Abstract
There is extensive evidence demonstrating that there is a clear inverse correlation between plasma high density lipoprotein cholesterol (HDL-C) concentration and cardiovascular disease (CVD). On the other hand, there is also extensive evidence that HDL functionality plays a very important role in atheroprotection. Thus, genetic disorders altering certain enzymes, lipid transfer proteins, or specific receptors crucial for the metabolism and adequate function of HDL, may positively or negatively affect the HDL-C levels and/or HDL functionality and subsequently either provide protection or predispose to atherosclerotic disease. This review aims to describe certain genetic disorders associated with either low or high plasma HDL-C and discuss their clinical features, associated risk for cardiovascular events, and treatment options.
Keywords: apolipoprotein A-I (ApoA-I), cardiovascular disease (CVD), genetic disorders, high density lipoprotein (HDL)
Introduction
There is extensive evidence demonstrating that there is a clear inverse correlation between plasma high density lipoprotein cholesterol (HDL-C) concentration and cardiovascular disease (CVD). HDL-C levels are considered a strong predictor for CVD independently to low density lipoprotein cholesterol (LDL-C) levels.1 Furthermore, studies have indicated that there is a 2–3% decrease in the risk for CVD for each 1 mg/dL increase in HDL-C levels.2 There is also extensive evidence that HDL functionality plays a very important role in atheroprotection.3
The antiatherogenic effects of HDL are mainly attributed to its role in reverse cholesterol transport (RCT) pathway by promoting the efflux of cholesterol from macrophages in the arterial wall, thus preventing foam cell formation, and therefore, the initial stages of atherogenesis.4
Hence, genetic disorders altering certain enzymes, lipid transfer proteins, or specific receptors crucial for the metabolism and adequate function of HDL, may positively or negatively affect the HDL-C levels and/or HDL functionality and subsequently either provide protection or predispose to atherosclerotic disease.
In this review, after providing a brief summary of HDL metabolism and its cardioprotective properties, we will describe certain genetic disorders associated with either low or high plasma HDL-C concentration and we will discuss their clinical features, associated risk for cardiovascular events, and treatment options.
HDL metabolism
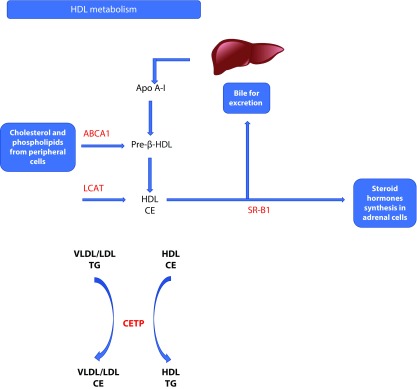
HDL is synthesized in the liver and small intestine and is the lipoprotein with the highest protein content (approximately 50% of total weight of the particle). When secreted, HDL contains only a small amount of cholesterol and no cholesteryl esters. HDL is formed by different apolipoproteins, the most important of which are the apolipoprotein A-I (ApoA-I), which is secreted predominantly by the liver and intestine as lipid-free ApoA-I and constitutes approximately 70% of HDL protein, and the apolipoprotein A-II (ApoA-II), which is only synthesized by the liver and constitutes around 20% of the HDL protein. Both apolipoproteins are required for the normal HDL biosynthesis.
The newly secreted ApoA-I must acquire lipids in the form of cholesterol or phospholipids (found in the peripheral cells) in order to generate the pre-β-HDL. This pathway is mediated by the action of the ATP binding cassette transporter A1 (ABCA1), which promotes the transfer of lipids to ApoA-I, resulting in lipid-poor ApoA-I.5,6
Once pre-β-HDL is in the circulation, lecithin:cholesterol acyltransferase (LCAT), using ApoA-I as cofactor, promotes the conversion of lecithin into lysolecithin and cholesterol into cholesteryl esters (CE). CE is a more hydrophobic form of cholesterol, which is then sequestered into the core of the HDL particle, resulting in the formation of the mature, spherical, α-migrating HDL (α-HDL).7
Finally, scavenger receptor class B type I (SR-BI) promotes the cholesterol uptake from mature HDL by the liver for secretion into the bile, or by the adrenal cells for the synthesis of steroid hormones, in a process that does not involve degradation of HDL apolipoproteins and is known as selective uptake.8,9
HDL-C can be alternatively metabolized and eventually transported by the liver, via the action of the cholesteryl ester transfer protein (CETP). This protein transfers triglycerides (TGs) from apolipoprotein B (ApoB)-containing lipoproteins, such as the very low density lipoprotein (VLDL) and LDL, in exchange for cholesteryl esters from HDL, thus leading to cholesteryl ester depletion and TG enrichment of HDL. Because TGs are not stable in HDL-C, it is degraded by lipoprotein lipase, leading to an overall net reduction in the size of the HDL particles.10
A schematic of HDL metabolism and the genes involved is shown in Figure 1.
