Abstract
Brain-derived neurotrophic factor (BDNF) has a central role in brain plasticity by mediating changes in cortical thickness and synaptic density in response to physical activity and environmental enrichment. Previous studies suggest that physical exercise can augment BDNF levels, both in serum and the brain, but no other study has examined how different types of activities compare with physical exercise in their ability to affect BDNF levels. By using a balanced cross over experimental design, we exposed nineteen healthy older adults to 35-minute sessions of physical exercise, cognitive training, and mindfulness practice, and compared the resulting changes in mature BDNF levels between the three activities. We show that a single bout of physical exercise has significantly larger impact on serum BDNF levels than either cognitive training or mindfulness practice in the same persons. This is the first study on immediate BDNF effects of physical activity in older healthy humans and also the first study to demonstrate an association between serum BDNF responsivity to acute physical exercise and working memory function. We conclude that the BDNF increase we found after physical exercise more probably has a peripheral than a central origin, but that the association between post-intervention BDNF levels and cognitive function could have implications for BDNF responsivity in serum as a potential marker of cognitive health.
Keywords: Aging, brain-derived neurotrophic factor, cognitive function, cognitive training, crossover design, exercise, geriatrics, intervention study, mindfulness, neuroplasticity
INTRODUCTION
Aging is often associated with cognitive decline but with large variation between individuals [1]. Age is also the primary risk factor for brain diseases that can severely and progressively impair cognitive functioning [2]. Consequently, with a rapidly growing proportion of elderly citizens worldwide, the increasing prevalence of cognitive impairment and dementia has become a major societal challenge, indicating a strong need for preventive strategies [3].
Although no medication exists to effectively halt or reverse the neuropathological progress of the most common dementia diseases, including Alzheimer’s disease, epidemiological association studies suggest that a number of lifestyle factors, including physical exercise, education, and a generally active lifestyle, may reduce the long-term risk of cognitive impairment and dementia [4]. Recent evidence from randomized intervention studies in older humans are consistent with some of these results by showing that both physical exercise [5, 6] and cognitive training [7, 8] may have beneficial effects on cognition [9]. In the first multidomain intervention study, a combination of physical and cognitive training, along with nutritional guidance and control of cardiovascular risk factors, improved cognitive performance over a two-year period in older adults at risk of developing dementia [10].
In the related field of environmental enrichment, several animal experiments have reported increased cortical thickness [11] and density [12] resulting from exposure to environmental enrichment [13]. Such effects are commonly regarded as manifestations of neuroplasticity, a commonly used concept to account for the brain’s ability to adapt itself to experience. Neural processes that reflect plasticity include modification of existing synapses, formation of new synapses, and neurogenesis. Neuroplasticity is thereby the reflection of learning processes at the neural level and a prerequisite for the ability to develop new behavior repertoires in response to changing environmental conditions [13]. Neuroplasticity is also important to counteract negative cognitive consequences of age-related changes and neuropathologies, and also for neurogenesis in the aging brain [14]. Several studies suggest that brain-derived neurotrophic factor (BDNF) may play a central role in neuroplasticity; animal experiments suggest that environmental enrichment, including environmental variation [15], physical exercise [16], and social enrichment [17] can result in elevated BDNF levels in the brain, that in turn has been associated with positive effects on memory and learning ability [18]. Some studies have demonstrated that physical exercise can also upregulate BDNF gene expression in rodent brains, especially in the hippocampal area [19, 20]. Other studies have found that cognitive activity may have a similar influence on BDNF gene expression in the brain; Tokuyama et al. [21] reported that a paired-associate learning task promoted increased expression of BDNF in monkeys, especially in inferior temporal areas [21], and Yamada et al. [22] found increased BDNF expression in the rat hippocampus after spatial learning [22]. Availability of BDNF may also be a prerequisite for neurogenesis to result after enrichment [23] and after physical exercise [24], and also a prerequisite for at least some types of learning to occur [25]. Thus, BDNF has been suggested as a key marker of neural plasticity [18, 26], synaptic growth [27], and neurogenesis [28-31].
In humans, there is evidence that physical exercise can increase BDNF levels in serum [32], both in younger [32] and older persons [33], even after a single bout of exercise[34-36]. To our knowledge, no previous studies have investigated how other types of training can compare with physical exercise in their capacity to alter BDNF levels. In this study we directly compared immediate effects on serum levels of BDNF in healthy older humans from a single session of physical exercise, cognitive training, and mindfulness practice, performed by the same individuals. We hypothesized that both physical and cognitive training would increase BDNF levels. Mindfulness was not only included as a reference, or a control condition, to the other activities. Several longitudinal studies have suggested that meditation practice may have beneficial effects on cognition [37], and specifically on executive control functions and cortical areas that underlie them [38], effects for which BDNF has been suggested as a key mediator [39]. A recent study found that a combination of yoga and meditation over a 12-week period improved visuospatial memory, improvements that were related to parallel changes in brain connectivity [40]. Some studies have reported that mindfulness has the ability to reduce stress levels as measured through within-subject decreases in cortisol levels [41], and one study found an inverse relation between stress and BDNF in the rat hippocampus [42]. To the extent that mindfulness and the different meditation techniques can be considered as related practices with similar effects, these studies suggest the possibility of a link between mindfulness and BDNF levels. Thus, although these studies [38, 39, 41, 42] constitute only indirect support, we could at least not exclude the possibility that mindfulness training may also have the potential to affect BDNF levels.
The relevance of BDNF for brain plasticity and cognitive function has led several researchers to look for an association between basal levels of serum BDNF and either cognitive function [43, 44] or cognitive impairment in neurodegenerative diseases [45-47], but the results have been inconsistent. Here, we compare BDNF basal circulating levels with BDNF response magnitudes to see if BDNF responsivity and post-intervention levels of BDNF may have a stronger association with memory function than basal levels of BDNF.
MATERIALS AND METHODS
Participants
The target group for this study was healthy persons between 65 and 85 years. We recruited a total of twenty-three prospective volunteers from south Sweden through advertisements in a local newspaper and through contacts with two volunteer senior organizations. Nineteen fully participated in the experiment: one was excluded because of poor vision, and the other three chose not to participate after having received full information about the experiment. Each of the remaining nineteen was randomly assigned to one of the possible orders of conditions to obtain a randomly balanced within-group (crossover) design. The demographic information of the participants (Table 1) confirms that our sample was a group of generally healthy older persons. Mean systolic blood pressure was mildly elevated (145 mm Hg) relative to recently recommended values [48], but not higher than normal for this age group in Sweden [49].
Table 1.
Characteristics of participants
| Variables | Mean value (±SEM) or % (N = 19) |
|---|---|
| Gender (% female) | 57.9 |
| Age (years) | 70.8 ± 0.8 |
| Formal education (years) | 8.02 ± 1.24 |
| Continued adult education (total time in years) | 2.63 ± 0.23 |
| MMSE score (range 0-30) | 28.9 ± 0.2 |
| Cognitive performance in CogMed, average ratio of correct answers | 0.72 ± 0.01 |
| Physical activities in daily life. Index score (range 0-40) | 19.1 ± 1.2 |
| Systolic blood pressure (mm Hg, average of three measures) | 145 ± 2.8 |
| Diastolic blood pressure (mm Hg, average of three measures) | 84 ± 1.4 |
| Resting pulse (average of three measures) | 75.8 ± 0.8 |
| Self-rated health (1-4, where 4 is best health) | 2.53 ± 0.77 |
| Health problems, average index score (0-1 on 35 symptoms) | 8.05 ± 1.22 |
Numbers are mean values with SEM for continuous variables and % participants for the nominal variable (gender).
