Abstract
Objective:
To evaluate the effects of different treatment methods for previous ectopic pregnancies (EP) on cryopreserved embryo transfer (CET) outcomes.
Materials and Methods:
This was a retrospective cohort study. Patients with EP histories were divided into four groups based on their previous EP treatments: Group 1–unilateral tubal removal; Group: 2–bilateral tubal removal or unilateral tubal removal with contralateral tubal ligation; Group: 3–conservative surgery group; and Group 4–conservative medication group. A total of 1333 women with previous histories of being admitted to the hospital for CET treatment were consecutively enrolled between January 2009 and December 2014.
Results:
Patients who underwent bilateral tubal ligation or removal had a lower miscarriage rate [8.88% vs. 3.46%, p = 0.006, odds ratio = 2.718, 95% confidence interval (CI) = 1.301–5.677] than those who underwent unilateral tubal removal. No significant difference was observed in the rate of EP after CET in the four groups in women with EP histories. (p1 = 0.258, 95%CI = 0.113–1.836; p2 = 0.137, 95%CI = 0.975–0.997; p3 = 0.314, 95%CI = 0.987–1.001; p4 = 0.198, 95%CI = 0.987–1.001). The groups were not different with regard to other pregnancy outcomes.
Conclusion:
There was no significant difference among EP treatment methods with regard to their impacts on CET outcomes in women with EP histories. Bilateral tubal ligation or removal surgery can decrease the miscarriage rate after CET.
Keywords: cohort study, cryopreserved embryo transfer, ectopic pregnancy, in vitro fertilization
Introduction
Ectopic pregnancy (EP) is a dangerous complication during early pregnancy. Pioneer studies have found that both previous EP and in vitro fertilization and embryo transfer (IVF-ET) are risk factors for EP recurrence.1,2,3,4,5,6,7,8,9,10 Thus, it can be speculated that women with EP histories who undergo IVF-ET have a higher risk of EP recurrence than other women. In general, there are three ways to cope with EP–medical treatment by methotrexate (MTX) injection, radical surgery (salpingectomy), and conservative surgery (salpingostomy).11 Many studies have investigated the influence of different EP treatment methods on pregnancy. Previous studies have reported that there was no significant difference in subsequent spontaneous fertility following different EP treatments.11,12 However, in two other studies, it was determined that conservative surgery is superior to radical surgery at preserving fertility.13,14 However, few studies have investigated the effects of the three main EP treatments on IVF-ET outcomes. According to current clinical knowledge, the cryopreserved embryo transfer (CET) cycle can significantly decrease the EP rate in IVF-ET.7,10,15,16 In this study, we discuss the effect of different treatment methods on the recurrence risk of EP in CET cycles.
Materials and methods
Patients
This retrospective cohort study was performed at the Center for Reproduction, Shandong University, Jinan, China. Our analysis of the data was approved by the Institutional Review Board of Shandong University. A total of 1333 women with previous histories of being admitted to the hospital for CET treatment were consecutively enrolled between January 2009 and December 2014. The method for identifying patients and assigning groups are shown in Figure 1. The authors already had access to identifying information during data collection.
Figure 1.
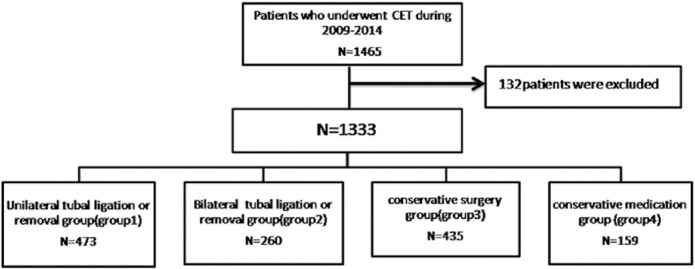
Database searching pathway and group divisions of women with ectopic pregnancy histories. CET = cryopreserved embryo transfer.
