Abstract
Study objective:
The aim of this study is to evaluate the efficacy of hormonal therapies for inhibiting an increase in uterine volume in patients with adenomyosis.
Design:
This was retrospective cohort study.
Setting:
This study was conducted at Nippon Medical School Musashikosugi Hospital.
Patients:
A total of 28 women diagnosed with adenomyosis using magnetic resonance imaging.
Methods:
After providing informed consent, patients were treated with gonadotropin-releasing hormone agonist (GnRHa group), a low-dose estrogen and progestin combination (LEP group), or dienogest (DNG group) for ≥16 weeks. Uterine volume was assessed using the formula for an ovoid; uterine volumes before and after 16 weeks of treatment were compared. A <5% increase in uterine volume at 16 weeks was considered to reflect inhibition of uterine volume increase and efficacy of the medication. We compared the efficacy rate among the groups.
Results:
In the GnRHa group, a significant reduction in uterine volume was noted, from 307.4 ± 230.1 to 177.9 ± 142.1 cm3 (P < 0.001). In the LEP and the DNG groups, there was no significant change (LEP: 226.7 ± 116.6 cm3 pre-treatment and 230.5 ± 128.6 cm3 post-treatment, P = 0.85; DNG: 232.6 ± 117.8 cm3 pre-treatment and 262.1 ± 136.8 cm3 post-treatment, P = 0.37). The number of responders (efficacy rate) in the GnRHa group, LEP group, and DNG group was 25/26 (96.2%), 7/15 (46.7%), and 6/11 (54.5%), respectively. The efficacy rate of GnRHa therapy was significantly higher than that of LEP or DNG therapy (P < 0.001 and P = 0.005, respectively).
Conclusion:
We conclude that the efficacy of GnRHa in reducing uterine volume should be considered when prescribing hormone therapy for adenomyosis.
Keywords: Adenomyosis, ethinyl estradiol, ferrous fumarate drug combination, dienogest, gonadotropin-releasing hormone, norethindrone acetate
INTRODUCTION
Adenomyosis has similarities to endometriosis, but it reveals clinically different characteristics.[1] Hormonal therapies effective in the treatment of patients with endometriosis are also generally effective for treating patients with adenomyosis.[2] These hormonal therapies are all comparatively effective for the treatment of menstrual pain, but the results have been inconsistent with regard to preventing an increase in uterine volume, and patients have experienced mixed results with regard to uterine enlargement.
It was reported that a large uterine volume could be a potential risk factor for an increase in the occurrence of moderate-to-severe lower urinary tract symptoms with adenomyosis.[3] It was also reported that larger tumors were more often complicated by deep venous thrombosis (DVT) in patients with uterine fibroids.[4] In our experience, a large uterus due to adenomyosis may lead to DVT.[5] A severe increase in uterine volume causes not only abdominal discomforts such as flatulence but also a visibly distended abdomen, which is esthetically displeasing to patients. In addition, uterine volume reduction by preoperative treatment with hormonal therapies leads to a decrease in blood loss during surgery and an improvement in the safety of surgery.[6] Furthermore, uterine volume reduction may enable the use of laparoscopic hysterectomy instead of laparotomy.
Therefore, preventing an increase in uterine volume should be one of the most important aspects of care in this condition. Till date, there have been a few reports regarding the efficacy of hormonal therapies used to treat endometriosis, including gonadotropin-releasing hormone agonist (GnRHa), low-dose estrogen-progestin (LEP) combinations, and dienogest (DNG)[7,8,9] on uterine volume. In addition, there have been no studies of how frequently these therapies inhibit an increase in uterine volume. Therefore, we conducted a retrospective cohort study to clarify the efficacy of hormonal therapies generally used for the treatment of patients with endometriosis for preventing an increase in uterine volume in patients with adenomyosis.
Purpose
The purpose was to evaluate the efficacy of hormonal therapies for inhibiting an increase in uterine volume in patients with adenomyosis.
MATERIALS AND METHODS
We conducted a retrospective cohort study of 28 patients who visited the Department of Obstetrics and Gynecology, Nippon Medical School Musashikosugi Hospital between August 2007 and July 2015 who had been diagnosed with adenomyosis with magnetic resonance imaging (MRI). After receiving detailed counseling and information on three kinds of hormonal therapy, the decision to use GnRHa, LEP, or DNG was made by the patients. The patients were administered one of the following therapies based on their decision as follows: GnRHa (GnRHa group), LEP (LEP group), and DNG group. Each hormonal therapy was administered for at least 16 weeks. Patients who had undergone hormonal therapy within 6 months before starting treatment and patients with multiple uterine fibroids measuring ≥2 cm in diameter were excluded from the study. This study was approved by the Institutional Review Board at Nippon Medical School Musashikosugi Hospital (#368-28-65).
