Abstract
Objective
To describe the demographic characteristics, health, and health care experiences of adult patients in primary care waiting rooms in Quebec, and to determine which pillars of the Patient’s Medical Home (PMH) are a priority to align primary care practices with the PMH model.
Design
Baseline survey of a prospective cohort study using self-administered on-site and mailed questionnaires.
Setting
Twelve primary care clinics within the geographic boundaries of 4 local health care networks in metropolitan, urban, rural, and remote settings in Quebec.
Participants
A total of 1029 adult patients aged between 25 and 75 who were selected during a 1-week period in the 12 primary care clinics; 789 returned questionnaires.
Main outcome measures
Patients’ health profiles, health behaviour patterns, reasons for the visit, and health care experiences.
Results
In this 2010 snapshot, 66.8% of patients waited longer than 2 weeks for their appointment, 71.0% of visits were for routine or follow-up care, and longer wait times and patient multimorbidity correlated with more reasons for the visit. After the visit, most patients reported being able to express their most important needs and that the doctor listened well; however, only 28.1% reported that the doctor had explored whether the recommendations would be realistic for them, and only 18.0% indicated that the doctor had explored the personal or family dimensions that affected their health. Among all patients, 56.9% reported having at least 3 chronic conditions (multimorbidity), and 30.3% reported having high or moderate levels of psychological distress. When describing their financial status, 30.7% of patients indicated it was “poor to squeezed or tight.” Slightly more than half of patients did not have complementary private health insurance to cover costs of psychological services.
Conclusion
In this study, the 4 priority pillars for practices to align with the PMH were timely access, team-based care, comprehensive care, and a patient-centred approach. Widespread implementation of advanced access is an urgent priority in light of persisting difficulties in timely access. Team-based and comprehensive care are needed to address the high prevalence of multimorbidity and psychological distress and to support health behaviour change. Finally, the patient-centred approach needs to underpin every care encounter.
Résumé
Objectif
Décrire les caractéristiques démographiques, l’état de santé et les expériences en matière de soins de santé de patients adultes dans la salle d’attente de cliniques de soins primaires au Québec, et déterminer les piliers du Centre de médecine de famille (CMF) auxquels il faut accorder la priorité pour que la pratique des soins primaires concorde avec ce modèle.
Conception
Enquête de référence d’une étude prospective de cohortes au moyen de questionnaires remplis sur place par les intéressés et de questionnaires envoyés par la poste.
Contexte
Douze cliniques de soins primaires situées dans les limites géographiques de 4 réseaux de soins primaires locaux dans des milieux métropolitains, urbains, ruraux et éloignés au Québec.
Participants
Au total, 1029 patients adultes âgés de 25 à 75 ans ont été choisis durant une période de 1 semaine dans les 12 cliniques de soins primaires; 789 ont retourné les questionnaires.
Principaux paramètres à l’étude
Le profil médical des patients, les habitudes comportementales des patients en matière de santé, les motifs de la visite, de même que les expériences sur le plan des soins de santé.
Résultats
Dans ce portrait réalisé en 2010, 66,8 % des patients avaient attendu plus de 2 semaines pour avoir un rendez-vous, 71,0 % des visites étaient pour des soins de routine ou de suivi. Les plus longs délais d’attente étaient corrélés avec la multimorbidité des patients ayant plus de raisons de consulter. Après la visite, la majorité des patients ont signalé qu’ils avaient été capables d’exprimer leurs plus importants besoins, et que le médecin les avait bien écoutés; par ailleurs, seulement 28,1 % des patients ont rapporté que le médecin avait demandé si ses recommandations étaient réalistes pour eux, et seulement 18,0 % ont dit que le médecin avait exploré les dimensions personnelles ou familiales qui affectaient leur santé. Dans l’ensemble des patients, 56,9 % ont rapporté avoir au moins 3 problèmes chroniques (multimorbidité), et 30,3 % ont indiqué avoir des degrés élevés ou modérés de détresse psychologique. Dans la description de leur situation financière, 30,7 % l’ont qualifiée de « mauvaise à difficile, ou serrée ». Un peu plus de la moitié des patients n’avaient pas d’assurance maladie privée complémentaire pour couvrir les coûts de services psychologiques.