All embryos were transferred in autologous cycles, and all outcomes were derived from the first CET cycle. We categorized the women into four groups based on the treatment methods used and whether the connection between the fallopian tubes and uterus had been severed. The women in Group 1 underwent unilateral removal surgery, and the women in Group 2 underwent bilateral tubal removal or unilateral tubal removal with contralateral tubal ligation. These women received ligation/removal of the contralateral tube because of a ruptured tubal ectopic pregnancy or serious adhesion and hydronephrosis. The women in Group 3 underwent conservative surgery (salpingostomy), and the women in Group 4 underwent conservative medical treatment with MTX and/or other drugs, such as Chinese herbal medicine and mifepristone, without any abdominal surgery. The outcome rates, including ectopic pregnancy rate, clinical pregnancy rate, delivery rate, miscarriage rate, implantation rate, and ongoing pregnancy rate, were calculated according to the methods described in our former study.17
Because patients in Groups 1, 3, and 4 had at least one fallopian tube, we compared pregnancy outcomes among them and compared data from Groups 1 and 2 separately.
The exclusion criteria were as follows: (1) the previous EP did not result from a natural pregnancy; (2) repeated implantation failure (underwent more than 3 cycles but did not become pregnant); (3) oocyte donor treatment cycles; and (4) the presence of other diseases, such as chromosome abnormalities, malignant intracavitary lesions, and a history of myomectomy. The baseline characteristics of the women studied are shown in Table 1.
Table 1.
Outcomes of cryopreserved embryo transfer in four groups.
| Items | EPR | DR | CPR | MR | IR | OPR |
|---|---|---|---|---|---|---|
| Group 1 | 3/473 (0.63) | 247/473 (52.22) | 302/473 (63.85) | 42/473 (8.88) | 366/750 (48.80) | 272/473 (57.51) |
| Group 2 | 0/260 (0) | 139/260 (53.46) | 159/260 (61.15) | 9/260 (3.46) | 204/420 (48.57) | 154/260 (59.23) |
| Group 3 | 6/435 (1.38) | 218/435 (50.11) | 271/435 (62.30) | 38/435 (8.74) | 343/722 (47.51) | 239/435 (54.94) |
| Group 4 | 0/159 (0) | 86/159 (54.09) | 99/159 (62.26) | 9/159 (5.66) | 131/262 (50.00) | 90/159 (56.60) |
Values are presented as n (%).
CPR = clinical pregnancy rate; DR = delivery rate; EPR = ectopic pregnancy rate; IR = implantation rate; MR = miscarriage rate; OPR = ongoing pregnancy rate.
Statistical analysis
Statistical analysis was conducted using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). The normally distributed data were expressed as mean ± standard deviation. The categorical data and the quantitative data were analyzed by χ2 tests and t tests, respectively. A p value < 0.05 was considered statistically significant. We used Cox regression to calculate the odds ratios (ORs) and 95% confidence intervals (CI) to investigate the associations between the treatment types. Additional factors that would influence pregnancy outcomes, including age, body mass index, polycystic ovarian syndrome, mycoplasma infection, tuberculosis infection, untreated hydrosalpinx, endometrium thickness, and endometriosis, were also evaluated. In order to evaluate the results from different groups, we defined the statistical outcomes of Group 1 versus Group 3 as p1, Group 3 versus Group 4 as p2, Group 1 versus Group 4 as p3, and Group 1 versus Group 2 as p4.
Ethics statement
This study was a retrospective analysis of clinical practice outcomes, and our analysis of the data was approved by the Institutional Review Board of Shandong University. We obtained informed consent from the patients before they participated in a clinical study or experiment.
Results
Unilateral tubal removal was performed in 473 women (35.48%), bilateral tubal removal or unilateral tubal removal with contralateral tubal ligation was performed in 260 women (19.50%), conservative surgery was performed in 435 women (32.63%), and conservative medication was administered to 159 women (11.93%). The baseline data are shown in Table 2.