Data for the study were obtained from patient medical records. Menorrhagia was assessed subjectively by asking the patients whether they felt their periods were excessively heavy or not. Dysmenorrhea was assessed by asking the patients if they experienced the pain of Grade 2 or 3 on the Andersch and Milsom scale.[10] Pain grades were defined as follows: Grade 2 pain affects daily activity, requires analgesics, can be sufficiently relieved so absence from work is unusual, and is described as “moderate pain;” Grade 3 pain clearly inhibits daily activity, is not relieved by analgesics, is associated with vegetative symptoms (e.g., headache, tiredness, nausea, vomiting, and diarrhea), and is described as “severe pain.” Chronic pelvic pain is nonmenstrual pelvic pain of 6 or more months duration severe enough to cause functional disability or require medical or surgical treatment.[11] The blood data (carcinoma antigen [CA] 125 and hemoglobin levels) were recorded when menstruation was not occurring and before hormonal therapy was started.
The administration regimen for GnRHa was 1.88 mg of leuprorelin acetate or 1.8 mg of goserelin acetate started on day 5 of the menstrual cycle and injected subcutaneously every 4 weeks. LEP was administered as a norethindrone 1 mg/ethinyl estradiol 0.035 mg combination (Lunabell® combination tablets LD, Nippon-Shinyaku., Kyoto, Japan), one tablet orally daily starting on day 1–5 of the menstrual cycle and continuing for 21 consecutive days followed by a 7-day break. DNG (Dinagest,® Mochida Pharmaceutical Co., Ltd., Tokyo, Japan) was administered as 1 mg DNG tablets, two tablets daily on days 2 through 5 of the menstrual cycle without interruption for at least 4 months. We checked for the existence of menorrhagia, dysmenorrheal and chronic pelvic pain, and serum CA 125 levels before and after treatment.
Ultrasonography was used to compare uterine volume, which was calculated by measuring the length of the long- and short-axis of the sagittal section and the long-axis of the cross-section of the uterus. Uterine volume was assessed using the formula for an ovoid (length × width × depth × 0.52).[12] The calculated results were evaluated comparing the uterine volume within each group before treatment and after 16 weeks of treatment. When the uterine volume had increased by <5% compared to the volume before starting treatment, it was determined that an increase in volume had been inhibited (effective treatment), and the incidence of this observation was set as the efficacy rate. We then compared the efficacy rate among each of the three treatment groups.
IBM® (IBM, New York, United States) SPSS® Statistics version 21 was used for the statistical analysis. One-way analysis of variance and the Kruskal–Wallis test were used to compare pretreatment values between each group. Fisher's exact test was used for evaluating the efficacy rate in the LEP and DNG groups, using the GnRHa group as the control. P < 0.05 was considered to be a statistically significant difference.
RESULTS
Twenty-six of the study participants were administered GnRHa for ≥16 weeks (GnRHa group), 15 participants were administered LEP for ≥16 weeks (LEP group), and 11 participants were administered DNG for ≥16 weeks (DNG group). There were no significant differences among the three groups regarding age; body mass index; parity; incidence of menorrhagia, dysmenorrhea, or chronic pelvic pain; pretreatment CA 125 levels; or hemoglobin levels [Table 1]. We showed the results of existence of menorrhagia, dysmenorrheal and chronic pelvic pain, and CA 125 levels before and after treatment are shown in Table 2. CA 125 levels were significantly decreased after treatment in all groups in comparison with before treatment.
Table 1.