Conclusion
Dans cette étude, pour que la pratique concorde avec le CMF, les 4 piliers prioritaires étaient l’accès en temps opportun, les soins en équipe, les soins complets et une approche centrée sur le patient. À la lumière des problèmes persistants d’accès en temps opportun, la mise en œuvre généralisée des modes d’accès avancé est une urgente priorité. Des soins complets dispensés en équipe sont nécessaires pour répondre à la forte prévalence de la multimorbidité et de la détresse psychologique, de même que pour encourager des changements dans les comportements en matière de santé. Enfin, chaque rencontre médicale doit reposer sur une approche centrée sur le patient.
Across Canada, primary care delivery is moving away from the traditional entrepreneurial model funded through fee-for-service reimbursement toward new alternate payment models and teams that include a range of health professionals. The College of Family Physicians of Canada has provided a unified language and vision that encompasses the diverse labels of new delivery models in different provinces: the Patient’s Medical Home (PMH).1
There is an urgent need for primary care practice redesign, as international health system surveys consistently place Canada’s primary care performance as low or lowest among peer countries.2–5 Although practice redesign options might be constrained by health system structures, regulatory frameworks, and funding mechanisms, many redesign options are under the direct control of family physicians. In this article, we describe the demographic characteristics, health, and health care experiences of adult patients in the waiting rooms of primary care clinics in Quebec in order to determine the priority PMH pillars to align practices with the model.
METHODS
This is the baseline survey of a prospective cohort study of adult patients selected during a 1-week period from the waiting rooms of 12 primary care clinics within the geographic boundaries of 4 local health care networks in metropolitan, urban, rural, and remote settings in Quebec. In each network we purposefully selected 3 clinics that were typical of the dominant forms of primary health care organizations and that were not known as either positive or negative deviants by local primary care leaders. We selected private medical clinics, community health centres, and family medicine groups (the Quebec reform model). The proposed method for this program of research has been reported previously.6 The study obtained approval from the 4 health network governance structures and was granted ethics approval by the research ethics committee at the Charles LeMoyne Hospital in Greenfield Park, Que.
Study population
In each clinic, patients were recruited in the waiting room in March and April 2010 by research assistants during every type of scheduled and walk-in service offered by the clinic (during weekdays, evenings, and weekends). In small clinics, we recruited all consecutive patients; and in large clinics, we collected a systematic random sample of patients. We aimed for approximately 100 patients per clinic to reliably represent the patient experience at the clinic.7,8 Study participants were residents in the local health network territory, self-identified regular patients of the clinic, consulting for themselves, aged between 25 and 75, and able to respond to written and oral questions in English or French.
Data collection
At recruitment, eligible and consenting participants completed a brief questionnaire eliciting basic sociodemographic information, health care use, and a brief report of their visit. They were given a longer self-administered questionnaire, with a stamped addressed envelope, that elicited information about their current health, health behaviour patterns, and usual primary health care experience in the previous year. To enhance response rates, we sent 4 reminders by e-mail or mail.9
To examine health profiles, we used validated measurement tools: the Disease Burden Morbidity Assessment,10 the 6-item Kessler (K6) scale of psychological distress,11 and the 12-item Short Form Health Survey of functional health.12 Multimorbidity was defined as 3 or more conditions from the Disease Burden Morbidity Assessment list of 21 chronic conditions. We assessed health behaviour patterns with short validated measures of physical activity13 and fruit and vegetable consumption,14 as well as questions about smoking and alcohol use.15 We elicited patient reasons for the visit and patient experience with the visit using evaluation questionnaires such as the Patient Perception of Patient-Centred Care16 and the Patient Enablement Instrument.17
Analysis
General descriptive statistics were used to create a baseline portrait of patients in the waiting room, and averages by clinic were used to capture how waiting room portraits varied among clinics. Confidence intervals were adjusted for clustering of patients within clinics using hierarchical regression models (HLM 7 software).18
RESULTS
We recruited 1029 adult patients; there were between 64 and 121 patients per clinic. Of these eligible and consenting patients, 76.7% returned their questionnaires (789 of 1029) and formed the main basis for this analysis.
Patient characteristics
The demographic profile of the adults in the waiting rooms included predominantly women (67.8%, 95% CI 64.2% to 71.5%) and the mean age was 53.0 years (95% CI 51.8 to 54.1 years). We observed variation among clinics in both sex and age distribution (Table 1). Patient levels of education varied among clinics (range was 25.5% to 50.0% for those with higher than secondary education), as did patient financial status (21.4% to 42.4% self-reported as “poor to squeezed or tight”). For patients with complementary private health insurance, the range among the clinics was between 44.3% and 78.2% for medical prescriptions and between 30.0% and 60.6% for psychological services.