Table 2.
Odds ratios of four groups in cryopreserved embryo transfer.
| Items | p | OR | 95%CI | |
|---|---|---|---|---|
| Upper | Lower | |||
| EPR | p1 = 0.258 | 0.456 | 0.113 | 1.836 |
| p2 = 0.137 | 0.986 | 0.975 | 0.997 | |
| p3 = 0.314 | 0.994 | 0.987 | 1.001 | |
| p4 = 0.198 | 0.994 | 0.987 | 1.001 | |
| DR | p1 = 0.526 | 1.088 | 0.838 | 1.412 |
| p2 = 0.391 | 0.853 | 0.592 | 1.227 | |
| p3 = 0.683 | 0.928 | 0.647 | 1.330 | |
| p4 = 0.747 | 0.951 | 0.703 | 1.288 | |
| CPR | p1 = 0.629 | 1.069 | 0.816 | 1.400 |
| p2 = 0.994 | 1.001 | 0.688 | 1.457 | |
| p3 = 0.720 | 1.070 | 0.738 | 1.552 | |
| p4 = 0.470 | 1.122 | 0.821 | 1.533 | |
| MR | p1 = 0.939 | 1.018 | 0.643 | 1.612 |
| p2 = 0.219 | 1.595 | 0.753 | 3.379 | |
| p3 = 0.197 | 1.624 | 0.772 | 3.416 | |
| p4 = 0.006* | 2.718* | 1.301 | 5.677 | |
| IR | p1 = 0.620 | 1.053 | 0.858 | 1.292 |
| p2 = 0.489 | 0.905 | 0.682 | 1.201 | |
| p3 = 0.738 | 0.953 | 0.719 | 1.263 | |
| p4 = 0.940 | 1.009 | 0.795 | 1.282 | |
| OPR | p1 = 0.437 | 1.110 | 0.854 | 1.443 |
| p2 = 0.718 | 0.935 | 0.648 | 1.348 | |
| p3 = 0.842 | 1.037 | 0.722 | 1.491 | |
| p4 = 0.651 | 0.931 | 0.685 | 1.267 | |
* Statistical significance.
CI = confidence interval; CPR = clinical pregnancy rate; DR = delivery rate; EPR = ectopic pregnancy rate; IR = implantation rate; MR = miscarriage rate; OPR = ongoing pregnancy rate; OR = odds ratio; p1 = Group 1 vs. Group 3; p2 = Group 3 vs. Group 4; p3 = Group 1 vs. Group 4; p4 = Group 1 vs. Group2.
Among the 1333 women who attempted to conceive again, 831 (62.34%) were clinical pregnant after CET cycles. Of these pregnancies, 9 (1.08%) were EPs, 690 (83.03%) were delivered (term and preterm births), and 98 (11.79%) were miscarried. The pregnancy outcomes in the four groups are as follows.
After a crude analysis, the EP rates were 0.63%, 1.38%, and 0% for Groups 1, 3, and 4, respectively. Although there was no statistically significant differences among the three groups (p1 = 0.258, 95% CI = 0.113–1.836; p2 = 0.137, 95%CI = 0.975–0.997; p3 = 0.314, 95% CI = 0.987–1.001), conservative surgery resulted in the highest EP rate, and OR was 0.456 (95%CI = 0.113–1.836) for Groups 1 and 3 regarding EP. The implantation rates were 48.40%, 47.51%, and 50.00% for Groups 1, 3, and 4, respectively. Group 4 had the highest implantation rate, but this rate was not significantly different among the groups (p1 = 0.620, 95%CI = 0.858–1.292; p2 = 0.489, 95%CI = 0.682–1.201; p3 = 0.738, 95%CI = 0.719–1.263). We reached the same finding when we compared delivery rates, clinical pregnancy rates, miscarriage rates, and ongoing pregnancy rates among the three groups. The detailed results are shown in Tables 2 and 3.