The experiment was carried out at the Health Clinic at the Linnaeus University in south Sweden and had been approved by the regional ethical review board in Linköping (decision dnr 2013/154-31). The study was carried out in accordance with the approved guidelines. Each participant had one preparatory visit and three additional visits to carry out each of the three interventions. During the preparatory visit, we informed the participants in detail about the project, both orally and in writing, and obtained informed consent. We performed a general health screen to identify possible medical exclusion criteria, including screening for cognitive impairment, normal vision and hearing, history of major neurological or psychiatric illness and cardiovascular disease. The screening procedure included a general health status questionnaire, the Mini-Mental State Examination (MMSE) [50] for cognitive status (with a score of <26 as criterion for exclusion), and a physical examination by a physician. A physician also interviewed each person to identify any possible medical obstacles for participation. The purpose of these investigations was to ensure that each participant was in good health, and that there was no medical contraindication to participating in moderate physical exercise during 35 minutes. We asked the participants to complete a questionnaire at home on demographical information. The participants returned on three occasions to go through the different training conditions, distributed with a minimum of five days between them. The preparatory visit took around one hour and the three following visits lasted around 2.5 hours each, including the 35-minute training session.
Experimental procedure
We used a balanced within-subject crossover design, meaning that all participants went through the three different training programs in a sequence order that had been randomly assigned to each of them. The duration of each program was identical (35 minutes) and the distribution was automatized by the use of pre-programmed and/or recorded instructions. This was accomplished by selection and modification of exercises we found in three commercially available applications: (1) physical aerobic exercise at a moderate level by using the interactive Xbox Kinect™ application (http://en.wikipedia.org/wiki/Kinect), (2) cognitive training through a computerized working memory training program (CogMed™, http://www.cogmed.com/program), and (3) mindfulness practice through the use of the Mindfulness App (http://www.mindapps.se/themindfulnessapp/). After arrival to the study visit, a trained nurse informed each participant briefly about the nature of the exercise in the physical exercise and mindfulness conditions, while a psychologist gave the corresponding information in the cognitive training condition. Pulse and blood pressure were measured, the catheter for blood sampling inserted, and the first blood sample was collected. To get acquainted with each type of activity in the three different programs, the person carried out a sample exercise and was invited to ask any questions. Shortly thereafter, the intervention started.
Physical exercise
To program and carry out the physical exercises, we used a Swedish translation version of the EA Sports Active 2™ program on a Microsoft Xbox360™ game console connected to a Microsoft Kinect™ accessory and an ordinary TV set. The Kinect accessory includes a video camera that captures the movements of the person who is training and enables the person to see him/herself as a moving avatar in real time on the TV screen. The participants responds with physical actions to complete challenges presented on the screen, e.g., kicking a ball that approaches the avatar of the participant on the screen. Before the exercise we instructed the participant to make an effort corresponding to score 11–13 on the rating of perceived exertion (RPE) Scale by Borg [51] and explained the meaning of the different scale levels. During the physical exercise, the effort was monitored by visual observation by a nurse, and the participants also repeatedly rated their self-perceived exertion according to the RPE scale. The participants were told to slow down if they either perceived exertion above score 13 on the RPE scale or if observations by the nurse during the exercise indicated an effort above the target moderate level, and to increase the effort if a level below that was indicated. The exercises comprised of rotations of arms, bending forward, heel lifting, hip rotations, bending knees, bowing down and jumping up, kicking a ball, and a step aerobic exercise with music, all according to the translated instructions in the EA Sports Active 2™ program.
Cognitive training
CogMed™ is a program designed for working memory training that was developed by Torkel Klingberg at Karolinska Institutet, Sweden. The program continuously adapts the level of difficulty to the performance level of the user. It is widely used in clinical settings and has been validated by several studies [52]. For this experiment we selected eight different tasks from the CogMed™ program entitled “Grid”, “Twist”, “Cube”, “Letters”, “Rotating”, “Assembly”, “Numbers”, and “Hidden”. In summary, the first five of these were related to remembering and indicating a spatial position after a short delay, the “Assembly” item was related to word building from presented letters, while the “Number” and “Hidden” items were both related to reproducing presented numbers in reverse order. The sequence of items was repeated until the 35 minutes had passed, usually between 15 and 20 times depending on how fast each person responded. We ran the program on an internet-connected computer and the performance data were automatically registered on a server.
Mindfulness practice
Mindfulness builds on principles from eastern meditation practices based on attention and awareness. These principles have been adapted and popularized in the West under the umbrella term mindfulness. In our experiment we selected different exercises from a commercially available tablet and mobile phone application, “the Mindfulness App” (http://www.mindapps.se), and the participant followed the guided instructions for each exercise through headphones. The exercises were based on principles from mindfulness-based stress reduction (MBSR) [53]. The mindfulness condition in our experiment comprised a five minute relaxing “body scan” when the person let the attention wander through the body from top to toe, as guided by a recorded voice in the application. The body scan was followed by an exercise where the person attended to breath, mood, thoughts, emotions, and physical sensations. The general approach in the instruction was, according to the MBSR type of mindfulness practice, to observe these aspects of bodily and mental processes with a non-judgmental attitude.
Blood collection
For BDNF analysis we collected 8 mL of blood from a suitable lower arm vein into Vacutainer tubes at four points in time: immediately before, immediately after, and at 20 minutes and 60 minutes after each exercise. The blood samples were kept at room temperature for 30 min to allow for clotting, and were then centrifuged at 2000 g for 10 min at 4°C. Serum was collected, mixed by inverting, and aliquoted into 0.5 mL Eppendorf tubes. All measurements were performed in the morning hours between 08 a.m. and 12 to minimize impact of natural diurnal variations in BDNF levels [54]. The time of day was also kept as similar as possible within participants across the three conditions.
Serum BDNF analysis
Serum BDNF was measured using a sandwich enzyme-linked immunosorbent assay (Human BDNF Quantikine ELISA, DBD00, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The manufacturer of the ELISA kit we use claims 13% cross-reactivity for recombinant human pro-BDNF. The Elisa kit we used has a very low cross-reactivity for proBDNF compared to the other available ELISA kits (see Polacchini et al. [55] and Lim et al. [56]),
Briefly, the serum samples were diluted 20-fold in the supplied assay diluent and assayed against a standard curve with BDNF concentrations ranging from 62.5 pg/mL to 4000 pg/mL. Serum samples and BDNF standards were incubated for 2 hours on the captured anti-human BDNF coated microplate. Then, a monoclonal antibody specific for human BDNF conjugated to horseradish peroxidase was added to the wells. After a wash step, the supplied tetramethylbenzidine (TMB) substrate solution was added to the wells and a blue color developed in proportion to the amount of human BDNF present in the samples. Color development was stopped with 2 N sulfuric acid, turning the color in the wells to yellow. The absorbance was measured at 450 nm with a correction set at 540 nm on a spectrophotometric microplate reader (SpectraMax 340PC, Molecular Devices). All samples were tested in duplicates. Sample BDNF concentrations were determined by non-linear regression from the standard curves using GraphPad Prism v6 (GraphPad, La Jolla, CA). The persons who ran the analyses were blind to which experimental condition each sample belonged.