Table 1.
Profile of the sociodemographic characteristics of the study population as a whole and clinic means within clinics
| CHARACTERISTIC |
STUDY POPULATION (N = 789), N (%)* |
CLINIC MEAN (95% CI) OBTAINED BY MULTILEVEL ANALYSIS (N = 12), % |
MINIMUM AND MAXIMUM CLINIC MEANS, % |
|---|---|---|---|
| Female sex | 535 (67.8) | 67.8 (64.2–71.5) | 46.5–73.8 |
| Age, y | |||
| • < 40 | 139 (17.8) | 17.8 (14.5–21.0) | 8.2–27.9 |
| • 40–49 | 141 (18.0) | 18.2 (13.6–22.8) | 8.2–27.9 |
| • 50–59 | 236 (30.2) | 30.1 (25.1–35.1) | 14.6–42.9 |
| • ≥ 60 | 266 (34.0) | 33.9 (28.7–39.0) | 23.0–50.0 |
| Highest level of education | |||
| • Secondary education or lower | 416 (54.1) | 54.4 (48.1–60.7) | 41.0–74.6 |
| • Higher than secondary education | 353 (45.9) | 45.6 (39.3–51.9) | 25.5–59.0 |
| Perceived financial situation | |||
| • Poor to squeezed or tight | 236 (30.7) | 30.7 (27.0–34.3) | 21.4–42.4 |
| • Quite comfortable | 306 (39.7) | 39.7 (35.9–43.6) | 29.8–44.9 |
| • Comfortable or very comfortable | 228 (29.6) | 29.6 (26.0–33.2) | 21.4–40.5 |
| Have complementary health insurance† | |||
| • Medication coverage | 487 (62.8) | 62.8 (55.5–70.0)‡ | 44.3–78.2 |
| • Psychological services | 352 (48.1) | 48.2 (41.3–55.0) | 30.0–60.6 |
Not all respondents answered all questions.
More than 1 answer was possible.
Statistically significant variation at clinical level was based on hierarchical linear model.
Health profile
Mean (SD) number of reported chronic conditions per patient was 3.4 (2.9); 95% CI for clinic mean was 3.1 to 3.8; and clinic means ranged from 2.4 to 4.7. Table 2 shows the strikingly high burden of chronic illness multimorbidity, with a mean (SD) of 3.4 (2.9) illnesses per person and 56.9% of adult patients with at least 3 chronic conditions. The most commonly reported chronic conditions were hypertension, arthritic disorders, hypercholesterolemia, and depression or anxiety. Although multimorbidity was highest in older adults (82.9% of those aged between 65 and 75), 15.3% of those aged between 25 and 35 had multimorbidity.
Table 2.
Chronic illnesses among the study population as a whole and clinic averages
| CHARACTERISTIC |
STUDY POPULATION (N = 789),* N (%) |
CLINIC MEAN (95% CI)† (N = 12), % |
MINIMUM AND MAXIMUM CLINIC MEANS, % |
|---|---|---|---|
| Top reported chronic conditions | |||
| • Hypertension | 272 (34.8) | 34.8 (29.9–39.6) | 22.8–49.4 |
| • Arthritic disorders‡ | 236 (30.2) | 30.2 (26.6–33.8) | 18.2–38.3 |
| • Hypercholesterolemia | 225 (28.8) | 28.8 (24.1–34.0) | 15.5–40.9 |
| • Depression or anxiety | 190 (24.3) | 24.3 (20.5–28.4) | 17.9–38.1 |
| • Thyroid disorder | 117 (15.0) | 15.0 (11.8–18.2) | 7.1–21.4 |
| • Asthma | 95 (12.2) | 12.2 (8.8–15.4) | 4.3–25.4 |
| • Diabetes | 94 (12.0) | 12.0 (7.9–16.3) | 1.8–25.4 |
| Patients with multimorbidity (≤ 3 of 21 persisting diagnoses) | 445 (56.9) | 56.9 (52.1–62.4) | 41.7–70.5 |
| Percent with high or moderate psychological distress (score of ≥ 8 on the Kessler 6-item scale) | 237 (30.3) | 30.5 (25.7–35.3) | 22.6–50.0 |
Not all respondents answered all questions.
The 95% CIs were obtained by multilevel analysis; none had statistically significant between-clinic variance.
This includes osteoarthritis and rheumatoid arthritis.