Table 3.
Main characteristics in the four groups.
| Items | Age (y) | Body mass index | Polycystic ovarian syndrome | Mycoplasma infection | Tuberculosis infection | Hydrosalpinx untreated | Endometrium thickness (cm) | Endometriosis |
|---|---|---|---|---|---|---|---|---|
| Group 1 | 31.07 ± 4.218 | 23.19 ± 3.41 | 50/473 (10.57) | 165/473 (33.88) | 7/473 (1.48) | 43/487 (8.83) | 0.97 ± 1.60 | 6/473 (1.27) |
| Group 2 | 31.26 ± 4.130 | 23.20 ± 3.51 | 21/260 (8.08) | 97/260 (37.30) | 8/260 (3.08) | – | 0.96 ± 1.57 | 3/260 (1.15) |
| Group 3 | 30.59 ± 3.994 | 22.83 ± 3.07 | 58/435 (13.33) | 160/435 (36.78) | 18/435 (4.14) | 49/429 (11.42) | 0.97 ± 1.60 | 6/435 (1.38) |
| Group 4 | 31.11 ± 3.823 | 23.44 ± 3.43 | 28/159 (17.61) | 59/159 (37.11) | 2/159 (1.26) | 18/159 (11.46) | 0.98 ± 1.52 | 2/159 (1.26) |
| p | p1 = 0.080 | p1 = 0.094 | p1 = 0.199 | p1 = 0.551 | p1 = 0.014 | p1 = 0.193 | p1 = 0.950 | p1 = 0.884 |
| p2 = 0.159 | p2 = 0.031* | p2 = 0.190 | p2 = 0.942 | p2 = 0.838 | p2 = 0.987 | p2 = 0.686 | p2 = 0.909 | |
| p3 = 0.922 | p3 = 0.418 | p3 = 0.020 | p3 = 0.612 | p3 = 0.085 | p3 = 0.327 | p3 = 0.652 | p3 = 0.992 | |
| p4 = 0.561 | p4 = 0.823 | p4 = 0.275 | p4 = 0.512 | p4 = 0.144 | – | p4 = 0.437 | p4 = 0.893 |
Values are presented as mean ± standard error or n (%).
* Statistical significance.
p1 = Group 1 vs. Group 3; p2 = Group 3 vs. Group 4; p3 = Group 1 vs. Group 4; p4 = Group 1 vs. Group 2.
In order to compare the effects of tubal residue, we compared Groups 1 and 2. The results showed that Group 2 had a 61.03% lower miscarriage rate (8.88% vs. 3.46%, p = 0.006) than the Group 1, suggesting that the residual tube is a risk factor for miscarriage (OR = 2.718, 95%CI = 1.301–5.677).The rate of EP was not significantly different according to the treatment type (0.63% vs. 0%, p = 0.137, 95%CI = 0.975–0.997). Other pregnancy outcomes were not statistically significant different among groups. The detailed results are shown in Tables 2 and 3.
Discussion
We undertook this retrospective study to determine whether different EP treatments affect CET outcomes. We found that the occurrence of EP is very low in CET cycles (9/1333, 0.675%). Tubal removal is a protective factor for conservative surgery to treat EP, and the residual tube is a risk factor for later miscarriage. There are no significant differences in delivery rates, implantation rates, and ongoing pregnancy rates among the different EP treatment groups.