Figure 1.
Metabolism of HDL.
ABCA1, ATP-binding cassette transporter A1; ApoA-I, apolipoprotein A-I; CE, cholesteryl ester; CETP, cholesteryl ester transfer protein; HDL, high-density lipoprotein; LCAT, lecithin:cholesterol acyl-transferase; LDL, low-density lipoprotein; SR-BI, scavenger receptor class B type I; TG, triglyceride; VLDL, very-low-density lipoprotein.
Clinical significance of HDL
While the main cardioprotective property of HDL relates to its ability to promote efflux of cholesterol from macrophages in the artery wall, a process known as reverse cholesterol transport (RCT), HDL may also prevent and/or inhibit the development of atherosclerosis via several other cardioprotective mechanisms.
HDL has anti-inflammatory effects11 and there is extensive evidence that inflammation plays an important role in the initiation and progression of CVD.12 Furthermore, HDL has antioxidant, anticoagulant, and antiaggregating effects; it promotes angiogenesis and enhances endothelial function and repair.4
In addition, HDL may also have beneficial effects on glucose metabolism, as there is evidence that both HDL and ApoA-I increase glucose uptake in human skeletal muscle cells, leading to reduced plasma glucose levels in diabetic patients.13
Conditions that cause high HDL-C levels
Genetic conditions associated with elevated HDL-C levels, are also described as primary familial or secondary hyperalphalipoproteinemia (HALP) due to the elevated Apo-AI and Apo-AII levels observed in these conditions.
Patients with HALP tend to be asymptomatic, aside from some rare reported cases of juvenile or premature corneal opacities14 or multiple symmetric lipomatosis,15 and are characterized by high levels of HDL-C and low incidence of CVD.16
Due to its lack of clinical symptoms, patients are usually identified through the routine assessment of a lipid profile or may have history of a relative found with elevated HDL-C levels. No treatment is generally required.
In this review we will only describe the primary familial causes of HALP.
Primary familial HALP
Primary familial HALP is defined as HDL-C levels greater than the 90th percentile for age and gender, a history of relatives with high HDL-C, and the absence of secondary causes of increased HDL-C levels, such as medications, malignancies, or liver disease. This condition is thought to coexist with longevity and to provide some type of protection from atherosclerotic disease.17 Primary familial HALP is an autosomal-dominant condition and may result from genetic mutations of ApoA-I, causing overproduction, or from certain variants of apolipoprotein C-III (ApoC-III). It is diagnosed incidentally with plasma HDL-C levels above 80 mg/dL.18
Selective upregulation of ApoA-I
ApoA-I is the major protein of HDL. It acts as a structural protein, mediates RCT, and activates LCAT.19
In addition to its function in the HDL-C metabolism, this apolipoprotein also has anti-inflammatory properties, which may contribute to its cardioprotective role.20
The selective upregulation of ApoA-I results in ApoA-I overproduction. It is characterized by elevated HDL-C and ApoA-I levels and has been linked to a reduced risk of CVD.21
ApoC-III variants
ApoC-III is a small apolipoprotein, which is synthesized mainly in the liver, is carried in the circulation by VLDL and HDL, and regulates plasma TG homeostasis. This apolipoprotein impairs the hydrolysis of triglyceride-rich lipoproteins (TRL) by inhibiting the activity of the lipoprotein lipase (LPL) and delaying the hepatic uptake of TRL by remnant receptors, thus resulting in elevated plasma TG levels.22,23
Beyond its role on TG metabolism, ApoC-III has been also associated with increased risk of CVD. It promotes HDL dysfunction, facilitates the interaction of monocytes and endothelial cells, stimulates smooth muscle cell proliferation, and alters platelet activity, thus promoting atherosclerosis.24
Furthermore, plasma ApoC-III concentration is directly related with the plasma TG concentration. Therefore, mutations that interrupt ApoC-III function or loss-of-function ApoC-III mutations are associated with very low plasma TG levels and elevated HDL-C levels, resulting in a reduction of the risk for CVD.25
Studies have described that carriers of ApoC-III loss-of-function mutations have up to 39% lower plasma TG levels, 22% higher plasma HDL-C levels, and 16% lower plasma LDL-C levels. Of note, carriers of ApoC-III loss-of-function mutations enjoy a 40% lower risk of coronary heart disease (CHD), as compared to non-carriers.26