Statistical analyses
The purpose of the three post-intervention measures was to assess the progression in BDNF levels after each intervention. As a one-way ANOVA did not show any significant differences between the three different post-intervention time points at 0, 20, and 60 minutes after each exercise, we collapsed these measures in the main calculations to obtain a single more robust and representative estimation of the post-intervention BDNF levels. The within-subject differences between the BDNF changes in the three conditions were tested through repeated measures ANOVA. One set of blood samples was lost, due to errors in handling, for one person in the mindfulness condition, limiting the number of participants to 18 in the repeated ANOVA analysis. The crossover design with four blood samples from each intervention thus means that these analyses were based on BDNF values from a total of 216 blood samples. We used one-sample t-tests to test the changes within each condition against 0 (N = 19 participants in two conditions and N = 18 in the mindfulness condition). Finally we carried out exploratory correlational analyses to tentatively trace sources of variation in intervention effects between individuals with a focus on gender differences, baseline BDNF levels and working memory performance.
To investigate the effect of gender, we performed a separate mixed ANOVA with gender as the between-subjects factor and time (pre- versus post-intervention) as the within-subjects factor. The relation between baseline BDNF and BDNF response magnitude was estimated through Pearson correlations. Because cognitive performance was not a primary outcome of interest, we did not a priori regard measurement of cognitive performance as relevant for our research question. Based on previous studies, both on the correlation between BDNF in serum and brain [57] and on BDNF in serum and cognitive function [43-45], we hypothesized that individual differences in cognitive function might explain part of the BDNF response variability in our sample. As we had indirectly generated a measure of working memory function through the performance in the cognitive training intervention, we were able to use the CogMed scores to explore the association between this dimension of cognitive function and the differences in BDNF responsivity that we had found. To obtain a stable estimation of working memory function and avoid contamination of warm-up effects, we followed the recommendations provided by CogMed (S. Söderqvist, personal communication, 2015) to use an index score that represented the average difficulty level of three correct responses at the highest level(s) for each CogMed item, i.e., a weighted score of the persons maximum performance on the eight CogMed items. The total working memory performance score was calculated as the mean of these eight weighted scores.
We used non-parametric (Spearman) correlations in all calculations that involved associations between working memory and BDNF levels.
All statistical analyses were performed in IBM SPSS Statistics v22 for Mac.
RESULTS
We found an average variability of 25% in BDNF levels between all measurements in our sample, similar to what other studies have reported [34, 58]. At baseline the mean concentration of serum BDNF was 20.2±0.95 ng/mL across the three conditions, and the average baseline level was also similar between conditions (F (2, 34) = 1.559, p = 0.23). The average BDNF levels at baseline and at the three post-intervention time points are presented for each condition, along with the average post-intervention levels, in Table 2. Physical exercise produced a significant increase in BDNF levels; the mean post-intervention BDNF level was 22.5±0.99 ng/mL after the physical exercise session, compared to 19.2±1.17 ng/mL at baseline (p = 0.004, paired t-test, Cohen’s d = 0.75). This increase is in contrast to the non-significant changes we found from cognitive training and mindfulness (Table 2). Figure 1 depicts these changes in BDNF levels from baseline in relation to each of the three post-intervention time points (at 0, 20, and 60 minutes), and in addition to the average of these three post-intervention measures.
Table 2.
Serum BDNF levels before and at three time points after each type of intervention, stratified for gender.
| BDNF levels | Before intervention (ng/mL±SEM) | 0 minutes after (ng/mL±SEM) | 20 minutes after (ng/mL±SEM) | 60 minutes after (ng/mL±SEM) | Average post intervention level (ng/mL±SEM) | Average change*/ (ng/mL±SEM) | 95% confidence interval Exact p-value | Effect size (Cohen’s d) |
|---|---|---|---|---|---|---|---|---|
| Physical exercise (N = 19) | 19.21±1.17 | 22.73±1.17 | 21.73±1.2 | 23.44±1.38 | 22.5±0.99 | 3.29±1.00 | 1.19–5.40 p = 0.004 | 0.75 |
| Cognitive training (N = 19) | 20.06±0.95 | 20.81±1.35 | 19.89±1.35 | 20.12±1.13 | 20.28±1.03 | 0.21±1 | -1.89–2.31 p = 0.83 | 0.05 |
| Mindfulness (N = 18) | 21.6±1.58 | 20.41±1.48 | 21.76±1.05 | 20.86±1.74 | 21.05±1.26 | -0.55±1.27 | -3.23–2.13 p = 0.67 | -0.10 |
Values are mean levels of BDNF at each measure point with standard error of mean (SEM). The 95% confidence interval is for the difference between the average post-intervention BDNF level (from three measures) and the baseline pre-intervention level immediately before the intervention started (from paired t-test). Cohen’s d was calculated by dividing the average change with the standard deviation of the change.
Fig. 1.
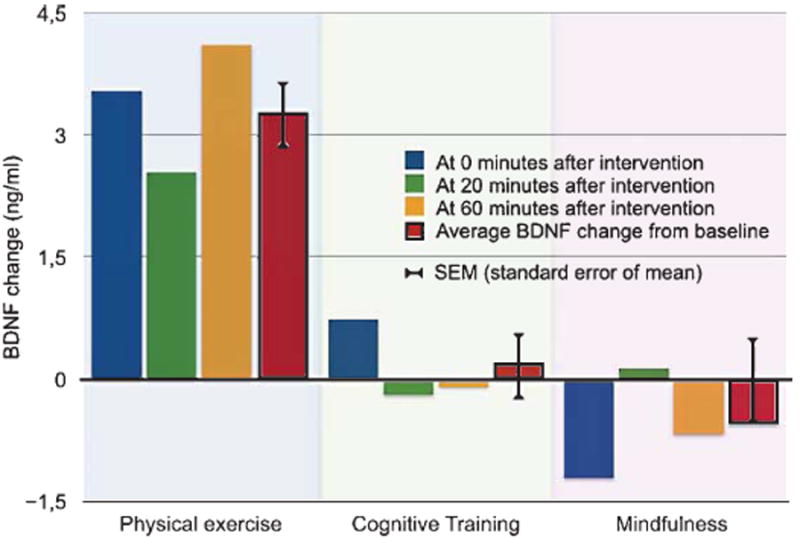
BDNF level changes from baseline at 0, 20, and 60 minutes after each intervention. Bars represent the difference in ng/ml serum between baseline and each post-intervention time point.
