 % predicted values were comparable for patients receiving mono long-acting bronchodilator, ICS/long-acting bronchodilator, dual long-acting bronchodilator, or other therapies (Figure 3A). When stratified by GOLD 2017 group, the highest post-bronchodilator FEV1 % predicted values were reported for patients in groups A (70.3%) and C (69.4%), and the lowest value was reported for patients in group D (54.7%; Figure 3B).
Figure 3.
Post-bronchodilator FEV1 % predicted by current treatment regimen (A) and GOLD 2017 group (B).
Notes: Error bars represent SD. aNumber of patients with FEV1 data available who were classified by treatment regimen. bOther treatments included, but were not limited to ICS, short-acting bronchodilators in combination with other treatments, and phosphodiesterase-4 inhibitors or oral corticosteroids alone or in combination with other treatments. cNumber of patients with FEV1 data available who were classified by GOLD 2017.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; LA, long-acting bronchodilator; SA, short-acting bronchodilator.
Relationship between functional performance, and lung function and symptoms
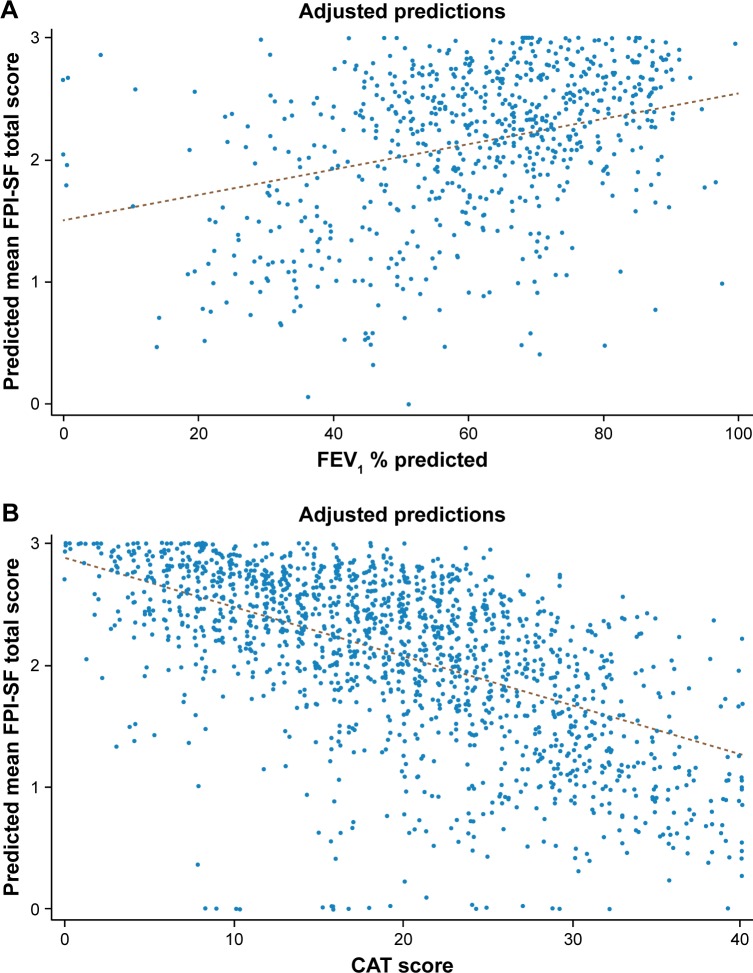
Multiple linear regression analysis was performed to evaluate the relationship between FPI-SF and FEV1 % predicted, and indicated that patients with a higher FEV1 % predicted values had a higher FPI-SF score for all domains (r2 value 0.3299; Figure 4A). The regression model suggested that for each unit increase in FEV1 % predicted, the FPI-SF total score would increase by 0.0104 (all P<0.01; Table 4). Regression analysis was also performed to evaluate the association between FPI-SF and CAT score. This analysis indicated that patients with a lower CAT score had a higher FPI-SF score for all FPI-SF domains (r2 value 0.4335; Figure 4B), with a one-unit decrease in CAT score resulting in an increase of 0.0403 (all P<0.01; Table 5) in FPI-SF total score.
Figure 4.
Multiple linear regression analysisa of FPI-SF and FEV1 % predicted (A) and CAT score (B).