We support the opinion of Horcajadas et al18 that a lower EP rate in CET cycles is associated with a negative effect on ovarian stimulation and endometrial receptivity. It has been reported that an improved endometrial environment leads to intrauterine implantation because the embryo must develop further within the uterine cavity.15
The pregnancy outcomes among the treatment groups were not significantly different. Although ORs (OR = 0.456, 95% CI = 0.113–1.836) were slightly different between Groups 1 and 3, the statistical p values did not differ (p = 0.258). As is well known, it is difficult to avoid tubal trauma during surgery, and concurrent salpingemphraxis and hydrosalpinx are more likely to occur after surgery, which are both important factors for EP recurrence.19 However, in our data, we compared women with hydrosalpinx to keep the interference factors to a minimum (Table 1).We speculated that a higher incidence of EP may be related to the residual tube. The lower miscarriage rate in Group 2 (8.88% vs. 3.46%, p = 0.006, OR = 2.718, 95% CI = 1.301–5.677) is probably attributable to salpingitis and latent hydrosalpinx on the other side. The selective bias is another interpretation for these outcomes.
In general, the CET outcome is related to the quality of embryos, age, endometrial receptivity, and the microenvironment.20,21 However, in our research, we compared these factors to keep the interference elements to a minimum. Rashid et al22 showed that the term pregnancy rates between the radical and conservative surgery treatment groups were not significantly different.13 However, Li et al23 proved that the interstitial pregnancy rate increased after laparoscopic salpingestomy. The results of our study are similar to those of Rashid et al22 in which different types of previous EP treatment did not significantly affect the main CET outcomes.
Overall, the three methods of EP treatment are used in different situations. Salpingectomy was typically performed in emergency cases in which the tube was already ruptured or the patient was critically bleeding. However, a recent opinion has speculated that salpingectomy may lead to spontaneous rupture of the pregnant uterus.24 Salpingostomy is the best conservative surgery for EP, and the main indications are that women are fertile, their vital signs are stable, and the sac diameter of the mass is < 6 cm. Patients are excluded from this type of treatment if the tube has already ruptured or they are critically bleeding. The major argument against conservative surgery is the possibility of incomplete fetus removal and the increased risk of EP recurrence as the damaged tube is left behind. In 2012, the National Collaborating Center for Women’s and Children’s Health published that salpingectomy is preferred over salpingostomy, except when patients present with other infertility risks. As for conservative medical treatment, MTX is suitable for women who have a definite diagnosis or are highly suspected of ectopic pregnancy, have stable hemodynamics, and an unbroken mass (ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy). The main advantages of this method are as follows: (1) a greater antitrophoblastic effect; (2) a shorter treatment period; (3) reduced dosage; and (4) the absence of side effects.25 In 2014, it was reported that MTX treatment for EP does not seem to affect subsequent fertility treatment.26 However, incomplete abortions often occur with this method.
Tubal factor infertility was the most common indication for IVF.27 In our data, we observed a large number of women (56%) who underwent tubal ligation or removal surgery, but it is unclear whether these data are necessary to consider.
Strengths and limitations
Our study has some strengths as well as limitations. First, our research is valuable because 1333 women make the study a large reported sample size. Second, the cryopreservation of morula by vitrification, embryo splitting, and embryo transfer occurred in the same reproductive medicine center (Shandong Provincial Key Laboratory of Reproductive Medicine, Jinan, People’s Republic of China). Thus, there are no obvious differences among culture conditions and embryo quality. Third, although many scholars have studied different EP treatments with regard to fertility or spontaneous pregnancy, we have not yet found studies that have investigated the effect of different treatments on CET cycles. Our study is innovative and credible and suggests that clinicians reconsider the management of EP before IVF.
The limitations of our research cannot be ignored. This is a retrospective study; therefore, selection bias is inescapable to some extent. The data from the four groups were all collected from the Reproductive Hospital affiliated with Shandong University and were not extracted from multiple centers. Also, the number of tubal surgeries and the degree of tubal damage were not the same. We could not control the previous EP treatments performed at other hospitals for which detailed operation methods are lacking. In addition, we collected the women’s information as completely as possible; however, some individual details were possibly ignored. However, we believe these differences do not influence our conclusions.
Conclusion
Different EP treatment methods have no significant impact on the main pregnancy outcomes after CET. Bilateral tubal ligation or removal surgery can decrease the miscarriage rate but does not impact other pregnancy outcomes. The tube after conservative therapy for tubal EP might be a bad prognostic factor for future successful pregnancy. Our results suggest that hydrosalpinx should be actively processed during surgery. However, EP surgery requires further detailed study.