CETP deficiency
CETP mediates the exchange of CE for TG between HDL and VLDL/LDL and regulates the lipid composition and particle size of lipoproteins.10 CETP activity correlates directly with LDL-C concentration and inversely with HDL-C concentration.27
CETP deficiency is an autosomal recessive inherited metabolic disorder, which was first studied in Japan in the 1980s. It is considered one of the most important and frequent causes of HALP in the Japanese population.28,29
Two common mutations in the CETP gene have been described particularly in the Japanese population: intron 14 splicing defect (In14), a null mutation with strong effects on plasma CETP activity and levels and HDL-C levels, and a missense mutation in exon 15 (Ex15) with less pronounced effects on plasma HDL-C levels compared to In14. Other less common mutations described are the intron 10 splicing defect (In10) and the exon 6 nonsense mutation (Ex6).30
The loss-of-function CETP enzyme results in elevated levels of ApoA-I and ApoA-II due to decreased turnover, significantly elevated HDL-C levels in homozygotes (usually >100 mg/dL), and moderately elevated HDL-C levels in heterozygotes due to lack of HDL remodeling. Thus, the HDL particles in this condition are enriched with CE and apolipoprotein E (ApoE) and have a low TG content. Moreover, there is increased hydrolysis of LDL leading to decreased LDL-C levels.21,31,32
Even though CETP deficiency is associated with elevated HDL-C and decreased LDL-C, its antiatherogenic potential remains very controversial with some studies suggesting a decreased risk for CVD in such patients10 and others indicating that, despite their high HDL-C content, these particles have a decreased capacity for cholesterol efflux and may not have antiatherogenic properties.31,33,34
Several studies were conducted with CETP inhibitors, but their effects on CVD risk were detrimental, neutral, or at most slightly positive, despite a substantial increase in HDL-C levels.3 Only anacetrapib produced a small decrease in the risk for CVD when added to statin therapy; however, this was achieved mainly by decreasing the non-HDL-C, rather than by increasing the HDL-C.35 However, more studies are needed to definitely determine the therapeutic potential of CETP inhibitors in the treatment of CVD.36
Scavenger receptor class B type I (SR-BI) mutations
SR-BI, encoded by the SCARB1 gene, is a main component of the RCT pathway with high affinity for HDL-C. It mediates the selective uptake of CE from HDL-C in liver and steroidogenic tissues and facilitates the secretion of cholesterol into bile. SR-BI is expressed in the liver and in macrophages in atherosclerotic plaques.9,37 Furthermore, SR-BI are multi-ligand receptors binding other lipoproteins, such as LDL and VLDL.38
Certain mutations in the SR-BI gene have been described and among them is exon 8 rs5888 single nucleotide polymorphism (SNP), associated with a decreased SR-BI protein expression and function, resulting in altered lipid levels in humans.39 Another mutation described in the SR-BI gene is a missense mutation, in which leucine replaces proline at position 376 (P376L). This mutation impairs posttranslational processing of SR-BI and results in almost complete loss of its function and dysregulation of the selective HDL-C uptake in transfected cells.40
The decreased activity or loss of SR-BI function results in decreased HDL-C bile secretion, leading to elevated HDL-C levels. However, despite the high plasma HDL-C concentration observed in this condition, carriers exhibit increased risk of CVD due to impaired RCT pathway caused by the reduced hepatic SR-BI function.9,40,41 More specifically, carriers of the P376L mutation (described above) have been shown to have a 79% higher risk of CHD, as compared to non-carriers.40
SR-BI mutations may also be associated with an increased risk for adrenal glucocorticoid insufficiency and impaired platelet function.37,42
Endothelial lipase mutations
Endothelial lipase (EL) is a member of the triacylglycerol (TAG) lipase gene family, along with LPL, hepatic lipase, and pancreatic lipase. Interestingly, it is the only identified lipase that is synthesized and expressed by endothelial cells.43
It promotes HDL particle binding and uptake, in addition to the selective uptake of HDL-CE due to its phospholipase activity.44 EL also cleaves HDL-phospholipids resulting in the release of fatty acids and lysophospholipids, which are then taken up by the cells expressing this enzyme.45