Notes: y =1.5068+0.0104*FEV1; r2 value 0.3299 (A); y =2.8775–0.0403*CAT; r2 value 0.4335 (B). aThe regression analyses were adjusted for the following variables: patient age, sex, BMI, employment status, currently prescribed treatment, smoking status, and Charlson Comorbidity Index.
Abbreviations: BMI, body mass index; CAT, COPD assessment test; FPI-SF, Functional Performance Inventory-Short Form.
Table 4.
Multiple linear regression analysis between FPI-SF and FEV1 % predicted
| FPI-SF total (N=735) | Body care (N=738) | Maintaining the household (N=739) | Physical exercise (N=741) | Recreation: activities for personal pleasure (N=741) | Spiritual activities (N=739) | Social interaction; family and friends (N=740) | |
|---|---|---|---|---|---|---|---|
| Regression coefficient | 0.0104 | 0.0103 | 0.0132 | 0.0107 | 0.0086 | 0.0094 | 0.0110 |
| r2 value | 0.3299 | 0.2642 | 0.3163 | 0.3322 | 0.2496 | 0.0622 | 0.2605 |
| P-value | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.005 | 0.00 |
Abbreviation: FPI-SF, Functional Performance Inventory-Short Form.
Table 5.
Multiple linear regression analysis between FPI-SF and CAT scores
| FPI-SF total (N=1,691) | Body care (N=1,708) | Maintaining the household (N=1,704) | Physical exercise (N=1,709) | Recreation: activities for personal pleasure (N=1,712) | Spiritual activities (N=1,704) | Social interaction; family and friends (N=1,711) | |
|---|---|---|---|---|---|---|---|
| Regression coefficient | −0.0403 | −0.0339 | −0.0483 | −0.0490 | −0.0371 | −0.0273 | −0.0470 |
| r2 value | 0.4335 | 0.3202 | 0.3163 | 0.4492 | 0.2960 | 0.0787 | 0.3434 |
| P-value | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Abbreviations: CAT, COPD assessment test; FPI-SF, Functional Performance Inventory-Short Form.
Discussion
This cross-sectional, real-world, observational study provides insight into the relationship between self-reported functional performance, current treatment regimen, GOLD 2017 groups, and spirometric measurements of lung function in a large sample of patients with COPD who were consulting their treating physician in routine clinical practice.
Although current GOLD guidelines recommend spirometric assessments to determine the diagnosis and prognosis of COPD,5 data from spirometric lung function tests were available for fewer than half of the patients in this study (42.7%). This proportion is reflective of the real-world setting and similar to, or higher than, earlier studies in the USA, which reported that overall, 31%–42% of patients with COPD had undergone spirometry tests.14–18 However, use of spirometric assessments can vary considerably (higher than threefold) between regions within the USA,16 and between different types of physicians as reported in this study, with pulmonologists performing tests for FEV1 in almost three times the number of patients compared with primary care physicians.
An ICS/long-acting bronchodilator combination was the most commonly prescribed treatment regimen for patients in GOLD groups A, B, and C, although not being recommended for groups A and B, and in GOLD group C only for patients who experience persistent exacerbations despite treatment with a long-acting bronchodilator.5 Dual long-acting bronchodilator treatment was prescribed to only 9.6% or less of patients in GOLD groups B, C, and D, despite being recommended as one of the preferred treatments in these groups.5 Triple therapy was prescribed to around one-eighth of patients in GOLD group A and around a quarter in groups B and C, although it is only recommended for patients in group D who experience further exacerbations or persistent symptoms on dual therapies.5 It should be noted that as the GOLD 2017 report was only released at the end of 2016 and this study was performed over the first half of 2017, it is unlikely that physicians would have begun to implement the changes in recommendations into clinical practice. However, many physicians also did not follow the treatment paradigm in the GOLD 2016 report, which like the 2017 report did not recommend ICS/long-acting bronchodilator combinations for patients in GOLD groups A or B, and recommended triple therapy for patients in GOLD group D only.19 This is reflective of findings from previous real-world studies, which described high prescribing rates of ICS in combination with a long-acting bronchodilator or as part of triple therapy across all GOLD groups.20,21
The FPI-SF was used in this study to assess functional performance, as a proxy measurement of physical activity impairment, because this instrument is validated in patients with COPD12 and captures the patients’ perspectives on their difficulties with performing daily activities that patients consider to be important, including personal care, family, and social activities. Furthermore, the FPI-SF does not exclude items that patients do not perform (either for health reasons or by choice); instead they receive a score of 0,12 and therefore contribute to low functional performance scores. Patients who were receiving triple therapy had the worst disease status as shown by the high proportion of patients who were classified into GOLD group D and the low FEV1 % predicted values. In addition, patients receiving triple therapy also reported the lowest functional performance (mean FPI-SF total score: 1.9). This is likely due to the fact that patients who were prescribed triple therapy had a higher level of disease severity than those using other bronchodilator therapies.5
