Abstract
Coagulation factor (F)XI has been described as a component of the early phase of the contact pathway of blood coagulation, acting downstream of factor XII. However, patients deficient in upstream members of the contact pathway, including FXII and prekallikrein, do not exhibit bleeding complications, while FXI-deficient patients sometimes experience mild bleeding, suggesting FXI plays a role in hemostasis independent of the contact pathway. Further complicating the picture, bleeding risk in FXI-deficient patients is difficult to predict because bleeding symptoms have not been found to correlate with FXI antigen levels or activity. However, recent studies have emerged to expand our understanding of FXI, demonstrating that activated FXI is able to activate coagulation factors FX, FV, and FVIII, and inhibit the anti-coagulant tissue factor pathway inhibitor (TFPI). Understanding these activities of FXI may help to better diagnose which FXI-deficient patients are at risk for bleeding. In contrast to its mild hemostatic activities, FXI is known to play a significant role in thrombosis, as it is a demonstrated independent risk factor for deep vein thrombosis, ischemic stroke, and myocardial infarction. Recent translational approaches have begun testing FXI as an antithrombotic, with one promising clinical study showing that an anti-sense oligonucleotide against FXI prevented venous thrombosis in elective knee surgery. A better understanding of the varied and complex role of FXI in both thrombosis and hemostasis will help to allow better prediction of bleeding risk in FXI-deficient patients and also informing the development of targeted agents to inhibit the thrombotic activities of FXI while preserving hemostasis.
Introduction
Hemostasis is a process in which a damaged blood vessel wall is closed off by a fibrin-rich platelet plug to stop the loss of blood into the extracellular space and initiate the repair of the damaged endothelium. This process begins with the activation of coagulation factors in the blood and is classically divided into two pathways known as the intrinsic and the extrinsic pathways of coagulation, which converge on the generation of thrombin and fibrin to form a hemostatic plug or clot. The intrinsic pathway, also called the contact pathway, can be activated in vitro when blood is exposed to negatively charged substances or artificial surfaces, which causes the conversion of factor XII (FXII) into activated FXII (FXIIa) [1]. FXIIa cleaves the coagulation factor prekallikrein (PK) to generate active kallikrein, which in turn feeds back to activate additional FXII. Activated FXII initiates the activation of factor XI (FXI) to FXIa, which in turn activates factor IX (FIX) to FIXa. Activated FIX activates factor X (FX) to FXa, after which the contact pathway leads into the common or final pathway, resulting in the generation of thrombin and fibrin formation. Meanwhile, the extrinsic pathway of coagulation is initiated by the exposure of blood to tissue factor (TF) in complex with activated FVII (FVIIa), which induces the activation of FX and FIX and initiation of the common pathway leading to thrombin and fibrin formation [2]. Blood platelets also become activated and play an important role in hemostasis, releasing additional activating compounds, providing a surface to accelerate reactions, and aggregating to form the bulk of the clot.
Deficiency in some coagulation factors is associated with bleeding disorders. FIX deficiency is associated with the severe bleeding disorder hemophilia B, and FVIII deficiency causes hemophilia A. However, despite the ability of the contact activation pathway to initiate coagulation in vitro, FXI appears to be the only contact factor required for hemostasis. Deficiencies in FXII, PK or HK are not associated with bleeding tendencies, while FXI-deficient patients sometimes present with mild bleeding, suggesting that the role of FXI in hemostasis is independent of contact activation [1]. While FXI appears to play a modest role in hemostasis, clinical trials and epidemiologic data indicate that FXI is a key contributor to thromboembolic diseases. Studies with animal models also suggest that the activation of FXI by FXIIa promotes pathological thrombus formation [3]. In this review, we will discuss our emerging understanding of the role of FXI in both hemostasis and thrombosis and the important clinical implications of this research.