By decreasing the triglyceride and phospholipid content of HDL, EL is a strong negative regulator of plasma HDL-C levels46,47 and thus EL loss-of-function mutations lead to increased HDL-C levels. Notwithstanding, the cardiovascular connotations of EL loss-of-function mutations are still unclear with some studies describing some cardioprotective properties,48 whereas others do not show any associated reduction in the risk of CHD.49
Disorders that cause low HDL-C levels
Although rare in the overall population, primary extremely low HDL-C levels result from monogenic disorders, such as ApoA-I deficiency, several ApoA-I missense mutations (such as ApoA-I Milano, ApoA-I Paris and others), Tangier disease, LCAT deficiency, or ABCA1 deficiency. Aside from certain ApoA-I missense mutations (including ApoA-I Milano and ApoA-I Paris), these conditions are associated with premature CVD due to abnormal accumulation of cholesterol.
The early evaluation to assess the risk for atherosclerotic disease, as well as to rule out any secondary causes, plays a major role in the care for these patients. The high risk for CVD warrants comprehensive secondary prevention measures. These include achieving an LDL-C <70 mg/dL with high-dose statin therapy, alone or in combination with other lipid-lowering agents, and by optimizing the identification and management of traditional risk factors, such as cigarette smoking, diabetes mellitus, obesity, and physical inactivity. Furthermore, early screening is recommended in those patients to evaluate subclinical atherosclerosis with coronary artery calcium scanning and/or carotid intima-media thickness assessment.50
ApoA-I deficiency
ApoA-I deficiency is an autosomal recessive inherited metabolic disorder, which can result from the deletion of ApoA-I gene51 or nonsense mutations early in the coding portion of the gene in both ApoA-I alleles.52
It is characterized by undetectable plasma ApoA-I levels, markedly decreased HDL-C levels (<5 mg/dL), normal TG levels, normal LDL-C levels, and premature CVD.51,53,54
Affected individuals may present with corneal opacification, cutaneous xanthomas, and a tendency for extensive atherosclerosis due to impairment of RCT pathway, which results in accumulation of cholesterol owing to the defective peripheral cellular efflux.55
ApoA-I Milano
This ApoA-I variant was first described in 1980 in a family originating from Limone sul Garda, a small town outside Milan in northern Italy.56 The ApoA-I Milano results from the substitution of a single amino acid, arginine 173 to cysteine, leading to the formation of homodimers or heterodimers with ApoA-II. Carriers are heterozygous for the mutation and are characterized by having very low plasma ApoA-I and HDL-C levels along with moderately elevated TG levels.57,58
Despite a lipid profile that is conventionally associated with a high risk of premature atherosclerosis, carriers of the ApoA-I Milano variant have a reduced incidence of CVD.57 Studies have described ApoA-I Milano as a gain-of-function mutation with cardioprotective properties.59
Furthermore, experimental studies with repetitive intravenous infusions of recombinant ApoA-I Milano have demonstrated a rapid regression of existing atheromas by promoting the RCT and improving the endothelial function.60,61
ApoA-I Paris
ApoA-I Paris is another ApoA-I variant, which results from the substitution of an arginine at position 151 to cysteine, leading to the formation of homodimers or heterodimers with ApoA-II. Similar to ApoA-I Milano, ApoA-I Paris does not cause any deleterious health effects and may even improve cardioprotection despite abnormally low levels of plasma ApoA-I and HDL-C and high levels of HDL triacylglycerides in mutation carriers, which are all heterozygotes.62 Whereas in the ApoA-I Milano subjects the low levels of ApoA-I appear to be due to a rapid catabolism with a normal synthetic rate, on the other hand, the low levels of ApoA-I in the ApoA-I Paris subjects are caused by a low production rate of ApoA-I.63 Similar to ApoA-I Milano, ApoA-I Paris also exhibits a potent antioxidant activity distinct from that of wild-type ApoA-I.64
Other ApoA-I missense mutations
There are over 50 reported ApoA-I missense or in-frame deletion mutations. Some of these variants are associated with low HDL-C levels, whereas others are associated with low HDL-C levels and amyloidosis.65
LCAT deficiency familial/partial