The mean (SD) score for functional health status was below the normed population score of 50 for the physical component (46.1 [11.1]). A difference of 5 points is considered to be clinically significant.19 For the physical component, those with multimorbidity had clinically significant lower scores than those without (39.2 vs 49.1; P < .001), but there was no clinically or statistically significant difference by multimorbidity for the mental health component (46.7 vs 47.3).
Although the mental health component of functional status is close to the population norm (mean [SD] 47.1 [10.6]), about a third of patients reported having at least moderate psychological distress (score of 8 or higher, the cutoff for moderate distress, on the K6 scale), with an important variance among clinics. The mean (SD) psychological distress score on the K6 was 9.6 (5.0) in those reporting a diagnosis of depression or anxiety; 63.8% of these patients were in high or moderate distress. In those patients who did not report anxiety or depression, the mean (SD) K6 score was 4.6 (4.0), but 1 in 5 of these undiagnosed patients (19.7%) reported being in high or moderate distress. Only 48.6% of patients who reported psychological distress or a diagnosis of depression or anxiety had complementary insurance for psychological services (range was 23.5% to 71.4% among clinics).
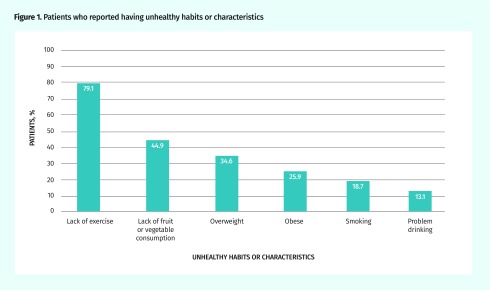
Most patients (88.0%) were not at recommended targets for at least 1 of the recommended health behaviour patterns; 56.1% were not at recommended targets for at least 2. Figure 1 shows the percentage of patients who reported having unhealthy habits or characteristics. Most patients needed to increase physical activity and daily consumption of fruits and vegetables, as well as lose weight. The prevalence of smoking was 18.7%, but another 13.5% of patients reported almost daily exposure to second-hand smoke.
Figure 1.
Patients who reported having unhealthy habits or characteristics
What happened at the visit?
Patients’ reasons for visits included consultation for routine visits (71.0%), annual checkup or pregnancy care (31.0%), and follow-up of an existing condition (40.0%). Among patients who made an appointment, 66.8% waited longer than 2 weeks. Almost one-fifth (17.6%) of patients reported having more than 1 reason for the visit. Longer appointment wait time (P=.002) and patient multimorbidity (P=.03) were significantly correlated with the number of reasons for the visit. Of the 21.0% of patients with same-day appointments (walk-in services), most of them reported a new health problem as the only reason for the visit.
When leaving the visit, 63.1% of patients had a medication prescription and half had prescriptions for more than 1 medication—a finding that was consistent across clinics (range was 53.6% to 73.3%). Only 26.8% of patients received written information about their health problem, but this proportion varied greatly among clinics (range was 14.3% to 49.2%). After their visit, 60.1% of patients had a referral for a diagnostic test (range among clinics was 42.9% to 73.2%) and 34.0% had a referral to a specialist. Following their visit, 23.8% of patients reported feeling enabled to take charge of their health problem; 32.6% felt no difference or even less enabled.
On the patient-centred communication scale, most patients gave top marks for being able to express their most important needs and sensing that the doctor listened well. They were less positive about a whole-person approach; only 28.1% reported that the doctor had explored whether the recommendations made would be realistic for them and only 18.5% reported that the doctor had addressed personal or family factors that might be affecting their health.
DISCUSSION
This snapshot of adult patients in the waiting room during a typical week in primary care clinics in Quebec points to 4 of the 10 pillars to prioritize in the PMH: timely access, comprehensive care, team-based care, and a patient-centred approach. Our findings apply principally to Quebec but they might resonate in other jurisdictions too.
First, access difficulty prioritizes the pillar of timely access. Timely access to primary health care is a persisting problem despite the introduction of alternate models designed to improve access. Our study found that most patients waited longer than 2 weeks for a scheduled appointment and that longer wait times accumulated more reasons for the visit, illustrating the importance of “doing today’s work today,” which is one of the mottoes of advanced access. In advanced access, physicians plan their schedules over a short term, reserving approximately 35% of their visits for same-day appointments by their own patients, ensuring both timely access and relational continuity.20–23 It requires considerable reorganization of clinic processes, but guides are readily available.24,25 Studies suggest that advanced access results in capacity to care for larger panel sizes, less time wastage by both physicians and patients, and higher satisfaction.26,27 Early family physician adoption of advanced access started in 2012 in Quebec,28 and deployment is accelerating, with more than 2000 physicians having received training by the Ministry of Health and Social Services in collaboration with the generalist physicians’ union.