Acknowledgments
This study was financially supported by the Science Research Foundation item of no-earnings health vocation (201402004) and the National Basic Research Program of China (973 Program) (2011CB944502).
Footnotes
Conflicts of interest: We declare that there are no conflicts of interest.
References
- 1.Li C, Zhao WH, Zhu Q, et al. Risk factors for ectopic pregnancy: a multi-center case-control study. BMC Pregnancy Childbirth. 2015;15:187. doi: 10.1186/s12884-015-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li C, Meng CX, Zhao WH, Lu HQ, Shi W, Zhang J. Risk factors for ectopic pregnancy in women with planned pregnancy: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2014;181:176–182. doi: 10.1016/j.ejogrb.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 3.Bastianelli C, Lucantoni V, Valente A, Farris M, Lippa A, Dionisi B. Risk factors for ectopic pregnancy. Case-control study. Minerva Ginecol. 1998;50:469–473. [PubMed] [Google Scholar]
- 4.Karaer A, Avsar FA, Batioglu S. Risk factors for ectopic pregnancy: a case-control study. Aust NZJ Obstet Gynaecol. 2006;46:521–527. doi: 10.1111/j.1479-828X.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- 5.Moini A, Hosseini R, Jahangiri N, Shiva M, Akhoond MR. Risk factors for ectopic pregnancy: A case-control study. J Res Med Sci. 2014;19:844–849. [PMC free article] [PubMed] [Google Scholar]
- 6.Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185–194. doi: 10.1093/aje/kwf190. [DOI] [PubMed] [Google Scholar]
- 7.Perkins KM, Boulet SL, Kissin DM, Jamieson DJ. National ARTSG. Risk of ectopic pregnancy associated with assisted reproductive technology in the United States, 2001-2011. Obstet Gynecol. 2015;125:70–78. doi: 10.1097/AOG.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weigert M, Gruber D, Pernicka E, Bauer P, Feichtinger W. Previous tubal ectopic pregnancy raises the incidence of repeated ectopic pregnancies in in vitro fertilization-embryo transfer patients. J Assist Reprod Genet. 2009;26:13–17. doi: 10.1007/s10815-008-9278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okohue JE, Ikimalo JI, Omoregie OB. Ectopic pregnancy following in vitro fertilisation and embryo transfer. West Afr J Med. 2010;29:349–351. [PubMed] [Google Scholar]
- 10.Londra L, Moreau C, Strobino D, Garcia J, Zacur H, Zhao Y. Ectopic pregnancy after in vitro fertilization: differences between fresh and frozen-thawed cycles. Fertil Steril. 2015;104:110–118. doi: 10.1016/j.fertnstert.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez H, Capmas P, Lucot JP, et al. Fertility after ectopic pregnancy: the DEMETER randomized trial. Hum Reprod. 2013;28:1247–1253. doi: 10.1093/humrep/det037. [DOI] [PubMed] [Google Scholar]
- 12.Bouyer J, Job-Spira N, Pouly JL, Coste J, Germain E, Fernandez H. Fertility following radical, conservative-surgical or medical treatment for tubal pregnancy: a population-based study. BJOG. 2000;107:714–721. doi: 10.1111/j.1471-0528.2000.tb13330.x. [DOI] [PubMed] [Google Scholar]
- 13.de Bennetot M, Rabischong B, Aublet-Cuvelier B, et al. Fertility after tubal ectopic pregnancy: results of a population-based study. Fertil Steril. 2012;98:1271–1276. doi: 10.1016/j.fertnstert.2012.06.041. e1271-1273. [DOI] [PubMed] [Google Scholar]