Role of FXI in the intrinsic and extrinsic pathway
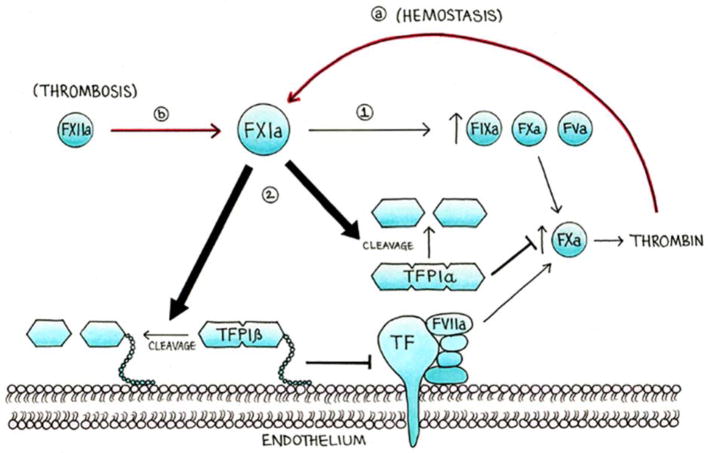
The role of FXI in activating the extrinsic pathway involves multiple mechanisms, due to the promiscuous interaction of FXI with a number of enzymatic substrates. The previous understanding that FXI only participated in the contact pathway was first questioned upon the discovery that FXI can be activated by thrombin downstream of the extrinsic pathway (Figure 1)[4,5]. Although FXIa’s primary substrate is the contact pathway factor FIX, more recently FXI has also been shown to activate FX in vitro and to promote thrombin generation by activating the cofactors FVIII and FV [6,7]. FXIa is also known to shorten the clotting time of recalcified FIX-depleted plasma, again revealing its pro-coagulant activity independent of FIX [8]. We have also recently discovered that FXIa promotes activation of the extrinsic pathway through proteolysis of tissue factor pathway inhibitor (TFPI) [9], a Kunitz-type protease that is the primary inhibitor of the TF/FVIIa/FXa complex (Figure 1)[10]. This inhibitory activity of FXI against TFPI may represent an important new mechanism by which FXI promotes coagulation independent of the contact pathway.
Figure 1. The role of FXI in thrombosis and hemostasis.
In addition to the activation of FIX, X and V (step 1), we hypothesize that FXIa promotes thrombin generation by enhancing the TF-FVIIa pathway (step 2), including via the inactivation of TFPIα from platelets and TFPIβ from endothelial cells. Moreover, we hypothesized that in hemostasis, the main activator of FXI is thrombin (a), while in thrombosis the main activator of FXI is FXIIa (b).
Additionally, it appears that the presence of platelets is required for FXIa to support hemostasis through the extrinsic pathway. Activated platelets release short-chain polyphosphates (polyP; 70–85-mer), which are linear polymers of orthophosphate linked by phosphoanhydride bonds. Platelet polyP can enhance the feedback activation of FXI by thrombin by approximately three thousand-fold [11,12] and has also been shown to enhance the activation of FV, a cofactor that promotes thrombin generation [13]. Interestingly, platelet-derived polyP has been shown to enhance the activation of FXI in a flow chamber model independently of FXIIa and the contact pathway, and this activation requires the participation of the extrinsic pathway [14]. In addition, we recently observed that short-chain polyP increases the capacity of FXIa to inactivate TFPI (unpublished observation), further supporting the combined role of platelet polyP and FXIa in promoting hemostasis.
FXI and Hemostasis
Hemophilia C, the congenital deficiency of coagulation FXI, is associated with postoperative or posttraumatic bleeding, especially in tissues with robust fibrinolytic activity such as the nose, oral cavity, and urinary tract [15]. However, current diagnostic tests are unable to accurately predict bleeding tendencies in FXI-deficient patients, as the symptoms are highly variable between patients and poorly correlated with plasma FXI levels. As a result, some FXI-deficient individuals may receive unnecessary FXI replacement therapy, which has been shown to enhance the risk of thrombosis or transfusion-related complications. Conversely, FXI-deficient patients left untreated can be at increased risk of life-threatening hemorrhage during surgery, presenting a serious dilemma for clinicians and patients.
One common diagnostic test, the activated partial thromboplastin time (aPTT) assay, which measures thrombin generation in platelet-poor plasma (PPP) following contact pathway activation, has not been found to accurately predict the bleeding phenotype of FXI-deficient patients. In contrast, a recent study demonstrated that a thrombin generation assay in platelet-rich plasma (PRP) in which the contact pathway was inhibited while the extrinsic pathway was activated via TF was able to successfully identify which FXI-deficient patients demonstrated a bleeding phenotype [16]. These differing results suggest that FXI does not play a role hemostasis via the contact pathway but that FXI can initiate coagulation via the extrinsic pathway in the presence of TF and platelets. This claim is supported by studies showing that patients deficient in the contact activation initiators FXII, prekallikrein and high-molecular-weight kininogen (HK) do not exhibit bleeding symptoms as do FXI-deficient patients. Therefore, the role of FXI in hemostasis is likely due to its involvement in the extrinsic, TF-mediated pathway rather than the contact pathway.