The BDNF change scores were normally distributed within each condition, as assessed by Shapiro-Wilk’s test (p = 0.57, p = 0.50, and p = 0.72), and Mauchly’s test of sphericity indicated that the sphericity assumption for ANOVA was also not violated, χ2(2) = 0.35, p = 0.84. The within-subjects ANOVA showed an overall difference in BDNF change scores between conditions, F (2. 34) = 4.62, p = 0.017, with an effect size of partial η2 = 0.21. Posthoc analyses showed only a negligible difference (0.473 ng/mL, SEM±1.450 ng/mL) between mindfulness and cognitive training (p = 1.00). When we contrasted the BDNF changes from physical exercise with the changes from cognitive training and mindfulness, we found a complex contrast estimate (the difference between physical exercise and the average of the other two conditions) of 3.60. F (1, 17) = 10.35, p = 0.010, and with an effect size of partial η2 = 0.3781. In summary, we found a significantly stronger acute effect on serum BDNF levels from physical exercise than from cognitive training and mindfulness, and the relative effect size of this difference was, by common standards for ANOVA [59], large.
In spite of the significant overall effect from physical exercise on BDNF levels in serum, we found considerable variability in response magnitudes between individuals, as depicted in Figs. 2-4. In the next step of analyses, we tried to further explore the origin of these individual differences.
Fig. 2.
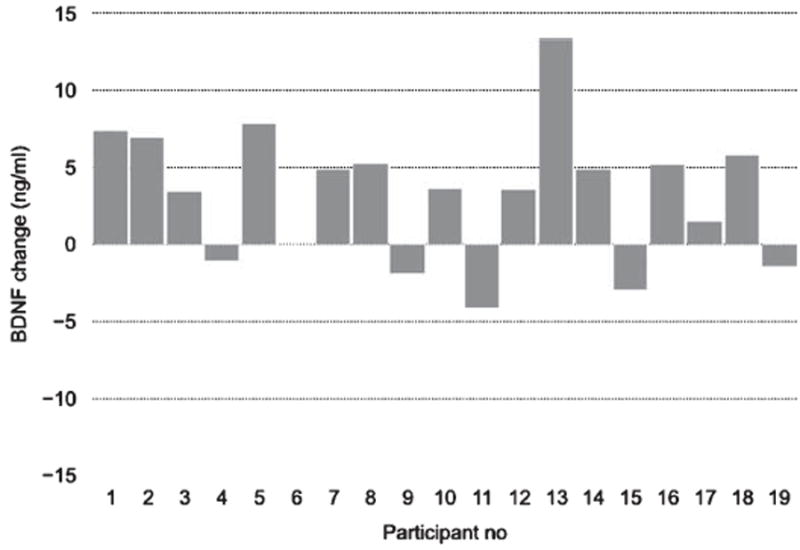
BDNF response to physical exercise for each participant. Each bar depicts the change in BDNF level between the average post-intervention BDNF level (across the three time points) in relation to the baseline (pre-session) level for each participant in the physical exercise condition.
Fig. 4.
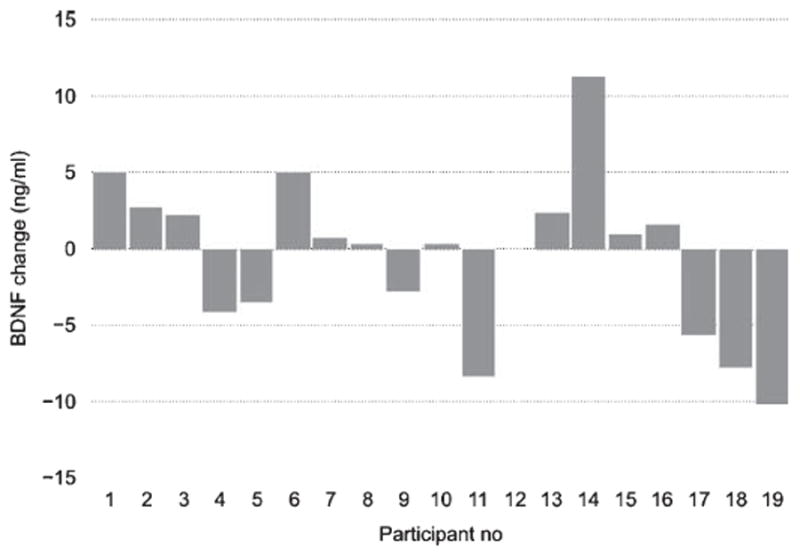
BDNF response to mindfulness practice for each participant. Each bar depicts the change in BDNF level between the average post-intervention BDNF level (across the three time points) in relation to the baseline (pre-session) level for each participant in the mindfulness condition.
Post-hoc analyses: Sources of variation in BDNF effects
Gender
Some studies have indicated a gender difference in BDNF effects from physical training [32, 60], in BDNF levels in platelets [61], and in the association between circulating BDNF levels and cognitive function [44]. We had eight men and ten women in our sample with complete BDNF data from all three conditions. To investigate if gender could account for part of the variation in response magnitudes in our sample, we used mixed within-subjects ANOVA after ensuring that all the statistical conditions for this analysis were fulfilled. The mixed ANOVA showed no significant interaction between gender and BDNF changes for the different conditions, F (2, 32) = 0.30, p = 0.74, partial η2 = 0.02. This strongly suggests that gender did not play a role in the variability of BDNF changes in our sample. A gender stratification of BDNF level changes in the different conditions produced a similar picture by showing that both men and women had the largest increases of serum BDNF in the physical exercise condition and also reached the highest post-intervention levels in that condition compared to cognitive training and mindfulness.
Baseline BDNF, time of day, and order effects
It seems likely that natural variability of BDNF levels [62], both within and between individuals, could be caused by various external and internal factors. With the assumption that a higher or lower BDNF baseline level could affect BDNF responses, i.e., the assumption of a ceiling effect, we investigated if BDNF responses would be more restricted in persons with a relatively higher BDNF level at baseline. This assumption was supported by an inverse correlation between the participant’s average baseline BDNF level and their overall BDNF response across conditions (rxy −0.49, p = 0.03), meaning that participants with higher baseline levels of BDNF tended to have a smaller BDNF response. The correlations between baseline levels and BDNF increases were significant also within each condition (rxy =−0.59, p = 0.008, for physical exercise, rxy = −0.45, p = 0.05, for cognitive training, and rxy = −0.63, p = 0.005, for mindfulness).
We tried to minimize extraneous effects between conditions by using only morning hours and in addition keep the hour as similar as possible between sessions within each subject. We found no association between the small variations in time of day that still occurred and neither baseline BDNF levels nor BDNF level changes from the different interventions. The order of conditions also had no effect on the magnitude of the BDNF response.