Second, this study confirms that multimorbidity is the new normal in primary care, with more than half of adult patients presenting with 3 or more chronic conditions, echoing other findings.29,30 This snapshot reveals the complexity of the management task faced by primary care clinicians on a regular basis. The challenge of reconciling practice guidelines for multiple chronic conditions is well recognized.31,32 The exit survey showed a high level of medical management; and although the survey does not provide any insight into the appropriateness of management, it is clear that patients also face considerable complexity in managing their health. Management of multimorbidity needs to be strongly anchored in a patient-centred approach that is minimally disruptive and promotes health.33
Third, we observe a high prevalence of common mental health disorders and undetected psychological distress. One-quarter of adult patients reported a diagnosis of depression or anxiety and 1 in 5 of those without such a diagnosis presented with moderate to high levels of psychological distress. In a subsequent analysis, we found that the combination of psychological distress and multimorbidity was associated with a more-rapid-than-expected decline in functional health over time (results available on request from the corresponding author). High multimorbidity and psychological distress prioritize 3 pillars of the PMH: comprehensive care, team-based care, and patient-centredness.
Team-based care is a mechanism to achieve comprehensiveness in managing chronic disease and addressing mental health. While family physicians hone their expertise in the medical management of multimorbidity, team members can support patient education and self-management and connections to external services. Even a 2-person physician-nurse “teamlet” can build a strong comprehensive care pillar and improve outcomes.34,35 Changes in regulatory frameworks and training for nurses position them to assume a proactive role in chronic disease management care on the primary care team,36,37 even though they are not consistently working at their full scope of practice in primary care.38 Team-based and comprehensive care can also include new care modalities such as group visits, peer coaches, Web-based self-care, and e-mail communication. These modalities are challenging but not impossible to implement in traditional fee-for-service models.
Fourth, but by no means last, is the centrality of patient-centredness. Although patient-centredness is not a practice redesign issue, per se, family physicians are called to consistently practise this value. The results suggest that although family physicians tend to elicit patients’ main concerns and listen well, they do not consistently elicit how the patient’s personal and family context affects the feasibility of carrying out physician recommendations. Person-centred care is not simply a “feel-good” quality; studies show that person-centredness is associated with better symptom resolution and lower use of specialist and diagnostic services.39–41 In a follow-up study, we also showed that over the subsequent 2 years, patients reporting higher levels of patient-centred care also received more health promotion and empowerment, shared-decision making around treatment, and safer care.42
Limitations
We used validated instruments on a robust sample size and rigorous analysis. However, the data were collected in 2010 and in predominantly rural networks in Quebec, so the findings might not be generalizable to the current state in Quebec or to the rest of Canada. Since 2010, Quebec has moved toward advanced access and increased multidisciplinarity of the primary care team, structural reforms that are favourable to the PMH.
Conclusion
While all pillars are critical in the PMH, this waiting room snapshot suggests that 4 of them might be a priority. Widespread adoption of advanced access will ensure timely access without sacrificing relational continuity of care. Comprehensive and person-focused management of multimorbidity and psychological distress requires team-based care—even a physician-nurse “teamlet”—with all providers in various disciplines working at their full scope of practice. Finally, we offer this study as an example of the PMH pillar of evaluation to support continuous quality improvement in primary care. We hope that our findings will provoke reflection among family physicians and encourage regular monitoring of these dimensions in future studies and quality improvement initiatives.
Acknowledgments
The research study was funded by the Canadian Health Services Research Foundation (now called the Canadian Foundation for Healthcare Improvement). During the study, Dr Haggerty was Canada Research Chair at the University of Sherbrooke. Dr Fortin held the Applied Chair in Health Services and Policy Research on Chronic Diseases in Primary Care, funded by the Canadian Institutes for Health Research from 2009 to 2013 and endowed by the Chicoutimi Health and Social Services Centre. During the study, Dr Breton was a postdoctoral fellow funded by the Canadian Health Services Research Foundation. The study and analysis was conducted by research teams under the supervision of Drs Haggerty and Fortin. Drs Haggerty and Fortin want to particularly acknowledge the study coordinators, Christine Beaulieu and Marie-Ève Poitras, and the statistician Fatima Bouharaoui, and they also want to acknowledge the guidance and advice from their decision-maker partners, especially from Dr Jean Rodrigue, Director of Medical Affairs for the health authority of Montérégie and the principal knowledge user for the study.