From a clinical perspective, the balance between TFPI and FXI may represent an important axis between bleeding and hemostasis, respectively. TFPI inhibitory activity appears to assume a central role in the pathogenesis of bleeding in patients with FVIII deficiency (hemophilia A). TFPI-blocking antibodies shorten the clotting time of FVIII-deficient plasma, restoring hemostasis, and administration of TFPI-blocking antibodies also improves hemostasis in hemophilic mice [17]. The anti-hemostatic potential of TFPI is also seen in east Texas bleeding disorder, in which the formation of an abnormal TFPI-FV complex results in a 10-fold increase in plasma TFPI, causing an impairment of coagulation with moderate-to-severe bleeding complications [18]. Meanwhile, our recent results demonstrating the ability of FXIa to inactivate TPFI suggest that FXI may play an important role in the counter-balancing the anti-hemostatic function of TFPI. It has been shown that patients with severe FXI deficiency and a history of bleeding exhibit higher levels of TFPI than asymptomatic patients [19]. TFPI levels may therefore serve as an important marker for bleeding risk in FXI-deficient patients.
FXI and Thrombosis
Despite the recent discoveries of the modest role of FXI in hemostasis, a number of studies have shown a significant role for FXI in promoting thrombosis. While mice lacking the contact factors FXII, HK, PK or FXI are resistant to experimental-induced thrombosis [20–23], in humans, FXI seems to be strongest candidate for playing a role in thrombosis. Patients with severe FXI deficiency were reported to exhibit protection from ischemic stroke and a lower incidence of venous thromboembolism (VTE) [24,25]. Conversely, higher levels of FXI were associated with increased risk of VTE and ischemic stroke [26,27]. Reducing factor XI levels in patients undergoing unilateral total knee arthroplasty has been shown to be an effective method for the prevention of postoperative VTE, suggesting FXI inhibition as an effective method of anticoagulation in surgery [28].
The contact pathway and the TF-FVIIa complex together have been shown to contribute to venous thrombosis in a mouse model, suggesting a combined role for the intrinsic and extrinsic pathways in thrombosis [29]. Our work suggests that FXI may provide the common link between these two pathways in thrombosis. We have observed that inhibition of FXI activation by FXIIa is protective in a mouse model of thrombosis (Figure 1) and also beneficial in mouse model of TF-induced pulmonary embolism [3]. Furthermore, we have shown that in a non-human primate model of thrombosis, the inhibition of FXI activation reduced intraluminal thrombus growth initiated by TF [30]. Our observations suggest this thrombotic potential of FXI can be explained by both the ability of FXIa to promote the extrinsic pathway of thrombin generation via inactivation of TFPI and by the feedback activation of FXI by thrombin to further amplify the extrinsic pathway.
The structure of FXI provides further insight into its pro-thrombotic tendencies. Each FXI subunit contains anion-binding sites (ABSs) on its A3 and catalytic domains that are required for heparin-mediated inhibition of FXIa by antithrombin (AT) and for polyP-mediated FXI activation by thrombin and FXIIa [31]. We recently observed that these regions are also required for polyP to enhance the pro-thrombotic capacity of FXIa to inhibit TFPI (unpublished observation). These mechanisms may partially explain why the loss of the FXI catalytic domain ABS has an antithrombotic effect in a mouse model of thrombosis [31]. Also, mice lacking both FIX and FXI are more resistant to chemical injury-induced arterial thrombosis than are mice deficient in FIX alone [6], further supporting the notion that FXI promotes thrombosis through mechanisms beyond the activation of FIX.
Research into the role of FXI in hemostasis and thrombosis is ultimately focused on identifying new therapeutic targets to prevent thrombosis without affecting hemostasis. Current anticoagulants inhibit the core pathways of thrombin generation and as a result, all are associated with major bleeding complications. The development of agents to inhibit FXI is fully underway, with the promise of safe and efficacious anti-thrombotic therapies due to the modest role of FXI in hemostasis relative to its role in thrombosis. Specifically, translational approaches are underway to inhibit the enzymatic function of FXI through monoclonal antibodies against FXI which block the activation of FIX or its activation by FXIIa, small-molecule inhibitors that block the active site of FXIa [32] or targets FXIa and allosterically inhibits its activation of FIX [33], or using an antisense oligonucleotide (ASO) to reduce the synthesis of FXI [28,32]. In particular, this antisense approach showed great promise in a clinical trial of patients to reduce the incidence of VTE associated with elective knee operation; the 200 mg and 300 mg ASO regimen was non-inferior and superior, respectively, to enoxaparin (P<0.001), indicating that lowering FXI levels, reduced the risk of VTE associated with knee operation. These translational efforts should take into account that efforts to prevent thrombosis by effectively inducing transient hemophilia C may potentiate a bleeding risk in some patients. Prediction of bleeding in asymptomatic FXI deficiency, whether inherited or pharmacologically induced, poses an important dilemma to surgeons and may become an increasing clinical problem with the development of FXIa inhibitors that fully block the promiscuous enzymatic activity of FXIa that may be required for effective hemostasis.