Patients within GOLD groups A and C reported comparable functional performance, while patients within groups B and D reported lower functional performance. This suggested that difficulty in performing physical activity may be related to the level of symptoms experienced rather than to the exacerbation history,5 although further studies investigating this assumption are required. However, the lowest functional performance (mean FPI-SF total score: 1.9) was reported in patients in GOLD group D who have both a high symptom burden and a high exacerbation risk,5 which is comparable with findings from previous studies.7,8 In addition, patients in GOLD group D had the lowest FEV1 % predicted values (54.7%). Irrespective of current treatment regimen or GOLD 2017 group, patients generally reported lower FPI-SF scores for the domains that are the most physically demanding (“physical exercise” and “maintaining the household”) than for the other domains. This is largely reflective of the findings from a previous small study validating FPI-SF which found that most patients had difficulties with activities that required high exertion, with the lowest FPI-SF scores reported for physical exercise, followed by spiritual activities and then maintaining the household.22
FPI-SF scores were found to be positively correlated with FEV1 % predicted, and longitudinal studies are required to elucidate if an improvement in lung function in patients with COPD could translate into an increase in patient physical activity. A correlation between FPI-SF score and CAT score was also observed, with a greater symptom burden (higher CAT score) associated with lower functional performance (lower FPI-SF score). This correlation was stronger than that between FPI-SF score and FEV1 % predicted, suggesting that CAT score may be a stronger predictor of patients’ physical activity impairment and that changes in CAT score could potentially relate to the effect of therapeutic interventions on physical activity. Together, these data suggest that achieving the maximum improvements in lung function and symptomatic relief through bronchodilator therapy could lead to patients with COPD having a more active lifestyle, which may in turn help to slow lung function decline.
A high proportion of patients in this study had comorbidities, with the type of comorbidities similar to previous findings in patients with COPD.23,24 A study in a small cohort of patients with COPD identified that those who had physical comorbidities such as obesity and musculoskeletal or neurological conditions had decreased physical activity levels in comparison with those who did not have physical comorbidities.6 Larger studies are required to further elucidate the effect of comorbidities on physical activity in patients with COPD.
Limitations of this study included that patients may have been visiting their physician due to an unusual flare up in symptoms, which may not have been indicative of their health status over the previous 12 months. A further limitation was that patients may have been seeing multiple physicians, but data were only recorded at one setting. However, physicians were asked to extract specific parts of the medical records that were available to them, which included visits to other physicians. While the patient sample was representative of the consulting population, due to the recruitment process, the patient sample was not strictly random, and it is unknown whether there would have been any differences between the patients who did and did not participate. In addition, although common and accepted for real-world studies, diagnosis of COPD was physician confirmed. Together, these factors may have limited the generalizability of the findings in this study to all COPD patients. However, the large number of patients included in this study provides reassurance that these data are representative of the real world.
Conclusion
Patients with COPD across all treatment regimens and GOLD 2017 groups experienced impairments in functional performance. Patients who had the worst lung function as measured by FEV1 % predicted, and those in GOLD group D, were typically prescribed loose triple therapy and tended to report the most difficulty with physical activity. Importantly, functional performance (FPI-SF score) was found to be correlated with lung function (FEV1 % predicted) and to a greater extent with symptoms (CAT score). Additional longitudinal randomized controlled trials, real-world evidence studies, and observational studies are needed to establish a correlation between the effect of treatment on symptom burden, lung function, and physical activity capacity.
Data availability
All relevant data analyzed during this study are included in this published article.
Supplementary material
Table S1.