Cognitive function and working memory performance
Klein et al. [57] have demonstrated a correlation between BDNF levels in serum and in the brain across three different species, although the mechanism behind such a serum–brain link has not been established. Based on the probable existence of such a link also in humans, and on the relevance of brain BDNF for cognitive functions [18, 25], previous studies have investigated the cognitive and clinical significance of low serum BDNF levels, but with inconsistent results. To exemplify, Komulainen et al. [44] found a negative association between serum levels of BDNF and cognitive performance in elderly women, but not in men, while Nettiksimmons et al. [43] failed to find an association between circulating BDNF levels and cognitive performance—and in addition found levels of circulating BDNF to be a poor predictor of cognitive decline [43]. One study found higher serum BDNF levels in patients with mild cognitive impairment and Alzheimer’s disease [45], while other studies found an association in the opposite direction [63] or no association at all [46]. In our study, when we compared the average baseline BDNF level across conditions for each participant with their overall working memory performance, we found a low and non-significant correlation of 0.12 (p = 0.62). This is in contrast to the associations we found between post-intervention BDNF levels and working memory function. From Table 3, it can be seen that all statistically significant associations with working memory performance, and also the strongest non-significant correlations, are found in relation to BDNF levels in the only condition in which we found a main effect, i.e., after physical exercise. Working memory performance showed a significant correlation with post-intervention BDNF values (rs = 0.50, p = 0.03), but not to basal levels or BDNF change (Table 3). When we, according to the hypothesis of a ceiling effect for the BDNF response (see above), used tertiles to stratify participants according to their to baseline BDNF values, we found a significant correlation with working memory performance also for BDNF change within the subgroup with the lowest baseline values (rs = 0.89, p = 0.02), but still only in the physical exercise condition (Table 3).
Table 3.
Associations of working memory performance with BDNF levels at baseline, with BDNF response and with BDNF post-intervention levels for the different interventions, stratified according to baseline BDNF levels
| Intervention | Physical exercise | Cognitive training | Mindfulness | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BDNF outcomes | Baseline level | Response | Post-interv level | Baseline level | Response | Post-interv level | Baseline level | Response | Post-interv level | |
| All participants (N = 19) | 0.25 p = 0.31 | 0.22 p = 0.38 | 0.50 p = 0.03 | 0.08 p = 0.74 | 0.09 p = 0.72 | 0.03 p = 0.90 | −0.12 p = 0.63 | −0.13 p = 0.60 | −0.09 p = 0.72 | |
| Stratified by baseline BDNF | Participants with lower levels (N = 6) | −0.60 p = 0.21 | 0.89 p = 0.02 | 0.71 p = 0.11 | 0.07 p = 0.88 | 0.36 p = 0.43 | 0.32 p = 0.48 | −0.03 p = 0.96 | −0.20 p = 0.70 | −0.37 p = 0.47 |
| Participants with higher levels (N = 6) | 0.71 p = 0.11 | 0.31 p = 0.54 | 0.77 p = 0.07 | 0.26 p = 0.63 | −0.66 p = 0.16 | −0.43 p = 0.40 | 0.26 p = 0.62 | −0.37 p = 0.47 | 0.14 p = 0.79 | |
All correlations were calculated between BDNF levels and the average maximum score on all CogMed items (see Methods).
Numbers are coefficients from Spearman correlations with exact p-values beneath.
The stratification of participants into lower versus higher baseline levels of BDNF was according to first and third tertiles.
It should be pointed out that these correlations were based on only nineteen subjects, and even fewer after the stratification shown in the lower part of Table 3. Although the participants were the same across conditions, this means that the statistical strength of the cross over design does not fully apply to these calculations, in contrast to the main calculations. The pattern of results could therefore be of greater interest than any single association, i.e., that strong associations with working memory performance were all found in the single condition where we also showed a main effect from the intervention.
In summary, when we explored possible sources of individual differences in BDNF responses, we found that participants with a higher BDNF level at baseline showed a more limited BDNF response to the subsequent intervention, we found no evidence for a gender difference, and we found that participants with higher working memory performance reached a higher BDNF level after physical exercise, independently of their baseline BDNF levels.
DISCUSSION
To the best of our knowledge, this is the first study on healthy older persons to investigate immediate effects of physical exercise on BDNF levels, and also the first study to contrast immediate effects of physical exercise with two other activities that could potentially alter BDNF levels. Our findings demonstrate a significant immediate increase in serum BDNF levels in healthy older individuals after a 35-minute physical exercise session, but not in the same individuals when they participated in either cognitive training or mindfulness practice for the same duration of time. Our results also indicate a positive association between serum BDNF response to physical exercise and working memory performance. All three exercises were distributed through automatized, commercially available programs, meaning that persons can perform these interventions independently in their homes.
Natural variability in baseline BDNF levels should present a strong case for the suitability of within-subject cross over designs with a high level of experimental control in order to reliably identify factors that affect serum BDNF. The statistical strength of a within-subjects cross over design includes the feature of each individual contributing at least one pair of values from each condition, rather than from only one condition. A further strength, compared to a parallel groups design, is that the BDNF responses of the same individual are compared across conditions, thereby eliminating noise from inter-individual differences that could otherwise mask a true effect from the intervention. Studies on BDNF are especially complicated by the fact that BDNF levels not only vary between individuals, but also within individuals over days and weeks [62]. This suggests that alternative study designs using parallel groups and/or extended intervention periods would require large sample sizes to obtain sufficient statistical power for evaluation of effects on BDNF levels.
We used a sample of healthy older persons, and when they participated in the physical exercise condition we ascertained, for health safety reasons, that their effort was only of moderate intensity. This is in contrast to a study on eighteen well-trained young men who performed an exercise of maximum intensity [64]. While these authors reported a higher increase of serum BDNF [64], other studies with maximum intensity training, found a similar increase as in our study [65, 66]. We have found only one study on acute effects of a single training session in “young” older persons. In this study, Laske et al. found an average increase of 4.1 ng/ml in a sample of 35 women with a depression diagnosis and a non-significant increase of 1 ng/ml in a non-depressed control group of 20 women [34]. In comparison, our participants were considerably older (mean age 70.8 versus 61.1 and 58.9 for the two samples in the study by Laske et al. [34]), and moreover, good health was a selection criterion for participation in our study. Thus, in spite of the well-established role of BDNF for healthy brain functioning and the urgent need to find means to maintain healthy brain functioning in a growing elderly population [67], our study seems to be the first to investigate acute effects on BDNF from physical and other types of activities in healthy older persons.
There was no detectable BDNF effect from the 35-minute session of mindfulness, nor, to our surprise, from cognitive training. If mindfulness or cognitive training still had a subtle effect that we were not able to identify statistically, it could be argued that contrasting physical exercise with these two other activities at least prevented us from overestimating the effect of physical exercise. This does not mean that mindfulness or cognitive training had no other beneficial effects, e.g., in terms of BDNF levels in the brain, cortisol levels, or other outcomes not related to our research question, and it is also important to recognize that from this study nothing can be said about the long-term effects of these activities.
Assuming that BDNF could have a central role in the mediation of learning-related structural changes in the brain [68], the absence of a BDNF effect from cognitive training, as opposed to physical exercise, was unexpected. There are several possible explanations for this, including the short training period; in order for cognitive training to cause a measurable increase in serum BDNF, prolonged training, i.e., over weeks, might be required. Promising and partial support for this approach already exists from two recent studies that found prolonged cognitive training to increase levels of serum BDNF in patients with heart failure [69] schizophrenia [70], and with Parkinson’s disease [71]. Related to length of training as a possible explanation for the lack of a BDNF effect from cognitive training and mindfulness in our study, is the possibility of a difference in serum BDNF origin between the different exercises we compared. BDNF can be produced both in the brain, and peripherally in, for example, blood platelets, muscle receptors, immune cells, salivary glands, and the kidney [72-74]. A correlation between levels of BDNF in blood and brain has previously been demonstrated across species [57], and previous research also provides strong evidence that an increase in serum BDNF after exercise has a favorable impact on brain BDNF levels [16, 19, 20].