Conclusion
Recent investigations have shown that FXIa can act on targets other than FIX, which may be relevant to its role in both hemostasis and thrombosis. The development of safe and effective antithrombotics targeting FXI should take into account that FXIa is able to activate factors of the extrinsic pathway such as FX, FV, and FVIII and also activate the TF-FVIIa pathway by inhibiting TFPI, and that polyP released from activated platelets can further enhance these activities of FXI significantly. In addition, companion diagnostics are required to identify which FXI-deficient patients and, in the future, FXI-anticoagulated patients could be at a risk of bleeding. Finally, further studies are needed to better understand the role of FXI in thrombosis and hemostasis to identify possible therapeutic targets against FXI that block its prothrombotic effect without affecting its hemostatic function.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01HL101972, R01GM116184). O.J.T. McCarty is an American Heart Association (AHA) Established Investigator (13EIA12630000). C.P. is an AHA Fellow (14POST18180011). We thank LoLo-ology for design of the artwork.
Footnotes
Authorship
C.P., R.R. and O.J.T.M. wrote the manuscript.
Disclosures & Conflict of Interest
none
References
- 1.Schmaier AH. Physiologic activities of the contact activation system. Thromb Res. 2014;133(Suppl):S41–4. doi: 10.1016/j.thromres.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arter Thromb Vasc Biol. 2007;27:1687–93. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 3.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun M-F, White-Adams TC, Smith SA, Hanson SR, McCarty OJT, Renné T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–9. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kravtsov DV, Matafonov A, Tucker EI, Sun M-F, Walsh PN, Gruber A, Gailani D. Factor XI contributes to thrombin generation in the absence of factor XII. Blood. 2009;114:452–8. doi: 10.1182/blood-2009-02-203604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gailani D, Broze GJ. Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–12. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 6.Matafonov A, Cheng Q, Geng Y, Verhamme IM, Umunakwe O, Tucker EI, Sun M-F, Serebrov V, Gruber A, Gailani D. Evidence for factor IX-independent roles for factor XIa in blood coagulation. J Thromb Haemost. 2013;11:2118–27. doi: 10.1111/jth.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puy C, Tucker EI, Wong ZC, Gailani D, Smith SA, Choi SH, Morrissey JH, Gruber A, McCarty OJT. Factor XII promotes blood coagulation independent of factor XI in the presence of long chain polyphosphate. J Thromb Haemost. 2013;11:1341–52. doi: 10.1111/jth.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whelihan MF, Orfeo T, Gissel MT, Mann KG. Coagulation procofactor activation by factor XIa. J Thromb Haemost. 2010;8:1532–9. doi: 10.1111/j.1538-7836.2010.03899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puy C, Tucker EI, Matafonov A, Cheng Q, Zientek KD, Gailani D, Gruber A, McCarty OJT. Activated factor XI increases the procoagulant activity of the extrinsic pathway by inactivating tissue factor pathway inhibitor. Blood. 2015;125:1488–96. doi: 10.1182/blood-2014-10-604587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baugh RJ, Broze GJ, Krishnaswamy S. Regulation of extrinsic pathway factor Xa formation by tissue factor pathway inhibitor. J Biol Chem. 1998;273:4378–86. doi: 10.1074/jbc.273.8.4378. [DOI] [PubMed] [Google Scholar]
- 11.Geng Y, Verhamme IM, Smith SB, Sun M-F, Matafonov A, Cheng Q, Smith SA, Morrissey JH, Gailani D. The dimeric structure of factor XI and zymogen activation. Blood. 2013;121:3962–9. doi: 10.1182/blood-2012-12-473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi SH, Smith SA, Morrissey JH. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood. 2011;118:6963–70. doi: 10.1182/blood-2011-07-368811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi SH, Smith SA, Morrissey JH. Polyphosphate accelerates factor V activation by factor XIa. Thromb Haemost. 2015;113:599–604. doi: 10.1160/TH14-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu S, Travers RJ, Morrissey JH, Diamond SL. FXIa and platelet polyphosphate as therapeutic targets during human blood clotting on collagen/tissue factor surfaces under flow. Blood. 2015;126:1494–502. doi: 10.1182/blood-2015-04-641472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seligsohn U. Factor XI deficiency in humans. J Thromb Haemost. 2009;7(Suppl 1):84–7. doi: 10.1111/j.1538-7836.2009.03395.x. [DOI] [PubMed] [Google Scholar]