Editor’s key points
▸ There is a need for primary care practice redesign. This study invites reflection on which of the Patient’s Medical Home (PMH) pillars should be prioritized to help strengthen the redesign of primary care practices.
▸ This snapshot of a typical week in primary care clinics in Quebec showed that patients have difficulty accessing care (eg, most patients waited longer than 2 weeks for appointments); that the prevalence of multimorbidity and psychological distress among patients is high, revealing the management complexity that family physicians face on a regular basis; and that family physicians do not consistently provide patient-centred care.
▸ To align primary care practices with the PMH model, this study found that timely access, team-based care, comprehensive care, and a patient-centred approach were the 4 priority PMH pillars. Although this study’s findings apply principally to Quebec, they might resonate in other jurisdictions too.
Points de repère du rédacteur
▸ Il est nécessaire de restructurer la pratique des soins primaires. Cette étude incite à une réflexion sur l’identification des piliers du Centre de médecine de famille (CMF) qu’il faut prioriser pour renforcer la restructuration de la pratique des soins primaires.
▸ Ce portrait d’une semaine typique dans les cliniques de soins primaires au Québec a révélé que les patients éprouvent des difficultés à accéder à des soins (p. ex. la plupart des patients ont dû attendre plus de 2 semaines pour avoir un rendez-vous); que la prévalence de la multimorbidité et de la détresse psychologique chez les patients est élevée, ce qui met en évidence la complexité à laquelle les médecins de famille sont confrontés sur une base régulière dans la prise en charge de leurs patients; et que les médecins de famille ne dispensent pas de manière uniforme des soins centrés sur le patient.
▸ Cette étude a fait valoir que l’accès en temps opportun, les soins en équipe, les soins complets et une approche centrée sur le patient sont les 4 piliers prioritaires du CMF pour que la pratique des soins primaires concorde avec ce modèle. Bien que les constatations de cette étude s’appliquent principalement au Québec, elles pourraient aussi s’appliquer à d’autres régions.
Footnotes
Contributors
All authors made significant contributions to the conception and design of the study, the interpretation of results, and drafting of the manuscript. Drs Haggerty and Fortin were principal investigators of the research study and were very involved in every aspect of the study, including the acquisition of data. Dr Breton contributed to the interpretation of results. Dr Haggerty drafted the initial version and took final responsibility for the manuscript, but it was extensively commented on by Drs Fortin and Breton and they have approved the final version.
Competing interests
None declared
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
References
- 1.College of Family Physicians of Canada. A vision for Canada. Family practice: the Patient’s Medical Home. Mississauga, ON: College of Family Physicians of Canada; 2011. Available from: http://patientsmedicalhome.ca/files/uploads/PMH_A_Vision_for_Canada.pdf. Accessed 2018 Jul 10. [Google Scholar]
- 2.Schoen C, Osborn R, Huynh PT, Doty M, Davis K, Zapert K, et al. Primary care and health system performance: adults’ experiences in five countries. Health Aff (Millwood) 2004;Suppl Web exclusive:W4-487–503. doi: 10.1377/hlthaff.w4.487. [DOI] [PubMed] [Google Scholar]
- 3.Schoen C, Osborn R, Doty MM, Bishop M, Peugh J, Murukutla N. Toward higher-performance health systems: adults’ health care experiences in seven countries, 2007. Health Aff (Millwood) 2007;26(6):w717–34. doi: 10.1377/hlthaff.26.6.w717. [DOI] [PubMed] [Google Scholar]
- 4.Squires D. International profiles of health care systems: Australia, Canada, Denmark, England, France, Germany, Italy, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and the United States. New York, NY: Commonwealth Fund; 2010. [Google Scholar]
- 5.Osborn R, Moulds D, Squires D, Doty MM, Anderson C. International survey of older adults finds shortcomings in access, coordination, and patient-centered care. Health Aff (Millwood) 2014;33(12):2247–55. doi: 10.1377/hlthaff.2014.0947. Epub 2014 Nov 19. [DOI] [PubMed] [Google Scholar]