- 14.Bangsgaard N, Lund CO, Ottesen B, Nilas L. Improved fertility following conservative surgical treatment of ectopic pregnancy. BJOG. 2003;110:765–770. [PubMed] [Google Scholar]
- 15.Ishihara O, Kuwahara A, Saitoh H. Frozen-thawed blastocyst transfer reduces ectopic pregnancy risk: an analysis of single embryo transfer cycles in Japan. Fertil Steril. 2011;95:1966–1969. doi: 10.1016/j.fertnstert.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro BS, Daneshmand ST, De Leon L, Garner FC, Aguirre M, Hudson C. Frozen-thawed embryo transfer is associated with a significantly reduced incidence of ectopic pregnancy. Fertil Steril. 2012;98:1490–1494. doi: 10.1016/j.fertnstert.2012.07.1136. [DOI] [PubMed] [Google Scholar]
- 17.Guo T, Qin Y, Gao X, et al. The role of male chromosomal polymorphism played in spermatogenesis and the outcome of IVF/ICSI-ET treatment. Int J Androl. 2012;35:802–809. doi: 10.1111/j.1365-2605.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- 18.Horcajadas JA, Minguez P, Dopazo J, et al. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab. 2008;93:4500–4510. doi: 10.1210/jc.2008-0588. [DOI] [PubMed] [Google Scholar]
- 19.Diquelou JY, Pia P, Tesquier L, Henry-Suchet J, Gicquel JM, Boyer S. The role of Chlamydia trachomatis in the infectious etiology of extra-uterine pregnancy. J Gynecol Obstet Biol Reprod (Paris) 1998;17:325–332. [PubMed] [Google Scholar]
- 20.Meldrum DR, Casper RF, Diez-Juan A, Simon C, Domar AD, Frydman R. Aging and the environment affect gamete and embryo potential: can we intervene? Fertil Steril. 2016;105:548–559. doi: 10.1016/j.fertnstert.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Evans J, Hannan NJ, Edgell TA, et al. Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Hum Reprod Update. 2014;20:808–821. doi: 10.1093/humupd/dmu027. [DOI] [PubMed] [Google Scholar]
- 22.Rashid M, Osman SH, Khashoggi TY, Kamal FA. Factors affecting fertility following radical versus conservative surgical treatment for tubal pregnancy. Saudi Med J. 2001;322:337–341. [PubMed] [Google Scholar]
- 23.Li B, Lin X, Kuang L, Huang D, Jin X, Zhang S. Effect of different laparoscopic procedures for hydrosalpinx on pregnancy outcome of embryo transfer. Zhonghua Yi Xue Za Zhi. 2014;94:2941–2944. [PubMed] [Google Scholar]
- 24.Stanirowski PJ, Trojanowski S, Slomka A, Cendrowski K, Sawicki W. Spontaneous rupture of the pregnant uterus following salpingectomy: a literature review. Gynecol Obstet Invest. 2015;80:73–77. doi: 10.1159/000398795. [DOI] [PubMed] [Google Scholar]
- 25.Mesogitis SA, Daskalakis GJ, Antsaklis AJ, Papantoniou NE, Papageorgiou JS, Michalas SK. Local application of methotrexate for ectopic pregnancy with a percutaneous puncturing technique. Gynecol Obstet Invest. 1998;45:154–158. doi: 10.1159/000009946. [DOI] [PubMed] [Google Scholar]
- 26.Ohannessian A, Loundou A, Courbiere B, Cravello L, Agostini A. Ovarian responsiveness in women receiving fertility treatment after methotrexate for ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod. 2014;29:1949–1956. doi: 10.1093/humrep/deu174. [DOI] [PubMed] [Google Scholar]
- 27.Pantoja M, Fernandes A. Indications for in vitro fertilization at a public center for reproductive health in Campinas, Brazil. Int J Gynaecol Obstet. 2015;128:14–17. doi: 10.1016/j.ijgo.2014.07.022. [DOI] [PubMed] [Google Scholar]


