Abstract
Epidermal growth factor receptor (EGFR) mutations are commonly observed in Glioblastoma (GBM) and have long posed as a target for new therapies. Trials involving erlotinib have shown mixed results, likely owing to a mechanism of the mutation that may instead favor other EGFR inhibitors, such as lapatinib. We aimed to determine whether or not pulse high-dose lapatinib was a safe and tolerable regimen in addition to standard therapy. We recruited adult patients with newly-diagnosed GBM who had Karnofsky Performance Status ≥60, normal baseline hematological, hepatic, and renal function tests, and no prior history of radiation or treatment with EGFR inhibitor. Lapatinib was administered at 2500 mg twice daily for two consecutive days per week on a weekly basis throughout concomitant and adjuvant standard therapy. The primary endpoints were tolerability and safety. 12 patients were enrolled in this study over 2 years. Of the non-hematological adverse events, there were 2 grade 3 events, fatigue and post-radiation cystic brain necrosis. The most common adverse events in general were fatigue, rashes, and diarrhea. Of the hematological adverse events, there were 13 grade 3 events, all of which were due to lymphopenia and affected 6 of 12 patients. Pulse high-dose lapatinib in addition to standard therapy for newly-diagnosed GBM is a tolerable and safe regimen, but higher rates of lymphopenia should be noted. However, further investigations will be required to evaluate the efficacy of this combination for the treatments of GBM.
Trial registration ClinicalTrials.gov Identifier: NCT01591577.
Keywords: Glioblastoma, Lapatinib, Temozolomide, EGFR
Introduction
Glioblastoma (GBM, World Health Organization Grade IV) is the most common primary malignant brain tumor in adults [1]. The standard treatment regimen consists of surgical resection, followed by chemotherapy and concurrent radiotherapy (RT), with continued adjuvant chemotherapy. Temozolomide (TMZ, Temodar®) is a well-tolerated alkylating agent that is considered standard chemotherapy. Even with combined chemotherapy and radiation, median overall survival is low at approximately 14.6 months [2]. New technologies, including tumor treating fields (Optune™, formerly novoTTF™) and immunotherapies, are emerging but there remains a strong need for greater improvement of outcomes [3].
The Epidermal Growth Factor Receptor (EGFR) gene has been shown to be abnormal in up to 57% of GBM, with approximately 19% showing at least low-level expression of EGFR variant III (EGFRvIII), which involves a deletion of exons 2–7 [4]. EGFR overexpression confers RT resistance and the inhibition of EGFR overexpression has been shown to sensitize tumors to RT [5, 6]. EGFRvIII has been shown to be associated with increased cell proliferation and decreased rates of apoptosis in vitro [7]. In addition, the overexpression of EGFRvIII has retrospectively been shown to be a prognostic indicator of poor overall survival in the setting of EGFR overexpression [8]. There have been clinical trials evaluating EGFR inhibitors, mostly erlotinib, as a treatment for GBM but most have shown mixed results. Two phase II clinical trials of erlotinib in combination with TMZ and RT for newly diagnosed GBM showed median overall survivals of approximately 15–19 months, comparable to standard therapy with TMZ and RT alone [9, 10]. However, a smaller phase II trial showed a median overall survival of 8.6 months and was halted early due to an excessive number of treatment-related deaths [11]. One phase II study showed a PFS-6 of 3% for single-agent erlotinib in recurrent GBM and was considered unsuccessful [12].
The mechanism for the poor response of small molecule inhibitors of EGFR in GBMs is likely related to site of the mutation, as well as site of inhibitor binding. The EGFRvIII mutation common in GBMs is located in the ectodomain, while other EGFR mutations common in lung cancer, a setting where erlotinib has proven highly efficacious, are located in the intracellular kinase domain. Vivanco et al. showed experimentally that Type II (inactive conformation) EGFR inhibitors, such as lapatinib, more effectively blocked ATP binding at the kinase domain than Type I (active conformation) EGFR inhibitors, such as erlotinib, in cell lines with EGFR ectodomain mutations. They also showed that GBM cell lines had higher rates of cell death when treated with lapatinib compared to erlotinib. This work reported results from a phase II trial where subjects received 750 mg daily of lapatinib for 7 days prior to resection for recurrent GBM. They found that lapatinib tumor concentrations were higher than the IC50 for inhibition of EGFR phosphorylation but lower than levels reported for cell death (approximately 1.5 μM) [13]. From this result, the authors hypothesized that a higher dose of lapatinib would be needed for better brain penetration, and conducted an experiment treating mice with pulse dosing of lapatinib. The mice treated with high pulse-dose lapatinib (1000 mg/kg every 5 days) had markedly greater tumor volume reduction than those treated with daily lapatinib (200 mg/kg daily).