As we mentioned earlier, LCAT is the enzyme responsible for the esterification of free cholesterol and plays a crucial role in the maturation of HDL.7
Mutations associated with a decreased production or absence of LCAT result in the accumulation of free cholesterol in plasma and peripheral cells (due to the inability to be converted to CE) and in the impaired formation of mature HDL particles, on top of an accelerated ApoA-I catabolism.17
There are two types of mutations in the LCAT gene in the homozygote state, both inherited in an autosomal recessive pattern, and result in either a complete deficiency, known as familial LCAT deficiency (FLD), or a partial deficiency, also known as fish-eye disease (FED). The molecular basis of the two types of LCAT deficiency lies on the fact that the inheritance of a mutated LCAT genotype causes a gene–dose-dependent alteration in the plasma lipid/lipoprotein profile, which is remarkably similar between subjects classified as FLD or FED.66
Both mutations are characterized by decreased HDL-C and ApoA-I levels (70–80% reduction), as well as low LDL-C levels, elevated TG levels, and early-onset corneal opacities. Heterozygous carriers are asymptomatic and may present only with low HDL-C levels.17,67,68
Apart from the characteristic lipid profile, affected individuals with the complete form of LCAT deficiency present with hepatosplenomegaly, hemolysis with subsequent normochromic normocytic anemia due to abnormal deposition of lipid in the red blood cells, proteinuria and kidney injury that can progress to end-stage renal disease. Kidney failure is the major cause of morbidity and mortality in these patients, and it is thought to be due to the accumulation in the renal glomeruli of an abnormal free cholesterol and phospholipid-rich particle described as apoprotein X (ApoX), which has been linked to the pathogenesis of the disease. The kidney disease caused by this condition may be treated with organ transplantation and, although recurrence of lesions after kidney transplantation can occur early, the function of the transplanted organ often persists long-term despite the presence of deposits.69
With regard to the cardiovascular impact of these mutations, it remains a very controversial subject despite the abnormal lipid pattern.70,71 Notwithstanding, in a very recent study, it was shown that complete and partial LCAT deficiency are differentially associated with atherosclerosis. More specifically, mutations leading to complete LCAT deficiency were shown to be associated with decreased atherosclerosis, whereas mutations leading to partial LCAT deficiency were shown to be associated with increased atherosclerosis. The authors proposed that this discrepancy may be related to the fact that the capacity of LCAT to generate cholesteryl esters on ApoB-containing lipoproteins is lost in complete LCAT deficiency, while it remains unaffected in partial LCAT deficiency.72
ATP binding cassette transporter A1 (ABCA1) deficiency
Also known as Tangier disease, it was first described in a family from Tangier Island in Virginia in 1961.73 Tangier disease is an autosomal recessive inherited metabolic disorder, which results from a loss-of-function mutation in the ABCA1 gene.74
The loss of enzyme activity leads to an impaired efflux of cholesterol and phospholipids to apolipoproteins, resulting in the inappropriate lipidation of ApoA-I, which is rapidly cleared, and in the deposition of cholesterol in tissues. This disorder is characterized by extremely low ApoA-I (~4 mg/dL) and HDL-C levels (<5 mg/dL), decreased LDL-C (about 50% of normal), and mildly elevated TG levels (>200 mg/dL). Affected individuals present with enlarged yellow-orange tonsils, thrombocytopenia, peripheral neuropathy (approximately 50% are affected), and hepatosplenomegaly due to ectopic deposition of cholesterol-laden macrophages in these tissues.54,67,75
Homozygous and compound heterozygous carriers of the disease who are more than 30 years of age have a six-fold higher risk of CVD,76 whereas heterozygotes exhibit a greater than three-fold increase in the frequency of coronary artery disease (CAD).77
To date, aside from the symptomatic management, no treatment has been found to prevent the progression of this disease, as drugs commonly used to increase the HDL-C have proven to be ineffective in these individuals. Patients should be encouraged to avoid any lifestyle habit that can promote further progression of atherogenesis.