Baseline patient characteristics in the gonadotropin-releasing hormone agonist, low-dose estrogen-progestin combination, and dienogest treatment groups
| GnRHa (26) | LEP (15) | DNG (11) | P | |
|---|---|---|---|---|
| Age (years)a | 40.0±6.1 | 37.7±5.3 | 38.9±7.8 | NS |
| BMI (kg/m2)a | 21.8±3.0 | 23.4±6.3 | 24.1±6.8 | NS |
| Paritya | 0.8±0.9 | 0.4±0.6 | 0.5±0.7 | NS |
| Menorrhagiab | 16 (61.5) | 9 (56.3) | 4 (36.4) | NS |
| Dysmenorrheab | 9 (34.6) | 9 (56.3) | 2 (18.2) | NS |
| Chronic pelvic painb | 6 (23.1) | 2 (12.5) | 4 (36.4) | NS |
| CA125 (U/mL)a | 203.1±163.8 | 237.5±162.8 | 255.9±181.3 | NS |
| Hgb (g/dL)a | 10.1±2.3 | 10.5±2.4 | 11.1±2.2 | NS |
aValues are given as mean±SD, bValues are given as n (%). GnRHa: Gonadotropin-releasing hormone agonist, LEP: Low-dose estrogen-progestin combination, DNG: Dienogest, BMI: Body mass index, CA125: Carcinoma antigen 125, Hgb: Hemoglobin, NS: Not significant, SD: Standard deviation
Table 2.
Changes of symptoms and biomarker in the gonadotropin-releasing hormone agonist, low-dose estrogen-progestin combination, and dienogest treatment groups
| GnRHa (26) | LEP (15) | DNG (11) | ||||
|---|---|---|---|---|---|---|
| Before | After 16 weeks of treatment | Before | After 16 weeks of treatment | Before | After 16 weeks of treatment | |
| Menorrhagiab | 16 (61.5) | 0 | 9 (56.3) | 7 (46.7) | 4 (36.4) | 2 (18.2) |
| Dysmenorrheab | 9 (34.6) | 0 | 9 (56.3) | 6 (40.0) | 2 (18.2) | 0 |
| Chronic pelvic painb | 6 (23.1) | 1 (3.8) | 2 (12.5) | 1 (6.3) | 4 (36.4) | 2 (18.2) |
| CA125 (U/mL)a | 203.1 | 36.5* | 237.5 | 25.2* | 255.9 | 26.4* |
aValues are given as mean, bValues are given as n (%), *P<0.01. GnRHa: Gonadotropin-releasing hormone agonist, LEP: Low-dose estrogen-progestin combination, DNG: Dienogest, CA125: Carcinoma antigen 125
The mean uterine volume before treatment in the GnRHa group was 307.4 ± 230.1 cm3, and the mean volume after 16 weeks of treatment was 177.9 ± 142.1 cm3, a statistically significant reduction (P < 0.001). The mean uterine volume before treatment in the LEP group was 226.7 ± 116.6 cm3, and the mean volume after 16 weeks of treatment was 230.5 ± 128.6 cm3, a slight nonsignificant increase (P = 0.85). The mean uterine volume before treatment in the DNG group was 232.6 ± 117.8 cm3, and the mean volume after 16 weeks of treatment was 262.1 ± 136.8 cm3, a slight nonsignificant increase (P = 0.37). About the uterine volume before and after treatment, the GnRHa group showed a significant reduction in uterine volume after treatment [Figure 1].
Figure 1.
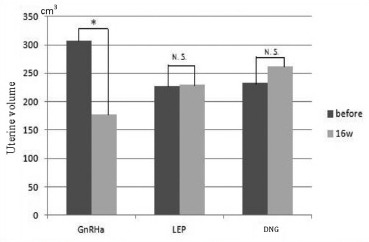
Uterine volume in each treatment group before and after 16 weeks of hormonal therapy. Uterine volumes before and after administration of each hormonal treatment indicates that uterine volume was significantly reduced in the GnRHa group, but in the LEP and DNG groups, there was a slight increase in uterine volume after treatment, but this increase was not statistically significant. *: P < 0.001
| Uterine volume | Before treatment (cm3) | After 16 weeks of treatment (cm3) | P |
|---|---|---|---|
| GnRHa (n=26) | 307.4±230.1 | 177.9±142.1 | <0.001 |
| LEP (n=15) | 226.7±116.6 | 230.5±128.6 | 0.85 |
| DNG (n=11) | 232.6±117.8 | 262.1±136.8 | 0.36 |
Value: Mean±SD. GnRHa: Gonadotropin-releasing hormone agonist, LEP: Low-dose estrogen-progestin combinations, DNG: Dienogest, SD: Standard deviation
The efficacy rate for the GnRHa group, LEP group, and DNG group was 96.2% (25/26), 46.7% (7/15), and 54.5% (6/11), respectively; therefore, the efficacy rate was significantly higher in the GnRHa group compared to that seen in the LEP and DNG groups (P < 0.001 and 0.005, respectively) [Figure 2].