- 6.Haggerty J, Fortin M, Beaulieu MD, Hudon C, Loignon C, Préville M, et al. At the interface of community and healthcare systems: a longitudinal cohort study on evolving health and the impact of primary healthcare from the patient’s perspective. BMC Health Serv Res. 2010;10:258. doi: 10.1186/1472-6963-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britt H, Miller GC, Charles J, Pan Y, Valenti L, Henderson J, et al. General practice activity in Australia 2005–06. Canberra, Aust: University of Sydney and the Australian Institute of Health and Welfare; 2007. General practice series no. 19. [Google Scholar]
- 8.Lyratzopoulos G, Elliott MN, Barbiere JM, Staetsky L, Paddison CA, Campbell J, et al. How can health care organizations be reliably compared? Lessons from a national survey of patient experience. Med Care. 2011;49(8):724–33. doi: 10.1097/MLR.0b013e31821b3482. [DOI] [PubMed] [Google Scholar]
- 9.Dillman DA. Mail and Internet surveys: the tailored design method. 2nd ed. New York, NY: Wiley; 2000. [Google Scholar]
- 10.Bayliss EA, Ellis JL, Steiner JF. Subjective assessments of comorbidity correlate with quality of life health outcomes: initial validation of a comorbidity assessment instrument. Health Qual Life Outcomes. 2005;3:51. doi: 10.1186/1477-7525-3-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32(6):959–76. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- 12.Kosinksi M, Ware JE, Turner-Bowker DM, Gandek B. User’s manual for the SF-v2 health survey: with a supplement documenting the SF-12 health survey. Lincoln, RI: QualityMetric Inc; 2007. [Google Scholar]
- 13.Institut national de santé publique du Québec. Portrait de santé du Québec et de ses régions 2006. Deuxième rapport national sur l’état de santé de la population du Québec. Quebec, QC: Gouvernement du Québec; 2006. [Google Scholar]
- 14.Resnicow K, Odom E, Wang T, Dudley WN, Mitchell D, Vaughan R, et al. Validation of three food frequency questionnaires and 24-hour recalls with serum carotenoid levels in a sample of African-American adults. Am J Epidemiol. 2000;152(11):1072–80. doi: 10.1093/aje/152.11.1072. [DOI] [PubMed] [Google Scholar]
- 15.Laforge RG, Velicer WF, Richmond RL, Owen N. Stage distributions for five health behaviors in the United States and Australia. Prev Med. 1999;28(1):61–74. doi: 10.1006/pmed.1998.0384. [DOI] [PubMed] [Google Scholar]
- 16.Stewart M, Belle Brown J, Weston WW, McWhiney IR, McWilliam CL, Freeman T. Patient-centered medicine. Transforming the clinical method. Oxford, UK: Radcliffe Publishing; 2003. [Google Scholar]
- 17.Howie JG, Heaney DJ, Maxwell M, Walker JJ, Freeman GK. Developing a ‘consultation quality index’ (CQI) for use in general practice. Fam Pract. 2000;17(6):455–61. doi: 10.1093/fampra/17.6.455. [DOI] [PubMed] [Google Scholar]
- 18.Raudenbush SW, Byrk AS, Cheong YF, Congdon R. HLM 5: hierarchical linear and nonlinear modeling. Lincolnwood, IL: Scientific Software International, Inc; 2001. [Google Scholar]
- 19.Ware JE, Jr, Kemp JP, Buchner DA, Singer AE, Nolop KB, Goss TF. The responsiveness of disease-specific and generic health measures to changes in the severity of asthma among adults. Qual Life Res. 1998;7(3):235–44. doi: 10.1023/a:1024946316424. [DOI] [PubMed] [Google Scholar]
- 20.Murray M, Tantau C. Same-day appointments: exploding the access paradigm. Fam Pract Manag. 2000;7(8):45–50. [PubMed] [Google Scholar]
- 21.Murray M, Bodenheimer T, Rittenhouse D, Grumbach K. Improving timely access to primary care: case studies of the advanced access model. JAMA. 2003;289(8):1042–6. doi: 10.1001/jama.289.8.1042. [DOI] [PubMed] [Google Scholar]
- 22.Pope C, Banks J, Salisbury C, Lattimer V. Improving access to primary care: eight case studies of introducing advanced access in England. J Health Serv Res Policy. 2008;13(1):33–9. doi: 10.1258/jhsrp.2007.007039. [DOI] [PubMed] [Google Scholar]
- 23.Schall MW, Duffy T, Krishnamurthy A, Levesque O, Mehta P, Murray M, et al. Improving patient access to the Veterans Health Administration’s primary care and specialty clinics. Jt Comm J Qual Saf. 2004;30(8):415–23. doi: 10.1016/s1549-3741(04)30047-x. [DOI] [PubMed] [Google Scholar]