Comorbidities
| Patients with comorbidities, n (%) | N=1,775 |
|---|---|
| None | 947 (53.4) |
| Cardiovascular condition | 935 (52.7) |
| Angina pectoris | 32 (1.8) |
| Cardiac arrhythmias | 105 (5.9) |
| Coagulated blood clotting disorder | 16 (0.9) |
| Congestive heart failure | 154 (8.7) |
| Coronary heart disease | 240 (13.5) |
| Elevated cholesterol/hyperlipidemia | 399 (22.5) |
| Hypertension | 733 (41.3) |
| Myocardial infarction | 75 (4.2) |
| Peripheral vascular disease | 93 (5.2) |
| Cerebrovascular disease | 32 (1.8) |
| Thrombosis | 20 (1.1) |
| Other cardiovascular condition | 26 (1.5) |
| Dementia | 65 (3.7) |
| Endocrine disorder | 333 (18.8) |
| Diabetes mellitus | 255 (14.4) |
| No end organ damage | 214 (12.1) |
| End organ damage | 41 (2.3) |
| Hyperthyroidism | 31 (1.7) |
| Osteoporosis | 48 (2.7) |
| Other endocrine disorders | 41 (2.3) |
| Glaucoma | 96 (5.4) |
| Gastrointestinal condition | 266 (15.0) |
| Crohn’s disease | 10 (0.6) |
| Dyspepsia/stomach pain | 39 (2.2) |
| Gastroesophageal reflux disease | 205 (11.5) |
| Peptic ulcer disease | 13 (0.7) |
| Other gastrointestinal condition | 25 (1.4) |
| Hematological disorder | 40 (2.3) |
| Anemia | 31 (1.7) |
| Leukemia | 4 (0.2) |
| Other hematological disorder | 6 (0.3) |
| HIV/AIDS | 8 (0.5) |
| AIDS | 2 (0.1) |
| HIV | 6 (0.3) |
| Pulmonary condition | 211 (11.9) |
| Chronic bronchitis | 87 (4.9) |
| Cystic fibrosis | 2 (0.1) |
| Emphysema | 80 (4.5) |
| Idiopathic pulmonary fibrosis | 8 (0.5) |
| Pulmonary arterial hypertension | 19 (1.1) |
| Lung cancer | 17 (1.0) |
| Other pulmonary condition | 37 (2.1) |
| Hemiplegia | 8 (0.5) |
| Malignant lymphoma | 13 (0.7) |
| Obesity | 255 (14.4) |
| Prostate disorder | 137 (7.7) |
| Psychiatric disorder | 279 (15.7) |
| Anxiety | 164 (9.2) |
| Depression | 184 (10.4) |
| Other psychiatric disorder | 19 (1.1) |
| Renal disorder | 85 (4.8) |
| Moderate/severe chronic kidney disease | 75 (4.2) |
| Renal failure | 7 (0.4) |
| Other renal disorder | 7 (0.4) |
| Rhinosinusitis | 113 (6.4) |
| Rheumatic disorder | 57 (3.2) |
| Arthritis | 48 (2.7) |
| Connective tissue/rheumatological disease | 11 (0.6) |
| Other rheumatic disorders | 1 (0.1) |
| Sleep apnea | 199 (11.2) |
| Sleep apnea | 55 (3.1) |
| Obstructive sleep apnea | 157 (8.8) |
| Other sleep apnea | 1 (0.1) |
| Solid tumor | 36 (2.0) |
| Non-metastatic | 28 (1.6) |
| Metastatic | 4 (0.2) |
| Other solid tumor | 5 (0.3) |
| Substance abuse/addiction | 107 (6.0) |
| Systemic sclerosis | 4 (0.2) |
| Other | 38 (2.1) |
Acknowledgments
The authors thank all the patients and their families and the team of investigators involved in the study. The authors also thank James Pike (Adelphi Real World) for his valuable contributions. Medical writing support, under the direction of the authors, was provided by Pauline Craig, PhD, of CMC CONNECT, a division of Complete Medical Communications Ltd, Glasgow, UK, which was funded by AstraZeneca, Cambridge, UK, in accordance with Good Publication Practice (GPP3) guidelines. This work was previously published as a meeting abstract (Thorax 2017;72(Suppl 3):A1–278) in conjunction with poster presentation at the British Thoracic Society Winter Meeting, London, UK, 6–8 December, 2017.
Footnotes
Author contributions
All authors contributed toward conception and design of the study or analysis and interpretation of the data, drafting and revising the paper, and agree to be accountable for all aspects of the work.