The standard dosing in most studies of lapatinib ranges from 1250 to 1500 mg daily dosing. A phase I study of a 2-day pulse dosing of lapatinib prior to nab-paclitaxel infusion for solid tumors showed a maximum tolerated dose (MTD) of 5250 mg/day, given in twice daily dosing. The incidence of grade 1 and 2 toxicities was proportional to MTD but were generally manageable [14]. To date, there is no clinical trial evaluating the safety of pulse-dosing in patients with newly diagnosed or recurrent GBM. To evaluate whether pulse high dose lapatinib would be tolerable in combination with TMZ and RT, we conducted a pilot phase II open-label trial to evaluate the tolerability and safety of 2-day pulse dose lapatinib in addition to standard treatment in patients with newly-diagnosed GBM. This manuscript describes the safety data seen in this pilot study, and with limited number of patients, survival outcome data will be addressed at the conclusion of the larger phase II study.
Materials and methods
The pilot study was an open-label, phase II single-arm, single center study for safety evaluation. Patients were treated and enrolled between November 7, 2012 and July 8, 2014. Inclusion criteria included recent diagnosis of biopsy-proven GBM, age ≥18 years old, Karnofsky Performance Score (KPS) ≥60, normal baseline hematological, hepatic, and renal function tests. Exclusion criteria included prior chemotherapy or radiation for GBM, prior use of any EGFR inhibitors, and concurrent use of any enzyme-inducing antiepileptic drugs. All patients were evaluated with a baseline echocardiogram and electrocardiogram. EGFR mutation or amplification status were not required inclusion criteria in this trial, but all patients did receive routine evaluation of EGFR amplification through Fluorescent in situ hybridization at our institution. Per institutional common practice, only patients with EGFR amplification were recommended this study by the investigators. Each patient provided informed consent prior to enrollment. The trial and consent were reviewed and approved by the institutional review board.
All patients underwent surgical resection, followed by standard protocol concomitant radiotherapy and daily TMZ for 6 weeks. Patients were subsequently treated with adjuvant chemotherapy, which consisted of standard TMZ dosing administered in a 5/28 day dose-dense schedule for up to 12 cycles. Lapatinib was administered weekly at the beginning of concomitant therapy, continued along with standard adjuvant therapy, and continued as monotherapy until progression. Lapatinib was administered at 2500 mg twice daily for two consecutive days of each week (Fig. 1). Patients were followed with brain MRI at least every 8 weeks to monitor for progression of disease. Imaging evaluation was measured using the Modified Levin’s scale. Functional status was measured using the Karnofsky Performance Scale (KPS) [15].
Fig. 1.
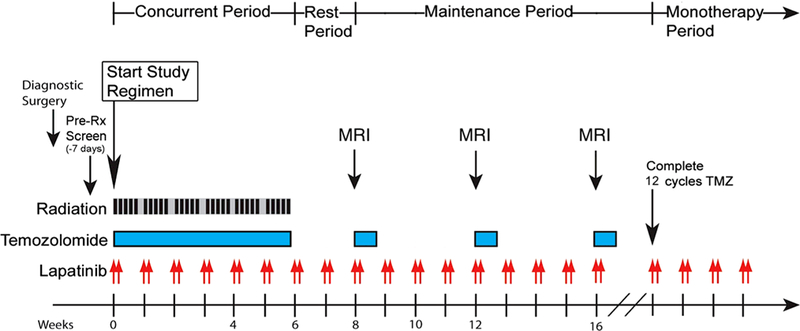
Trial schema
Adverse events (AEs) were recorded at each clinical appointment. The treating oncologist graded each event in accordance with the grading scale by the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4 [16] AEs were grouped into three distinct time periods: (1) concomitant therapy, (2) 4 weeks post-radiation therapy, and (3) maintenance therapy. For the pilot phase, if no more than 4 out of 10 patients developed persistent grade 3/4 events that were deemed related to study drugs, we would than expand the study to complete all enrollments. AEs were categorized into hematological and non-hematological events. Temozolomide dose reduction was based on the package insert [17]. Lapatinib would be dose reduced up to 2 levels, as per supplementary data 1. The primary endpoint was to evaluate safety, including frequency of grade 3/4 adverse events.