Still, if and how BDNF can pass the blood-brain barrier is a controversial matter. One study induced increased mRNA BDNF activity in rodents, leading to higher BDNF levels in the brain, but with no detectable changes in plasma [75]. Rasmussen et al. [35] did find a measurable increase in brain efflux of BDNF into the blood stream, but only after several hours of continued exercise [35]. However, these findings do not rule out the reverse possibility that elevated BDNF levels in the blood may have a more immediate effect on BDNF levels in the brain, either directly via influx through the blood-brain barrier and/or by stimulating BDNF gene expression in different brain regions [19, 20]. Related to this, Pan et al. [76] found that BDNF efflux from the rodent brain is relatively slow, with a half-life clearance (disappearance) of around 45 minutes, compared to a much more rapid influx. As suggested by de Melo Coelho et al. [33], BDNF in blood may function as a BDNF reserve for the brain, and this may explain why the BDNF influx mechanism seems prioritized compared to BDNF efflux from the brain [76].
A possible scenario could thus be that the serum BDNF we measured directly after the physical exercise session had mainly or exclusively a peripheral origin. This could also explain why we did not find a significant serum BDNF increase from a single 35-minute session of cognitive training or mindfulness practice, even if BDNF may have increased in the brain. Based on the results of, for example, Pan et al. [76], the explanation for this could be that BDNF efflux from the brain into the blood stream is too slow to be immediately reflected after a 35-minute exercise, and based on the results of Pardridge [77], the reason could be that BDNF in the brain does not pass through the blood-brain barrier. A way to address this issue could be to compare the acute effects on serum BDNF levels that we found with effects from prolonged exposures of physical exercise and other types of training, a study that is now underway in our laboratory.
The association we found between working memory function and serum BDNF levels after physical exercise, indicates that the ability to increase peripheral BDNF through physical exercise is somehow related to brain function; participants in our study with higher serum levels of BDNF after physical exercise also performed significantly better in the working memory training task, measured on a separate occasion. These findings are consistent with a recent one year intervention study with physical exercise showing that improvement of cognitive function was dependent on serum BDNF levels in older adults [39]. Our data add to those results by demonstrating that serum levels of BDNF after a single 35-minute session of physical exercise are associated with working memory performance. The ability to produce BDNF in the brain is essential for cognitive functions, both according to previous animal studies [18, 23-31] and to a recent study on the association between BDNF gene expression in the human brain and cognitive impairment [78]. This study reported an inverse relation between severity of various forms of neuropathology, including Alzheimer’s disease, and BDNF expression in postmortem human brains [78]. The same study also found an association between higher levels of BDNF expression in the postmortem human brain and a slower rate of cognitive decline during the last six years of life [78]. Taken together, our findings suggest that the ability to respond to physical exercise with increased BDNF levels in serum is associated with cognitive function, and may mean that the ability to produce BDNF peripherally is a marker of BDNF availability also in the brain.
Fig. 3.
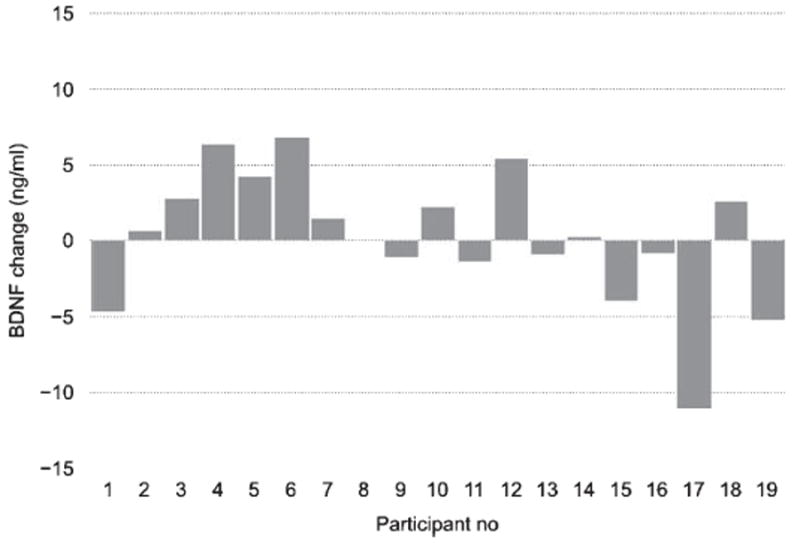
BDNF response to cognitive training for each participant. Each bar depicts the change in BDNF level between the average post-intervention BDNF level (across the three time points) in relation to the baseline (pre-session) level for each participant in the cognitive training condition.
Acknowledgments
We are grateful to Willemo Carlsson, our study nurse, who conducted baseline interviews and instructed the participants in all individual exercises, and together with nurse Leif Eriksson collected and prepared the blood samples, to Mhretab K. Tewele, who did the data programming and gave technical support related to the interventions, to Laura Columbo for excellent technical support in the BDNF analysis of the blood samples, to Sofia Jönsson-Ekström for her meticulous work of entering data into the database, and to Thomas Zucconi-Mazzini and Markus Ågren, our medical doctors who performed the health checks and interviewed all the participants. Above all, we thank our nineteen study participants without whom this study would not have been possible. We are very grateful to the main funder of this study, the Kamprad Family Foundation, Växjö, Sweden. We are also grateful for the funding provided by the Carroll A. Campbell Jr. Neuropathology Laboratory, South Carolina, USA and Demensfonden, Stockholm, Sweden.
Footnotes
As described in the Methods section, when we noticed that the three post-intervention values were very similar, we collapsed them to get a more robust estimation of the BDNF level after each intervention. When we repeated the calculation in relation to only the first post-intervention time point, the results were similar, but as expected, variations around the means were somewhat larger. As a result, the overall ANOVA had F = 3.29 instead of 4.62 (p = 0.049 instead of 0.017) and the complex contrast had F = 8.59 instead of 10.35 (p = 0.009 instead of 0.005).
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0593r2).