Therapeutic strategies favorably affecting HDL-C levels and/or HDL function and metabolism
Several therapeutic strategies have been tried to increase levels of HDL-C and/or to improve HDL function and favorably alter HDL metabolism.
As it was mentioned earlier, several clinical studies have been conducted with CETP inhibitors, but their effects on CVD risk were detrimental, neutral, or at most slightly positive despite a substantial increase in HDL-C levels.3
Several trials have been conducted to test the therapeutic efficacy of ApoA-I infusions. As it was mentioned earlier in this review, infusions of recombinant ApoA-I Milano have been shown to cause a significant regression of coronary atherosclerosis in patients with acute coronary syndrome (ACS),60 but manufacturing difficulties and contamination from host-derived proteins delayed subsequent clinical development of the product.78 Notwithstanding, in a pilot trial, MDCO-216 (a recently manufactured recombinant ApoA-I Milano without contamination by host-derived proteins) did not produce plaque regression in statin-treated patients following an ACS,79 and the sponsor company abandoned its further development.
The infusion of plasma-derived, wild-type, human ApoA-I into humans has been also tested in several trials since the 1990s. In an earlier small trial, lipid-free ApoA-I infusion into men with low HDL-C level led to an increase in plasma total ApoA-I concentration without any significant adverse effects, and this increase was confined to the pre-beta region in the plasma.78,80 In another trial, short-term infusions of purified wild-type ApoA-I from human plasma linked to soybean phosphatidylcholine (CSL-111) resulted in no significant reductions in percentage change in atheroma volume or nominal change in plaque volume compared with placebo, but did result in statistically significant improvement in the plaque characterization index and coronary score on quantitative coronary angiography.78,81 Infusions of CSL-112 (a successor of CSL-111 with an enhanced ability to accept cholesterol from ABCA1) have been proven to be safe, well tolerated, and produced increases in ApoA-I concentration in a dose-dependent manner.78 CSL-112 has also been shown to increase HDL-C level and preferentially ABCA1-dependent cholesterol efflux capacity.78,82 In a randomized, double-blind, placebo-controlled, dose-ranging phase 2b trial, which was designed to assess the safety and tolerability of CSL-112 after acute myocardial infarction (AEGIS-I trial), it was shown that 4 weekly infusions of CSL-112 were feasible, well tolerated, and not associated with any significant impairment in liver or kidney function or other safety concerns.3,83 A phase 3 trial to assess the potential benefit of CSL-112 to reduce major adverse cardiovascular events has already been initiated and began recruiting patients in early 2018 (AEGIS-II trial – ClinicalTrials.gov Identifier: NCT03473223).3
Finally, in a prospective, double-blinded, randomized trial, infusions of CER-001, an engineered pre-β-HDL mimic consisting of recombinant human ApoA-I and ApoA-II different phospholipid carriers, did not reduce coronary atherosclerosis on intravascular ultrasonography (IVUS) and quantitative coronary angiography (QCA) when compared with placebo.78,84 However, CER-001 was able to stimulate cholesterol mobilization and reduced artery wall dimension and inflammation in patients with familial hypoalphalipoproteinemia (FHA), thus supporting further evaluation of CER-001 in patients with FHA.85
Although infusions of recombinant ApoA-I may be safe and potentially clinically effective, the high cost of large-scale production of ApoA-I and the need for repeated intravenous administration limits the potential of this therapy for broad clinical application. Gene therapy may represent an alternative approach due to its possible long-term effect and some preclinical studies have provided some evidence of benefit. However, further studies are required for more definitive clinical validation of this approach.3,78 Recent progress in recombinant adeno-associated virus (AAV) technology appears promising in this regard.3,78,86
ABCA1 upregulation may represent another future therapeutic direction. As it was mentioned earlier, ABCA1 is a key transporter which mediates cellular cholesterol and phospholipid efflux to lipid-poor ApoA-I in HDL synthesis and plays a crucial role in RCT. Thus, ABCA1 may be considered as a promising therapeutic target for the prevention of atherosclerosis. In a recent experimental study, allicin, a novel antiatherosclerotic molecule, induced upregulation of ABCA1, promoted cholesterol efflux, and reduced lipid accumulation via peroxisome proliferator-activated receptor γ (PPARγ)/liver X receptor α (LXRα) in THP-1 macrophage-derived foam cells. Thus, treatment with allicin may represent a novel future therapeutic strategy for the prevention and/or treatment of atherosclerosis.87