- 16.Pike GN, Cumming AM, Hay CRM, Bolton-Maggs PHB, Burthem J. Sample conditions determine the ability of thrombin generation parameters to identify bleeding phenotype in FXI deficiency. Blood. 2015;126:397–405. doi: 10.1182/blood-2014-12-616565. [DOI] [PubMed] [Google Scholar]
- 17.Maroney SA, Cooley BC, Ferrel JP, Bonesho CE, Nielsen LV, Johansen PB, Hermit MB, Petersen LC, Mast AE. Absence of hematopoietic tissue factor pathway inhibitor mitigates bleeding in mice with hemophilia. Proc Natl Acad Sci USA. 2012;109:3927–31. doi: 10.1073/pnas.1119858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent LM, Tran S, Livaja R, Bensend TA, Milewicz DM, Dahlbäck B. Coagulation factor V(A2440G) causes east Texas bleeding disorder via TFPIα. J Clin Invest. 2013;123:3777–87. doi: 10.1172/JCI69091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zucker M, Seligsohn U, Salomon O, Wolberg AS. Abnormal plasma clot structure and stability distinguish bleeding risk in patients with severe factor XI deficiency. J Thromb Haemost. 2014;12:1121–30. doi: 10.1111/jth.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langhauser F, Göb E, Kraft P, Geis C, Schmitt J, Brede M, Göbel K, Helluy X, Pham M, Bendszus M, Jakob P, Stoll G, Meuth SG, Nieswandt B, McKrae KR, Kleinschnitz C. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood-brain-barrier damage and inflammation. Blood. 2012;120:4082–92. doi: 10.1182/blood-2012-06-440057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revenko AS, Gao D, Crosby JR, Bhattacharjee G, Zhao C, May C, Gailani D, Monia BP, MacLeod AR. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood. 2011;118:5302–11. doi: 10.1182/blood-2011-05-355248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renné T, Pozgajová M, Grüner S, Schuh K, Pauer H-U, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–81. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Cheng Q, Xu L, Feuerstein GZ, Hsu M-Y, Smith PL, Seiffert DA, Schumacher WA, Ogletree ML, Gailani D. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 24.Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost. 2011;105:269–73. doi: 10.1160/TH10-05-0307. [DOI] [PubMed] [Google Scholar]
- 25.Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–7. doi: 10.1182/blood-2007-10-120139. [DOI] [PubMed] [Google Scholar]
- 26.Meijers JC, Tekelenburg WL, Bouma BN, Bertina RM, Rosendaal FR. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342:696–701. doi: 10.1056/NEJM200003093421004. [DOI] [PubMed] [Google Scholar]
- 27.Yang DT, Flanders MM, Kim H, Rodgers GM. Elevated factor XI activity levels are associated with an increased odds ratio for cerebrovascular events. Am J Clin Pathol. 2006;126:411–5. doi: 10.1309/QC259F09UNMKVP0R. [DOI] [PubMed] [Google Scholar]
- 28.Büller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, Segers A, Verhamme P, Weitz JI. Factor XI Antisense Oligonucleotide for Prevention of Venous Thrombosis. N Engl J Med. 2014;372:232–40. doi: 10.1056/NEJMoa1405760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Köllnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–35. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruber A, Hanson SR. Factor XI-dependence of surface- and tissue factor-initiated thrombus propagation in primates. Blood. 2003;102:953–5. doi: 10.1182/blood-2003-01-0324. [DOI] [PubMed] [Google Scholar]
- 31.Geng Y, Verhamme IM, Smith SA, Cheng Q, Sun M, Sheehan JP, Morrissey JH, Gailani D. Factor XI anion-binding sites are required for productive interactions with polyphosphate. J Thromb Haemost. 2013;11:2020–8. doi: 10.1111/jth.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong PC, Quan ML, Watson CA, Crain EJ, Harpel MR, Rendina AR, Luettgen JM, Wexler RR, Schumacher WA, Seiffert DA. In vitro, antithrombotic and bleeding time studies of BMS-654457, a small-molecule, reversible and direct inhibitor of factor XIa. J Thromb Thrombolysis. 2015;40:416–23. doi: 10.1007/s11239-015-1258-7. [DOI] [PubMed] [Google Scholar]
- 33.Karuturi R, Al-Horani RA, Mehta SC, Gailani D, Desai UR. Discovery of allosteric modulators of factor XIa by targeting hydrophobic domains adjacent to its heparin-binding site. J Med Chem. 2013;56:2415–28. doi: 10.1021/jm301757v. [DOI] [PMC free article] [PubMed] [Google Scholar]