Disclosure
BD and SS are employees of AstraZeneca. DJ, MS, and NB-E are employees of Adelphi Real World. This work was supported by AstraZeneca. The funder of the study was involved in study design, data analysis, data interpretation, and writing of the report. All authors had full access to all the data in the study and the corresponding author had the final responsibility for the decision to submit for publication. No restrictions were placed on authors regarding the statements made in the manuscript. The authors report no other conflicts of interest in this work.
References
- 1.GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2015 Chronic Respiratory Disease Collaborators Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vorrink SN, Kort HS, Troosters T, Lammers JW. Level of daily physical activity in individuals with COPD compared with healthy controls. Respir Res. 2011;12:33. doi: 10.1186/1465-9921-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimeno-Santos E, Frei A, Steurer-Stey C, et al. Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax. 2014;69(8):731–739. doi: 10.1136/thoraxjnl-2013-204763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Initiative for Chronic Obstructive Lung Disease [homepage on the Internet] Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. [Accessed November 1, 2017]. Available from: http://www.goldcopd.org.
- 6.McNamara RJ, McKeough ZJ, McKenzie DK, Alison JA. Physical comorbidities affect physical activity in chronic obstructive pulmonary disease: a prospective cohort study. Respirology. 2014;19(6):866–872. doi: 10.1111/resp.12325. [DOI] [PubMed] [Google Scholar]
- 7.Demeyer H, Gimeno-Santos E, Rabinovich RA, et al. Physical activity characteristics across GOLD quadrants depend on the questionnaire used. PLoS One. 2016;11(3):e0151255. doi: 10.1371/journal.pone.0151255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durheim MT, Smith PJ, Babyak MA, et al. Six-minute-walk distance and accelerometry predict outcomes in chronic obstructive pulmonary disease independent of Global Initiative for Chronic Obstructive Lung Disease 2011 Group. Ann Am Thorac Soc. 2015;12(3):349–356. doi: 10.1513/AnnalsATS.201408-365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen HQ, Bailey A, Coleman KJ, et al. Patient-centered physical activity coaching in COPD (Walk On!): a study protocol for a pragmatic randomized controlled trial. Contemp Clin Trials. 2016;46:18–29. doi: 10.1016/j.cct.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Pitta F, Takaki MY, Oliveira NH, et al. Relationship between pulmonary function and physical activity in daily life in patients with COPD. Respir Med. 2008;102(8):1203–1207. doi: 10.1016/j.rmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Zogg S, Dürr S, Miedinger D, Steveling EH, Maier S, Leuppi JD. Differences in classification of COPD patients into risk groups A–D: a cross-sectional study. BMC Res Notes. 2014;7:562. doi: 10.1186/1756-0500-7-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leidy NK, Knebel A. In search of parsimony: reliability and validity of the Functional Performance Inventory-Short Form. Int J Chron Obstruct Pulmon Dis. 2010;5:415–423. doi: 10.2147/COPD.S13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 14.Damarla M, Celli BR, Mullerova HX, Pinto-Plata VM. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51(10):1120–1124. [PubMed] [Google Scholar]
- 15.Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132(2):403–409. doi: 10.1378/chest.06-2846. [DOI] [PubMed] [Google Scholar]
- 16.Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134(1):38–45. doi: 10.1378/chest.08-0013. [DOI] [PubMed] [Google Scholar]
- 17.Lee TA, Bartle B, Weiss KB. Spirometry use in clinical practice following diagnosis of COPD. Chest. 2006;129(6):1509–1515. doi: 10.1378/chest.129.6.1509. [DOI] [PubMed] [Google Scholar]
- 18.Mapel DW, Picchi MA, Hurley JS, et al. Utilization in COPD: patient characteristics and diagnostic evaluation. Chest. 2000;117(5 Suppl 2):346S–353S. doi: 10.1378/chest.117.5_suppl_2.346s. [DOI] [PubMed] [Google Scholar]
- 19.Global Initiative for Chronic Obstructive Lung Disease [homepage on the Internet] Global Strategy for the Diagnosis, Management and Prevention of COPD. 2016. [Accessed October 30, 2016]. Available from: http://www.goldcopd.org.