Infusion of recombinant LCAT has been tried as a means to increase plasma LCAT concentration, which would potentially be beneficial for patients with CHD or FLD. In an earlier animal study, infusion of recombinant LCAT rapidly restored the normal lipoprotein phenotype in LCAT-knockout mice and increased cholesterol efflux, thus suggesting the possibility of using recombinant LCAT as an enzyme-replacement therapy agent for LCAT deficiency.88 Later on, in a phase 1b, open-label, single-dose escalation study, infusion of recombinant LCAT, administered in subjects with stable CHD and low HDL-C levels, was proven to have an acceptable safety profile and it favorably altered HDL metabolism. These data again provide support for the use of recombinant human LCAT in future clinical trials in CHD and FLD patients.89 Finally, in a first-in-human treatment with enzyme replacement in FLD, recombinant LCAT infusions led to an improvement of anemia and most parameters related to renal function in spite of advanced disease. In addition, plasma lipids transiently normalized, and there was rapid sequential conversion of small pre-β-HDL particles to mature spherical α-HDL particles.90 Notwithstanding, as it was mentioned earlier, further larger long-term clinical trials will be required to definitely establish the clinical efficacy of human recombinant LCAT administration in patients with CHD and FLD.
Conclusions
From the above review of the scientific epidemiological and clinical data, it becomes apparent that there are several primary genetic disorders affecting the concentration and/or functionality of HDL. It is to be noted that, in certain cases, disorders associated with high HDL-C levels, such as certain SR-BI mutations, ‘paradoxically’ lead to an increased risk for CVD, whereas, in other cases, disorders associated with low HDL-C levels, such as ApoA-I Milano and ApoA-I Paris, ‘paradoxically’ lead to a reduced risk for CVD. This observation provides again clear and unequivocal evidence that the functionality of HDL plays a crucial role for its cardioprotective effect. Obviously, no treatment is required when the specific primary HDL disorder leads to a reduction of cardiovascular risk. On the other hand, when the specific primary HDL disorder leads to an increase of cardiovascular risk, in most cases no treatment has been found to prevent the progression of the disease, as drugs commonly used to increase the HDL-C have proven to be ineffective in these individuals. Some new promising therapeutic strategies that may favorably affect HDL-C levels and/or HDL function and metabolism, such as ApoA-I infusion, ABCA1 upregulation and LCAT infusion, have emerged but have not yet been incorporated into the everyday clinical practice. However, as it was mentioned earlier, the high risk for CVD warrants comprehensive secondary prevention measures. These include achieving an LDL-C <70 mg/dL with high-dose statin therapy, alone or in combination with other lipid-lowering agents, and by optimizing the identification and management of traditional risk factors, such as cigarette smoking, diabetes mellitus, obesity, and physical inactivity. Furthermore, early screening is recommended in those patients to evaluate subclinical atherosclerosis with coronary artery calcium scanning and/or carotid intima-media thickness assessment.
Acknowledgments
None.
Footnotes
Contributions: Conceived the concepts: CEK; analyzed the data: CEK; wrote the first draft of the manuscript: DS and CEK; contributed to the writing of the manuscript: AS, FG, PDM, EG; agreed with manuscript results and conclusions: CEK, DS, AS, FG, PDM, EG; jointly developed the structure and arguments for the paper: CEK; made critical revisions and approved final version: CEK; all authors reviewed and approved the final manuscript.
Disclosure and potential conflicts of interest: Constantine E Kosmas and Eliscer Guzman are members of the Dyslipidemia Speaker Bureau of Amgen, Inc. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors are available for download at http://www.drugsincontext.com/wp-content/uploads/2018/09/dic.212546-COI.pdf
Correct attribution: Copyright © 2018 Kosmas CE, Silverio D, Sourlas A, Garcia F, Montan PD, Guzman E. https://doi.org/10.7573/dic.212546. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/primary-genetic-disorders-affecting-high-density-lipoprotein-hdl
Provenance: invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Peer review comments to author: 9 August 2018
Funding declaration: There was no funding associated with the preparation of this article.