Figure 2.
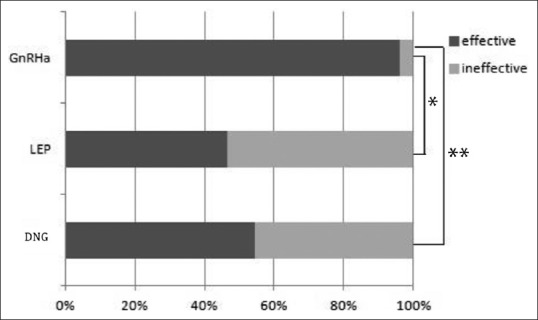
Efficacy rate for each hormonal treatment group. The GnRHa group had a significantly higher efficacy rate than that seen in the LEP and DNG groups (P < 0.001, P = 0.005). Efficacy ratio. GnRHa: 96.2% (25/26). LEP: 46.7% (7/15)*, DNG: 54.5% (6/11)**, *<0.001, ** = 0.005. GnRHa: Gonadotropin-releasing hormone agonist, LEP: Low-dose estrogen-progestin combinations, DNG: Dienogest
DISCUSSION
Nowadays medical therapy shows increasing efficacy in patients complaining of symptoms or requiring fertility treatments. However, no drug is currently labeled for adenomyosis, and there are no specific guidelines to follow for best management.[13] Two important observations can be made from this study. First, GnRHa therapy significantly reduced the volume of adenomyosis, while LEP or DNG therapy resulted in neither a significant reduction nor increase in uterine volume. Second, the efficacy rate of GnRHa therapy (96.2%) in reducing uterine volume was significantly higher than the efficacy rates of LEP or DNG therapy (46.7% and 54.5%, respectively).
As previously mentioned, severely increased uterine volume causes abdominal discomfort, with symptoms including flatulence, distended abdomen, and lower urinary tract symptoms with adenomyosis.[3] Furthermore, a larger uterus may lead to DVT. When surgical intervention is necessary, total laparoscopic hysterectomy (TLH) is the preferred surgical treatment, and it has been found that reducing the uterine volume can expand the indications for and reduce the difficulty of TLH.[14] Therefore, preventing an increase in uterine volume should be considered as a high priority in the treatment of this condition. Previous reports have shown that GnRHa significantly reduces adenomyosis volume,[7] but there have been no definitive reports on the effects of LEP therapy on uterine volume in patients with adenomyosis. DNG has been reported to be effective for treating symptomatic adenomyosis but ineffective for reducing uterine volume.[8] The results of our study validate the findings of previous studies. The study data reveal that GnRHa may be the only effective hormonal therapy for patients with subjective symptoms of abdominal distension. Conversely, all hormonal therapies in this study were effective for the subjective symptoms of menorrhagia, dysmenorrheal, and chronic pelvic pain in most cases [Table 2]. Additional studies are necessary to establish the role of LEP and DNG for inhibiting an increase in uterine volume in patients with adenomyosis. Adenomyosis can be associated with abnormalities of coagulation and fibrinolysis,[15] and previous reports found that the administration of LEP for adenomyosis can cause cerebral venous sinus thrombosis;[16] therefore, the treatment of adenomyosis with LEP requires close monitoring for complications such as thrombosis. The administration of DNG for adenomyosis can also pose the risk of excessive bleeding;[17] thus, DNG therapy for adenomyosis should be cautiously administered in patients with comparatively large uterine volumes and in patients presenting with pretreatment anemia.[18] The present study suggests that GnRHa should be the first-line therapy in patients with comparatively large adenomyosis.