- 20.Ding B, Small M, Holmgren U. A cross-sectional survey of current treatment and symptom burden of patients with COPD consulting for routine care according to GOLD 2014 classifications. Int J Chron Obstruct Pulmon Dis. 2017;12:1527–1537. doi: 10.2147/COPD.S133793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–905. doi: 10.2147/COPD.S62750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leidy NK, Hamilton A, Becker K. Assessing patient report of function: content validity of the Functional Performance Inventory-Short Form (FPI-SF) in patients with chronic obstructive pulmonary disease (COPD) Int J Chron Obstruct Pulmon Dis. 2012;7:543–554. doi: 10.2147/COPD.S32032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:95–109. doi: 10.2147/COPD.S54473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jo YS, Choi SM, Lee J, et al. The relationship between chronic obstructive pulmonary disease and comorbidities: a cross-sectional study using data from KNHANES 2010–2012. Respir Med. 2015;109(1):96–104. doi: 10.1016/j.rmed.2014.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Comorbidities
| Patients with comorbidities, n (%) | N=1,775 |
|---|---|
| None | 947 (53.4) |
| Cardiovascular condition | 935 (52.7) |
| Angina pectoris | 32 (1.8) |
| Cardiac arrhythmias | 105 (5.9) |
| Coagulated blood clotting disorder | 16 (0.9) |
| Congestive heart failure | 154 (8.7) |
| Coronary heart disease | 240 (13.5) |
| Elevated cholesterol/hyperlipidemia | 399 (22.5) |
| Hypertension | 733 (41.3) |
| Myocardial infarction | 75 (4.2) |
| Peripheral vascular disease | 93 (5.2) |
| Cerebrovascular disease | 32 (1.8) |
| Thrombosis | 20 (1.1) |
| Other cardiovascular condition | 26 (1.5) |
| Dementia | 65 (3.7) |
| Endocrine disorder | 333 (18.8) |
| Diabetes mellitus | 255 (14.4) |
| No end organ damage | 214 (12.1) |
| End organ damage | 41 (2.3) |
| Hyperthyroidism | 31 (1.7) |
| Osteoporosis | 48 (2.7) |
| Other endocrine disorders | 41 (2.3) |
| Glaucoma | 96 (5.4) |
| Gastrointestinal condition | 266 (15.0) |
| Crohn’s disease | 10 (0.6) |
| Dyspepsia/stomach pain | 39 (2.2) |
| Gastroesophageal reflux disease | 205 (11.5) |
| Peptic ulcer disease | 13 (0.7) |
| Other gastrointestinal condition | 25 (1.4) |
| Hematological disorder | 40 (2.3) |
| Anemia | 31 (1.7) |
| Leukemia | 4 (0.2) |
| Other hematological disorder | 6 (0.3) |
| HIV/AIDS | 8 (0.5) |
| AIDS | 2 (0.1) |
| HIV | 6 (0.3) |
| Pulmonary condition | 211 (11.9) |
| Chronic bronchitis | 87 (4.9) |
| Cystic fibrosis | 2 (0.1) |
| Emphysema | 80 (4.5) |
| Idiopathic pulmonary fibrosis | 8 (0.5) |
| Pulmonary arterial hypertension | 19 (1.1) |
| Lung cancer | 17 (1.0) |
| Other pulmonary condition | 37 (2.1) |
| Hemiplegia | 8 (0.5) |
| Malignant lymphoma | 13 (0.7) |
| Obesity | 255 (14.4) |
| Prostate disorder | 137 (7.7) |
| Psychiatric disorder | 279 (15.7) |
| Anxiety | 164 (9.2) |
| Depression | 184 (10.4) |
| Other psychiatric disorder | 19 (1.1) |
| Renal disorder | 85 (4.8) |
| Moderate/severe chronic kidney disease | 75 (4.2) |
| Renal failure | 7 (0.4) |
| Other renal disorder | 7 (0.4) |
| Rhinosinusitis | 113 (6.4) |
| Rheumatic disorder | 57 (3.2) |
| Arthritis | 48 (2.7) |
| Connective tissue/rheumatological disease | 11 (0.6) |
| Other rheumatic disorders | 1 (0.1) |
| Sleep apnea | 199 (11.2) |
| Sleep apnea | 55 (3.1) |
| Obstructive sleep apnea | 157 (8.8) |
| Other sleep apnea | 1 (0.1) |
| Solid tumor | 36 (2.0) |
| Non-metastatic | 28 (1.6) |
| Metastatic | 4 (0.2) |
| Other solid tumor | 5 (0.3) |
| Substance abuse/addiction | 107 (6.0) |
| Systemic sclerosis | 4 (0.2) |
| Other | 38 (2.1) |
Data Availability Statement
All relevant data analyzed during this study are included in this published article.