References
- 1.Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends Cogn Sci. 2012;16:292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Cummings J. Alzheimer’s disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 3.Wimo A, Winblad B, Jonsson L. The worldwide societal costs of dementia: Estimates for 2009. Alzheimers Dement. 2010;6:98–103. doi: 10.1016/j.jalz.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Deckers K, van Boxtel MPJ, Schiepers OJG, de Vugt M, Muñoz Sánchez JL, Anstey KJ, Brayne C, Dartigues J-F, Engedal K, Kivipelto M, Ritchie K, Starr JM, Yaffe K, Irving K, Verhey FRJ, Köhler S. Target risk factors for dementia prevention: A systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30:234–246. doi: 10.1002/gps.4245. [DOI] [PubMed] [Google Scholar]
- 5.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 6.Hindin SB, Zelinski EM. Extended practice and aerobic exercise interventions benefit untrained cognitive outcomes in older adults: A meta-analysis. J Am Geriatr Soc. 2012;60:136–141. doi: 10.1111/j.1532-5415.2011.03761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lampit A, Hallock H, Valenzuela M. Computerized cognitive training in cognitively healthy older adults: A systematic review and meta-analysis of effect modifiers. PloS Med. 2014;11:e1001756. doi: 10.1371/journal.pmed.1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebok GW, Ball K, Guey LT, Jones RN, Kim H-Y, King JW, Marsiske M, Morris JN, Tennstedt SL, Unverzagt FW, Willis SL, ACTIVE Study Group Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62:16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daffner KR. Promoting successful cognitive aging: A comprehensive review. J Alzheimers Dis. 2009;19:1101–1122. doi: 10.3233/JAD-2010-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER):a randomised controlled trial. The Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 11.Rosenzweig MR. Environmental complexity, cerebral change, and behavior. Am Psychol. 1966;21:321–332. doi: 10.1037/h0023555. [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Ye L, Ruan Q. Environmental enrichment induces synaptic structural modification after transient focal cerebral ischemia in rats. Exp Biol Med (Maywood) 2009;234:296–305. doi: 10.3181/0804-RM-128. [DOI] [PubMed] [Google Scholar]
- 13.Mohammed A, Zhu S, Darmopil S, Hjerling-Leffler J, Ernfors P, Winblad B, Diamond M, Eriksson P, Bogdanovic N. Environmental enrichment and the brain. Prog Brain Res. 2002;138:109–133. doi: 10.1016/S0079-6123(02)38074-9. [DOI] [PubMed] [Google Scholar]
- 14.Park DC, Bischof GN. The aging mind: Neuro-plasticity in response to cognitive training. Dialogues Clin Neurosci. 2013;15:109–119. doi: 10.31887/DCNS.2013.15.1/dpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- 16.Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 17.Branchi I, D’Andrea I, Sietzema J, Fiore M, Di Fausto V, Aloe L, Alleva E. Early social enrichment augments adult hippocampal BDNF levels and survival of BrdU-positive cells while increasing anxiety- and “depression-” like behavior. J Neurosci Res. 2006;83:965–973. doi: 10.1002/jnr.20789. [DOI] [PubMed] [Google Scholar]
- 18.Agranoff BW, Cotman CW, Uhler MD. Synaptic Plasticity as a Model for Learning and Memory Research. Lippincott-Raven; Philadelphia: 1999. [Google Scholar]
- 19.Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109–109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 20.Ieraci A, Mallei A, Musazzi L, Popoli M. Physical exercise and acute restraint stress differentially modulate hippocampal brain-derived neurotrophic factor transcripts and epigenetic mechanisms in mice. Hippocampus. 2015;25:1380–1392. doi: 10.1002/hipo.22458. [DOI] [PubMed] [Google Scholar]
- 21.Tokuyama W, Okuno H, Hashimoto T, Xin Li Y, Miyashita Y. BDNF upregulation during declarative memory formation in monkey inferior temporal cortex. Nat Neurosci. 2000;3:1134–1142. doi: 10.1038/80655. [DOI] [PubMed] [Google Scholar]
- 22.Yamada K, Mizuno M, Nabeshima T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002;70:735–744. doi: 10.1016/s0024-3205(01)01461-8. [DOI] [PubMed] [Google Scholar]
- 23.Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neuroge-nesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 24.Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999;835:259–265. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- 26.Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res. 1999;58:76–87. [PubMed] [Google Scholar]
- 27.Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology. 2014;76(Pt C):628–638. doi: 10.1016/j.neuropharm.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Waterhouse EG, An JJ, Orefice LL, Baydyuk M, Liao G-Y, Zheng K, Lu B, Xu B. BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J Neurosci. 2012;32:14318–14330. doi: 10.1523/JNEUROSCI.0709-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 30.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2009;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatric Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Melo FG, Coelho FG, Gobbi SS, Andreatto CAAC, Corazza DID, Pedroso RVR, Santos-Galduróz RFR. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): A systematic review of experimental studies in the elderly. Arch Gerontol Geriatr. 2012;56:10–15. doi: 10.1016/j.archger.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Laske C, Banschbach S, Stransky E, Bosch S, Straten G, Machann J, Fritsche A, Hipp A, Niess A, Eschweiler GW. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. Int J Neuropsychopharmacol. 2010;13:595–602. doi: 10.1017/S1461145709991234. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 36.Tsai C-L, Chen F-C, Pan C-Y, Wang C-H, Huang T-H, Chen T-C. Impact of acute aerobic exercise and cardiores-piratory fitness on visuospatial attention performance and serum BDNF levels. Psychoneuroendocrinology. 2014;41:121–131. doi: 10.1016/j.psyneuen.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Lavretsky H, Epel ES, Siddarth P, Nazarian N, Cyr NS, Khalsa DS, Lin J, Blackburn E, Irwin MR. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: Effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28:57–65. doi: 10.1002/gps.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox KCR, Nijeboer S, Dixon ML, Floman JL, Ellamil M, Rumak SP, Sedlmeier P, Christoff K. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48–73. doi: 10.1016/j.neubiorev.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, Phillips SM, Gothe NP, Mailey E, Vieira-Potter VJ, Martin SA, Pence BD, Lin M, Parasuraman R, Greenwood PM, Fryxell KJ, Woods JA, McAuley E, Kramer AF, Erickson KI. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci. 2014;8:985. doi: 10.3389/fnhum.2014.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eyre HA, Acevedo B, Yang H, Siddarth P, Van Dyk K, Ercoli L, Leaver AM, Cyr NS, Narr K, Baune BT, Khalsa DS, Lavretsky H. Changes in neural connectivity and memory following a yoga intervention for older adults: A pilot study. J Alzheimers Dis. 2016;52:673–684. doi: 10.3233/JAD-150653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Leary K, O’Neill S, Dockray S. A systematic review of the effects of mindfulness interventions on corti-sol. J Health Psychol. 2016;21:2108–2121. doi: 10.1177/1359105315569095. [DOI] [PubMed] [Google Scholar]