To the best of our knowledge, our study is the first to report the efficacy rate of GnRHa, LEP, and DNG for reducing uterine volume in patients with adenomyosis. Despite the clear efficacy of LEP and DNG for the treatment of subjective symptoms,[8,9] these hormonal therapies are ineffective for reducing uterine volume and frequently, patients may even experience an increase in uterine volume. In this study, the uterus enlarged in eight of 15 patients in the LEP group and five of 11 patients in the DNG group. For the LEP and DNG groups, these therapies were ineffective for reducing uterine volume in 53.3% and 44.5% of patients, respectively. The largest increase in uterine volume with LEP treatment was 2-fold (pretreatment, 187.2 cm3; after 16 weeks of LEP therapy, 385.2 cm3), and one patient experienced a 2.1-fold increase in uterine volume with DNG therapy (pretreatment, 151.6 cm3; after 16 weeks of DNG therapy, 325.9 cm3). Most prior studies[8,9] used the posttreatment mean uterine volume value; however, when the therapeutic effects vary widely and comparing the mean values becomes meaningless. The reason for the varying therapeutic effects of LEP and DNG on uterine volume in adenomyosis, in contrast with the definitive effects of both treatments on endometriosis, is unclear. Adenomyosis reveals clinically different characteristics; although, it is similar to endometriosis.[1] The pathological differences between endometriosis and uterus adenomyosis have been empirically shown in the human leukocyte antigen system,[19] which plays a key role in the immune response, and membrane protein p63, which is associated with epithelial proliferation and differentiation.[20] Recently, four subtypes of adenomyosis assessed by MRI were shown to have different pathogeneses (endometrial invasion, endometriotic invasion, de novo metaplasia, or a heterogeneous mixture of very advanced disease).[21] The varying therapeutic effects of LEP and DNG on uterine volume in adenomyosis may be caused by differences in pathogenesis. Unfortunately, we were unable to clarify the subtypes of adenomyosis wherein LEP or DNG was effective in reducing uterine swelling. Further studies are needed to clarify the factors predicting a patient's response to hormonal therapy. Until that time, LEP and DNG treatment of patients with adenomyosis should be continued only if the treatment has been shown to be effective in reducing that patient's uterine volume.
This study had several limitations. Our results were based on a dosing period of approximately 16 weeks, but there may be patients in whom a longer period of hormonal treatment is effective for increasing uterine volume. This study also had a small number of participants, and we were unable to determine what patient characteristics were predictive of a positive or negative therapeutic response. Larger studies are needed to further clarify the patient characteristics predictive of a decrease in uterine volume in response to therapy.
CONCLUSION
GnRHa therapy showed the greatest efficacy for reducing or limiting the increase in uterine volume in patients with adenomyosis. Although approximately half of the participants in the LEP-and DNG-treated groups experienced an inhibitory effect, some participants in these two groups experienced an increase in volume. Thus, GnRHa should be selected rather than LEP or DNG to reduce uterine volume.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Editage (www.editage.jp) for English language editing.
REFERENCES
- 1.Benagiano G, Brosens I, Habiba M. Adenomyosis: A life-cycle approach. Reprod Biomed Online. 2015;30:220–32. doi: 10.1016/j.rbmo.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Pontis A, D’Alterio MN, Pirarba S, de Angelis C, Tinelli R, Angioni S, et al. Adenomyosis: A systematic review of medical treatment. Gynecol Endocrinol. 2016;32:696–700. doi: 10.1080/09513590.2016.1197200. [DOI] [PubMed] [Google Scholar]
- 3.Li T, Xu XX, Dai Y, Zhang JJ, Lang JH, Leng JH, et al. Menorrhagia and uterine volume associated with lower urinary tract symptoms in patients with adenomyosis. Chin Med J (Engl) 2017;130:1552–6. doi: 10.4103/0366-6999.208232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiota M, Kotani Y, Umemoto M, Tobiume T, Tsuritani M, Shimaoka M, et al. Deep-vein thrombosis is associated with large uterine fibroids. Tohoku J Exp Med. 2011;224:87–9. doi: 10.1620/tjem.224.87. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Iwasaki N, Ichikawa M, Mine K, Kuwabara Y, Takeshita T, et al. Successful long-term management of adenomyosis associated with deep thrombosis by low-dose gonadotropin-releasing hormone agonist therapy. Clin Exp Obstet Gynecol. 2009;36:123–5. [PubMed] [Google Scholar]