- 24.College of Family Physicians of Canada. Best advice. Timely access to appointments in family practice. Mississauga, ON: College of Family Physicians of Canada; 2012. [Google Scholar]
- 25.Institute for Healthcare Improvement [website]. Primary care access. Boston, MA: Institute for Healthcare Improvement; 2018. Available from: www.ihi.org/Topics/PrimaryCareAccess/Pages/default.aspx. Accessed 2018 Jul 10. [Google Scholar]
- 26.Bundy DG, Randolph GD, Murray M, Anderson J, Margolis PA. Open access in primary care: results of a North Carolina pilot project. Pediatrics. 2005;116(1):82–7. doi: 10.1542/peds.2004-2573. [DOI] [PubMed] [Google Scholar]
- 27.Hudec JC, MacDougall S, Rankin E. Advanced access appointments. Effects on family physician satisfaction, physicians’ office income, and emergency department use. Can Fam Physician. 2010;56:e361–7. Available from: www.cfp.ca/content/cfp/56/10/e361.full.pdf. Accessed 2018 Jul 10. [PMC free article] [PubMed] [Google Scholar]
- 28.Breton M, Maillet L, Paré I, Abou Malham S, Touati N. Perceptions of the first family physicians to adopt advanced access in the province of Quebec, Canada. Int J Health Plann Manage. 2017;32(4):e316–32. doi: 10.1002/hpm.2380. Epub 2016 Sep 8. [DOI] [PubMed] [Google Scholar]
- 29.Stewart M, Fortin M, Britt HC, Harrison CM, Maddocks HL. Comparisons of multi-morbidity in family practice—issues and biases. Fam Pract. 2013;30(4):473–80. doi: 10.1093/fampra/cmt012. Epub 2013 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10(2):142–51. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace E, Salisbury C, Guthrie B, Lewis C, Fahey T, Smith SM. Managing patients with multimorbidity in primary care. BMJ. 2015;350:h176. doi: 10.1136/bmj.h176. [DOI] [PubMed] [Google Scholar]
- 32.Fortin M, Contant E, Savard C, Hudon C, Poitras ME, Almirall J. Canadian guidelines for clinical practice: an analysis of their quality and relevance to the care of adults with comorbidity. BMC Fam Pract. 2011;12:74. doi: 10.1186/1471-2296-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. doi: 10.1136/bmj.b2803. [DOI] [PubMed] [Google Scholar]
- 34.Bodenheimer T, Pham HH. Primary care: current problems and proposed solutions. Health Aff (Millwood) 2010;29(5):799–805. doi: 10.1377/hlthaff.2010.0026. [DOI] [PubMed] [Google Scholar]
- 35.Chen EH, Thom DH, Hessler DM, Phengrasamy L, Hammer H, Saba G, et al. Using the teamlet model to improve chronic care in an academic primary care practice. J Gen Intern Med. 2010;25(Suppl 4):S610–4. doi: 10.1007/s11606-010-1390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canadian Nurses Association. Position statement: interprofessional collaboration. Ottawa, ON: Canadian Nurses Association; [Google Scholar]
- 37.Martin-Misener R, Bryant-Lukosius D. Optimizing the role of nurses in primary care in Canada. Final report. Ottawa, ON: Canadian Nurses Association; 2014. [Google Scholar]
- 38.Commissaire à la santé et au bien-être. Rapport d’appréciation de la performance du système de santé et de services sociaux 2010. L’appréciation globale et intégrée de la performance: analyse des indicateurs de monitorage. Quebec, QC: Commissaire à la santé et au bien-être; 2010. [Google Scholar]
- 39.Stewart M, Brown JB, Donner A, McWhinney IR, Oates J, Weston WW, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796–804. [PubMed] [Google Scholar]
- 40.Little P, Everitt H, Williamson I, Warner G, Moore M, Gould C, et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7381):908–11. doi: 10.1136/bmj.323.7318.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dwamena F, Holmes-Rovner M, Gaulden CM, Jorgenson S, Sadigh G, Sikorskii A, et al. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database Syst Rev. 2012;(12):CD003267. doi: 10.1002/14651858.CD003267.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouharaoui F, Haggerty JL, Hudon C, Fortin M. Patient-centered care: more than feel-good care. Toronto, ON: Canadian Association for Health Services and Policy Research; 2014. Available from: http://cahspr.ca/en/conferences/past/2014/abstracts/poster. Accessed 2018 Aug 22. [Google Scholar]