Results
Thirteen patients were enrolled in this study between November 2012 and July 2014. One patient failed initial screening and was excluded from this report. Twelve patients were included in the final enrollment of the pilot phase of the study (Table 1), and to date, all patients have progressed and only 2 patients are still alive. Of the 12 patients, 9 were male and 3 were female. Median age at diagnosis was 58 years old. Median KPS was 90 (range 90–100). The median time from diagnosis to treatment was 34.5 days. All patients completed their 6-week concomitant therapy phase. Following concomitant therapy, the median number of completed adjuvant TMZ cycles was 5.5 (range 1–12). One patient only underwent 1 cycle of adjuvant TMZ before opting to withdraw consent due to abdominal pain which continued after lapatinib was stopped. All other patients stopped chemotherapy upon evidence of disease progression.
Table 1.
Patient Characteristics
| Characteristics | N median (range) |
|---|---|
| Number of patients | 12 |
| Gender | |
| Male | 9 |
| Female | 3 |
| Age | 58 (37–71) |
| KPS | 90 (90–100) |
| Days from diagnosis to treatment | 34.5 (25–42) |
| Extent of resection | |
| Gross total resection | 8 |
| Subtotal resection | 4 |
| Biopsy | 0 |
KPS Karnofsky performance status
Of the non-hematological AEs, there were 64 grade 1, 21 grade 2, and 2 grade 3 events (Table 2). There were no grade 4 AEs reported. The most commonly involved systems were gastrointestinal (35.6%) and skin (20.7%). The three overall most common non-hematological AEs were fatigue (n = 18) and diarrhea (n = 15), followed by rash (n = 8). Of the grade 1 AEs, 27 occurred during the concomitant phase, 12 during the post-radiation phase, and 25 during the maintenance phase. The most common of these were diarrhea, fatigue, a papular rash, and nausea. Grade 1 diarrhea was observed in 10 of our 12 patients and grade 1 fatigue was observed in 7 of our 12 patients. Two patients suffered from grade 1 radiodermatitis during the concomitant therapy phase. Of the grade 2 AEs, 6 occurred during the concomitant phase, 5 during the post-radiation phase, and 10 during the maintenance phase. The most common grade 2 AEs were consistent with the most common grade 1 AEs, with the addition of dehydration (n = 4). There were only 2 non-hematological grade 3 AEs, both occurring during the maintenance phase. One was fatigue and the other was enlargement of a cyst due to brain necrosis post-concomitant treatments.
Table 2.
Non-hematologic adverse events
| Adverse event category | Adverse event | Concomitant therapy | 4 weeks post RT | Maintenance phase | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3/4 | Grade 1 | Grade 2 | Grade 3/4 | Grade 1 | Grade 2 | Grade 3/4 | ||
| General disorders | Fatigue | 3 | 2 | 0 | 1 | 1 | 0 | 5 | 5 | 1 |
| Skin | Rash | 3 | 0 | 0 | 1 | 2 | 0 | 2 | 0 | 0 |
| Hyperpigmentation | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Facial edema | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Xerosis | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Skin fragility | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Periungual irritation | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | |
| Erythema | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Gastrointestinal | Diarrhea | 10 | 1 | 0 | 1 | 0 | 0 | 3 | 0 | 0 |
| Nausea | 1 | 1 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | |
| Anorexia | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | |
| Oral mucositis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Abdominal pain | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vomiting | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | |
| Xerostomia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| CNS Disorders | Hypersomnia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| CNS necrosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Tremors | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | |
| Musculoskeletal | Cramping | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Metabolism and Nutrition Disorders | Dehydration | 0 | 1 | 0 | 1 | 2 | 0 | 2 | 1 | 0 |
| Procedural complications | Radiodermatitis | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eye disorders | Epiphora | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Investigations | Weight loss | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 |
RT radiation therapy
The hematological AEs consisted of lymphopenia, and thrombocytopenia (Table 3). Of these hematological AEs, there were 24 grade 1, 14 grade 2, and 13 grade 3 (lymphopenia n = 13). Lymphopenia accounted for 88% of all hematological AEs and affected 75% of patients. Of the grade 1 and 2 events, the vast majority occurred either in the concomitant therapy phase or the maintenance phase. Of the grade 3 lymphopenia events, 3 occurred during the concomitant therapy phase, 2 during the post-radiation phase, and 8 during the maintenance phase. 6 of our 12 patients experienced grade 3 lymphopenia.