- 6.Uccella S, Cromi A, Bogani G, Casarin J, Formenti G, Ghezzi F, et al. Systematic implementation of laparoscopic hysterectomy independent of uterus size: Clinical effect. J Minim Invasive Gynecol. 2013;20:505–16. doi: 10.1016/j.jmig.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Grow DR, Filer RB. Treatment of adenomyosis with long-term GnRH analogues: A case report. Obstet Gynecol. 1991;78:538–9. [PubMed] [Google Scholar]
- 8.Hirata T, Izumi G, Takamura M, Saito A, Nakazawa A, Harada M, et al. Efficacy of dienogest in the treatment of symptomatic adenomyosis: A pilot study. Gynecol Endocrinol. 2014;30:726–9. doi: 10.3109/09513590.2014.926882. [DOI] [PubMed] [Google Scholar]
- 9.Shaaban OM, Ali MK, Sabra AM, Abd El Aal DE. Levonorgestrel-releasing intrauterine system versus a low-dose combined oral contraceptive for treatment of adenomyotic uteri: A randomized clinical trial. Contraception. 2015;92:301–7. doi: 10.1016/j.contraception.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Andersch B, Milsom I. An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol. 1982;144:655–60. doi: 10.1016/0002-9378(82)90433-1. [DOI] [PubMed] [Google Scholar]
- 11.Howard FM. Chronic pelvic pain. Obstet Gynecol. 2003;101:594–611. doi: 10.1016/s0029-7844(02)02723-0. [DOI] [PubMed] [Google Scholar]
- 12.Sheth SS, Hajari AR, Lulla CP, Kshirsagar D. Sonographic evaluation of uterine volume and its clinical importance. J Obstet Gynaecol Res. 2017;43:185–9. doi: 10.1111/jog.13189. [DOI] [PubMed] [Google Scholar]
- 13.Vannuccini S, Luisi S, Tosti C, Sorbi F, Petraglia F. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109:398–405. doi: 10.1016/j.fertnstert.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Saito A, Hirata T, Koga K, Takamura M, Fukuda S, Neriishi K, et al. Preoperative assessment of factors associated with difficulty in performing total laparoscopic hysterectomy. J Obstet Gynaecol Res. 2017;43:320–9. doi: 10.1111/jog.13198. [DOI] [PubMed] [Google Scholar]
- 15.Yamanaka A, Kimura F, Yoshida T, Kita N, Takahashi K, Kushima R, et al. Dysfunctional coagulation and fibrinolysis systems due to adenomyosis is a possible cause of thrombosis and menorrhagia. Eur J Obstet Gynecol Reprod Biol. 2016;204:99–103. doi: 10.1016/j.ejogrb.2016.07.499. [DOI] [PubMed] [Google Scholar]
- 16.Matsushima T, Akira S, Asakura H, Takesita T. Low-dose gonadotropin-releasing hormone agonist therapy (draw-back therapy) for successful long-term management of adenomyosis associated with cerebral venous and sinus thrombosis from low-dose oral contraceptive use. Clin Exp Obstet Gynecol. 2017;44:143–5. [PubMed] [Google Scholar]
- 17.Nishino K, Hayashi K, Chaya J, Kato N, Yamamuro O. Effective salvage of acute massive uterine bleeding using intrauterine balloon tamponade in a uterine adenomyosis patient on dienogest. J Obstet Gynaecol Res. 2013;39:738–41. doi: 10.1111/j.1447-0756.2012.02005.x. [DOI] [PubMed] [Google Scholar]
- 18.Nagata C, Yanagida S, Okamoto A, Morikawa A, Sugimoto K, Okamoto S, et al. Risk factors of treatment discontinuation due to uterine bleeding in adenomyosis patients treated with dienogest. J Obstet Gynaecol Res. 2012;38:639–44. doi: 10.1111/j.1447-0756.2011.01778.x. [DOI] [PubMed] [Google Scholar]
- 19.Koumantakis EE, Panayiotides JG, Goumenou AG, Ziogos EC, Margariti A, Kalapothaki V, et al. Different HLA-DR expression in endometriotic and adenomyotic lessons: Correlation with transvaginal ultrasonography findings. Arch Gynecol Obstet. 2010;281:851–6. doi: 10.1007/s00404-009-1168-z. [DOI] [PubMed] [Google Scholar]
- 20.Poli Neto OB, Ferreira HM, Ramalho LN, Rosa e Silva JC, Candido dos Reis FJ, Nogueira AA, et al. Expression of p63 differs in peritoneal endometriosis, endometriomas, adenomyosis, rectovaginal septum endometriosis, and abdominal wall endometriosis. Arch Pathol Lab Med. 2007;131:1099–102. doi: 10.5858/2007-131-1099-EOPDIP. [DOI] [PubMed] [Google Scholar]
- 21.Kishi Y, Suginami H, Kuramori R, Yabuta M, Suginami R, Taniguchi F, et al. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol. 2012;207:114.e1–7. doi: 10.1016/j.ajog.2012.06.027. [DOI] [PubMed] [Google Scholar]