- 42.Lakshminarasimhan H, Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PloS One. 2012;7:e30481. doi: 10.1371/journal.pone.0030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nettiksimmons J, Simonsick EM, Harris T, Satterfield S, Rosano C, Yaffe K, for the Health ABC Study The associations between serum brain-derived neurotrophic factor, potential confounders, and cognitive decline: A longitudinal study. PLoS One. 2014;9:e91339. doi: 10.1371/journal.pone.0091339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komulainen P, Pedersen M, Hanninen T, Bruunsgaard H, Lakka TA, Kivipelto M, Hassinen M, Rauramaa TH, Pedersen BK, Rauramaa R. BDNF is a novel marker of cognitive function in ageing women: The DR’s EXTRA Study. Neurobiol Learn Mem. 2008;90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Angelucci F, Spalletta G, di Iulio F, Ciaramella A, Salani F, Colantoni L, Varsi AE, Gianni W, Sancesario G, Caltagirone C, Bossú P. Alzheimer’s disease (AD) and Mild Cognitive Impairment (MCI) patients are characterized by increased BDNF serum levels. Curr Alzheimer Res. 2010;7:15–20. doi: 10.2174/156720510790274473. [DOI] [PubMed] [Google Scholar]
- 46.Ziegenhorn AA, Schulte-Herbrüggen O, Danker-Hopfe H, Malbranc M, Hartung H-D, Anders D, Lang UE, Steinhagen-Thiessen E, Schaub RT, Hellweg R. Serum neurotrophins–a study on the time course and influencing factors in a large old age sample. Neurobiol Aging. 2007;28:1436–1445. doi: 10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Laske C, Stransky E, Leyhe T, Eschweiler GW. Stage-dependent BDNF serum concentrations in Alzheimer’s disease. J Neural Transm. 2006;113:1217–1224. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- 48.Aronow WS. Blood pressure goals and targets in the elderly. Curr Treat Options Cardiovasc Med. 2015;17:394. doi: 10.1007/s11936-015-0394-x. [DOI] [PubMed] [Google Scholar]
- 49.Qiu C, Strauss von E, Fastbom J, Winblad B, Fratiglioni L. Low blood pressure and risk of dementia in the Kung-sholmen project: A 6-year follow-up study. Arch Neurol. 2003;60:223–228. doi: 10.1001/archneur.60.2.223. [DOI] [PubMed] [Google Scholar]
- 50.Folstein M, Folstein S, McHugh P. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 51.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 52.Shinaver CS, Entwistle PC, Söderqvist S. Cogmed WM training: Reviewing the reviews. Appl Neuropsychol Child. 2014;3:163–172. doi: 10.1080/21622965.2013.875314. [DOI] [PubMed] [Google Scholar]
- 53.Kabat-Zinn J. Some reflections on the origins of MBSR, skillful means, and the trouble with maps. Contemporary Buddhism. 2011;12:281–306. [Google Scholar]
- 54.Giese M, Beck J, Brand S, Muheim F, Hemmeter U, Hatzinger M, Holsboer-Trachsler E, Eckert A. Fast BDNF serum level increase and diurnal BDNF oscillations are associated with therapeutic response after partial sleep deprivation. J Psychiatric Res. 2014;59:1–7. doi: 10.1016/j.jpsychires.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Polacchini A, Metelli G, Francavilla R, Baj G, Florean M, Mascaretti LG, Tongiorgi E. A method for reproducible measurements of serum BDNF: Comparison of the performance of six commercial assays. Sci Rep. 2015;5:17989. doi: 10.1038/srep17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim Y, Zhong JH, Zhou XF. Development of mature BDNF-specific sandwich ELISA. J Neurochem. 2015;134:75–85. doi: 10.1111/jnc.13108. [DOI] [PubMed] [Google Scholar]
- 57.Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. The Int J Neuropsychopharmacol. 2011;14:347–353. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- 58.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 60.Schuch FB, da Silveira LE, de Zeni TC, da Silva DP, Wollenhaupt-Aguiar B, Ferrari P, Reischak-Oliveira Á, Kapczinski F. Effects of a single bout of maximal aerobic exercise on BDNF in bipolar disorder: A gender-based response. Psychiat Res. 2015;229:57–62. doi: 10.1016/j.psychres.2015.07.072. [DOI] [PubMed] [Google Scholar]
- 61.Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 62.Trajkovska V, Marcussen AB, Vinberg M, Hartvig P, Aznar S, Knudsen GM. Measurements of brain-derived neurotrophic factor: Methodological aspects and demographical data. Brain Res Bull. 2007;73:143–149. doi: 10.1016/j.brainresbull.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 63.Laske C, Stransky E, Leyhe T. Decreased brain-derived neurotrophic factor (BDNF)-and beta-thromboglobulin (beta-TG)-blood levels in Alzheimer’s disease. Thromb Haemost. 2006;96:102–103. doi: 10.1160/TH06-03-0173. [DOI] [PubMed] [Google Scholar]
- 64.Cho H-C, Kim J, Kim S, Son YH, Lee N, Jung SH. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO2max performance in healthy college men. Neurosci Lett. 2012;519:78–83. doi: 10.1016/j.neulet.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 65.Heyman E, Gamelin FX, Goekint M, Piscitelli F, Roelands B, Leclair E, Di Marzo V, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans—Possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–851. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 66.Rojas Vega S, Hollmann W, Vera Wahrmann B, Strüder HK. pH buffering does not influence BDNF responses to exercise. Int J Sports Med. 2012;33:8–12. doi: 10.1055/s-0031-1285929. [DOI] [PubMed] [Google Scholar]
- 67.Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, Cedazo-Minguez A, Dubois B, Edvardsson D, Feldman H, Fratiglioni L, Frisoni GB, Gauthier S, Georges J, Graff C, Iqbal K, Jessen F, Johansson G, Jonsson L, Kivipelto M, Knapp M, Mangialasche F, Melis R, Nordberg A, Rikkert MO, Qiu C, Sakmar TP, Scheltens P, Schneider LS, Sperling R, Tjernberg LO, Waldemar G, Wimo A, Zetterberg H. Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol. 2016;15:455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 68.Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- 69.Pressler SJ, Titler M, Koelling TM, Riley PL, Jung M, Hoyland-Domenico L, Ronis DL, Smith DG, Bleske BE, Dorsey SG, Giordani B. Nurse-enhanced computerized cognitive training increases serum brain-derived neurotropic factor levels and improves working memory in heart failure. J Card Fail. 2015;21:630–641. doi: 10.1016/j.cardfail.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol Psychiatry. 2009;66:549–553. doi: 10.1016/j.biopsych.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Angelucci F, Peppe A, Carlesimo GA, Serafini F, Zabberoni S, Barban F, Shofany J, Caltagirone C, Costa A. A pilot study on the effect of cognitive training on BDNF serum levels in individuals with Parkinson’s disease. Front Hum Neurosci. 2015;9:130. doi: 10.3389/fnhum.2015.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phillips C, Baktir MA, Srivatsan M, Salehi A. Neuroprotective effects of physical activity on the brain: A closer look at trophic factor signaling. Front Cell Neurosci. 2014;8:170. doi: 10.3389/fncel.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brunelli A, Dimauro I, Sgro P, Emerenziani GP, Magi F, Baldari C, Guidetti L, Di Luigi L, Parisi P, Caporossi D. Acute exercise modulates BDNF and pro-BDNF protein content in immune cells. Med Sci Sports Exerc. 2012;44:1871–1880. doi: 10.1249/MSS.0b013e31825ab69b. [DOI] [PubMed] [Google Scholar]
- 74.Smith PA. BDNF: No gain without pain? Neuroscience. 2014;283:107–123. doi: 10.1016/j.neuroscience.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 75.Lanz TA, Bove SE, Pilsmaker CD, Mariga A, Drummond EM, Cadelina GW, Adamowicz WO, Swetter BJ, Carmel S, Dumin JA, Kleiman RJ. Robust changes in expression of brain-derived neurotrophic factor (BDNF) mRNA and protein across the brain do not translate to detectable changes in BDNF levels in CSF or plasma. Biomarkers. 2012;17:524–531. doi: 10.3109/1354750X.2012.694476. [DOI] [PubMed] [Google Scholar]
- 76.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 77.Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 78.Buchman AS, Yu L, Boyle PA, Schneider JA, De Jager PL, Bennett DA. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology. 2016;86:735–741. doi: 10.1212/WNL.0000000000002387. [DOI] [PMC free article] [PubMed] [Google Scholar]