Table 3.
Hematologic adverse events
| Adverse event | Concomitant therapy | 4 weeks post RT | Maintenance phase | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3/4 | Grade 1 | Grade 2 | Grade 3/4 | Grade 1 | Grade 2 | Grade 3/4 | |
| Lymphopenia | 5 | 6 | 3 | 3 | 2 | 2 | 10 | 6 | 8 |
| Thrombocytopenia | 2 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
RT radiation therapy
Three patients had delays in their TMZ dosing. Two of these patients also received dose reductions in their TMZ, one due to fatigue (during cycle 3 when a TMZ dose was increased from 150 to 200 mg/m2) and one due to weight loss. The patient who suffered fatigue also received a dose reduction for lapatinib to 2000 mg twice a day. This patient completed five cycles of adjuvant chemotherapy, with the last cycle consisting of only lapatinib; the TMZ was held due to severe fatigue. There were no other patients with lapatinib dose changes. One patient developed thrombocytopenia and the next TMZ cycle was accordingly delayed. Another patient developed enlargement of a necrotic cyst after radiation, for which they required surgical resection after five cycles of adjuvant chemotherapy. Pathology of this resection was consistent with radiation necrosis.
Discussion
In this pilot study of pulse high dose lapatinib with radiation therapy and temozolomide chemotherapy, we found that this combination seemed tolerable and safe. The vast majority of AEs observed from our study were Grade 1 and 2 toxicities. The most common classes of non-hematological and hematological AEs remained consistent with those seen in studies of standard TMZ and radiation therapy. Fatigue, gastrointestinal events, and dermatological events were the most common classes of AEs in our study. In our study, grade 2 and 3 fatigue were seen at rates consistent with those seen in standard therapy. We observed grade 2 fatigue in 33% (4 of 12) of patients and grade 3 fatigue in 8.3% (1 of 12), compared to 38 and 13%, respectively, in Stupp 2005. Our study also showed 3 of 12 patients (25%) were affected by grade 2 dermatological AEs, compared to 12% in Stupp 2005. The rashes reported ranged from papular to diffuse macules, pruritic to non-pruritic, and often affected the forehead and trunk, consistent with typical rash caused by EGFR inhibitors. The rashes also often improved with topical steroids and are likely caused by the addition of lapatinib. 4 of 12 (33%) patients experienced some grade II gastrointestinal AEs, also consistent with the rate of nausea/vomiting (28%) seen by Stupp 2005 [2]. One gastrointestinal AE related to lapatinib, diarrhea, was mostly a grade 1–2 toxicity and was well-controlled with loperamide. The rate of diarrhea in our study is slightly higher than that reported in a phase 1 study of pulse-dose lapatinib at 5250 mg/day in conjunction with paclitaxel (83 vs. approximately 60%). The other notable non-hematological grade 3 AE was a necrotic brain cyst that grew following radiation and ultimately required surgical resection, but there is no current evidence to suggest this was due to the addition of lapatinib. Overall, the rates of non-hematological AEs with the addition of pulse-dosing lapatinib appear largely consistent with that seen with current standard of care except for the increase in diarrhea. Lapatinib has received a FDA black box warning for severe hepatotoxicity. No grade 3/4 elevated ALT or AST AEs were observed during our study. Also, no patient developed cardiac toxicity when evaluated by repeat echocardiogram at 6 months.
Of the hematological AEs, lymphopenia was the most common. 7 of 12 patients (58%) experienced grade 2 lymphopenia; 6 of those 7 patients also developed grade 3 lymphopenia at some point during treatment. In comparison to current standard radiation and chemotherapy, the addition of pulse high dose lapatinib showed no stark differences in non-hematological events but did show higher rates of grade 3 lymphopenia. For example, the Gilbert 2013 phase III trial showed a grade 3 lymphopenia rate of 12% [18]. Prados 2009, a phase II trial of TMZ/RT/erlotinib for upfront treatment of GBM, also found their predominant adverse event to be grade III lymphopenia (they reported 29 events from their 65 patients), which they attributed to TMZ [10]. Karavasilis 2013 performed a phase I study of TMZ and lapatinib 1000–1500 mg daily for recurrent high-grade gliomas and found hematological AEs to be both the primary dose-limiting toxicity and overall most common side effect, but at overall lower rates than seen in our study [19]. Our observed higher rates of lymphopenia are consistent with our higher dosages of lapatinib.
Our pilot study is the first known trial to evaluate pulse-dosing of high dose lapatinib in upfront treatment of newly-diagnosed GBM. The strengths of our study included strong adherence to the trial protocol and regular long-term follow up. The primary limitation of our study is a relatively small cohort size, which can make it difficult to compare our rates of AEs to those of other larger studies. This pilot trial was not designed to fully demonstrate efficacy of pulse-dosing lapatinib.
Conclusions
Through our study we have demonstrated that the addition of pulse high dose lapatinib to standard therapy for newly-diagnosed GBM is largely well tolerated and safe. The rates of fatigue, GI, and dermatological AEs are consistent with standard therapy, but the incidence of lymphopenia and diarrhea appears to be elevated. Since this study reports only a small number of patients, further studies would be needed to evaluate the efficacy of this combination in the treatment of glioblastoma. Because of the higher rate of diarrhea, we would recommend use of prophylactic loperamide in any patients with loose stools from the first treatment of lapatinib. Also because of the lack of cardiac toxicity, we did not require follow-up cardiac echo in this population unless a patient developed evidence of cardiac problems.
Supplementary Material
Acknowledgments
Funding Novartis (previously Glaxo-Smith Kline) partially supported this pilot study.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11060-017-2533-6) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors do not declare any conflicts of interest related to this work.
References
- 1.Quinn T, Ostrom HG, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2015) CBTRUS Statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol 17:iv1–iv62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996 [DOI] [PubMed] [Google Scholar]
- 3.Cloughesy TF, Lassman AB (2017) NovoTTF: where to go from here? Neuro-oncol 19:605–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan CW, Verhaak RG, McKenna A et al. (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker FG 2nd, Simmons ML, Chang SM et al. (2001) EGFR overexpression and radiation response in glioblastoma multi-forme. Int J Radiat Oncol Biol Phys 51:410–418 [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee B, McEllin B, Camacho CV et al. (2009) EGFRvIII and DNA double-strand break repair: a molecular mechanism for radioresistance in glioblastoma. Cancer Res 69:4252–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ (1996) A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res 56:5079–5086 [PubMed] [Google Scholar]
- 8.Shinojima N, Tada K, Shiraishi S et al. (2003) Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res 63:6962–6970 [PubMed] [Google Scholar]
- 9.Brown PD, Krishnan S, Sarkaria JN et al. (2008) Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol 26:5603–5609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prados MD, Chang SM, Butowski N et al. (2009) Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol 27:579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peereboom DM, Shepard DR, Ahluwalia MS et al. (2010) Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol 98:93–99 [DOI] [PubMed] [Google Scholar]
- 12.Raizer JJ, Abrey LE, Lassman AB et al. (2010) A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro-oncol 12:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivanco I, Robins HI, Rohle D et al. (2012) Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov 2:458–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chien AJ, Illi JA, Ko AH et al. (2009) A phase I study of a 2-day lapatinib chemosensitization pulse preceding nanoparticle albumin-bound Paclitaxel for advanced solid malignancies. Clin Cancer Res 15:5569–5575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin VA, Crafts DC, Norman DM, Hoffer PB, Spire JP, Wilson CB (1977) Criteria for evaluating patients undergoing chemo-therapy for malignant brain tumors. J Neurosurg 47:329–335 [DOI] [PubMed] [Google Scholar]
- 16.Institute NC (2009). Common Terminology Criteria for Adverse Events v4.0. NIH publication # 09–7473 [Google Scholar]
- 17.Reference PD (2015) 2016 Physicians’ Desk Reference. PDR Network, LLC, Montvale [Google Scholar]
- 18.Gilbert MR, Wang M, Aldape KD et al. (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31:4085–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karavasilis V, Kotoula V, Pentheroudakis G et al. (2013) A phase I study of temozolomide and lapatinib combination in patients with recurrent high-grade gliomas. J Neurol 260:1469–1480 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