References
- 1.Barter P, Gotto AM, LaRosa JC, et al. Treating to New Targets Investigators. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357(13):1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 2.Chapman MJ, Assmann G, Fruchart JC, Shepherd J, Sirtori C European Consensus Panel on HDL-C. Raising high-density lipoprotein cholesterol with reduction of cardiovascular risk: the role of nicotinic acid--a position paper developed by the European Consensus Panel on HDL-C. Curr Med Res Opin. 2004;20(8):1253–1268. doi: 10.1185/030079904125004402. [DOI] [PubMed] [Google Scholar]
- 3.Kosmas CE, Martinez I, Sourlas A, et al. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context. 2018;7:212525. doi: 10.7573/dic.212525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rye KA, Barter PJ. Cardioprotective functions of HDLs. J Lipid Res. 2014;55(2):168–179. doi: 10.1194/jlr.R039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsujita M, Wu CA, Abe-Dohmae S, Usui S, Okazaki M, Yokoyama S. On the hepatic mechanism of HDL assembly by the ABCA1/apoA-I pathway. J Lipid Res. 2005;46(1):154–162. doi: 10.1194/jlr.M400402-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116(12):3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rousset X, Vaisman B, Amar M, Sethi AA, Remaley AT. Lecithin: cholesterol acyltransferase--from biochemistry to role in cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2009;16(2):163–171. doi: 10.1097/med.0b013e328329233b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trigatti BL, Krieger M, Rigotti A. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23(10):1732–1738. doi: 10.1161/01.ATV.0000091363.28501.84. [DOI] [PubMed] [Google Scholar]
- 9.Trigatti B, Covey S, Rizvi A. Scavenger receptor class B type I in high-density lipoprotein metabolism, atherosclerosis and heart disease: lessons from gene-targeted mice. Biochem Soc Trans. 2004;32(Pt 1):116–120. doi: 10.1042/bst0320116. [DOI] [PubMed] [Google Scholar]
- 10.Barter PJ, Brewer HB, Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23(2):160–167. doi: 10.1161/01.ATV.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 11.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 12.Moreira DM, da Silva RL, Vieira JL, Fattah T, Lueneberg ME, Gottschall CA. Role of vascular inflammation in coronary artery disease: potential of anti-inflammatory drugs in the prevention of atherothrombosis. Inflammation and anti-inflammatory drugs in coronary artery disease. Am J Cardiovasc Drugs. 2015;15(1):1–11. doi: 10.1007/s40256-014-0094-z. [DOI] [PubMed] [Google Scholar]
- 13.Drew BG, Duffy SJ, Formosa MF, et al. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation. 2009;119(15):2103–2111. doi: 10.1161/CIRCULATIONAHA.108.843219. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzawa Y, Yamashita S, Kameda K, Kubo M, Tarui S, Hara I. Marked hyper-HDL2-cholesterolemia associated with premature corneal opacity. A case report. Atherosclerosis. 1984;53(2):207–212. doi: 10.1016/0021-9150(84)90196-5. [DOI] [PubMed] [Google Scholar]
- 15.Deiana L, Pes GM, Carru C, Campus GV, Tidore MG, Cherchi GM. Extremely high HDL levels in a patient with multiple symmetric lipomatosis. Clin Chim Acta. 1993;223(1–2):143–147. doi: 10.1016/0009-8981(93)90070-K. [DOI] [PubMed] [Google Scholar]
- 16.Patsch W, Kuisk I, Glueck C, Schonfeld G. Lipoproteins in familial hyperalphalipoproteinemia. Arteriosclerosis. 1981;1(2):156–161. doi: 10.1161/01.atv.1.2.156. [DOI] [PubMed] [Google Scholar]
- 17.Qasim A, Rader DJ. Human genetics of variation in high-density lipoprotein cholesterol. Curr Atheroscler Rep. 2006;8(3):198–205. doi: 10.1007/s11883-006-0074-0. [DOI] [PubMed] [Google Scholar]
- 18.Glueck CJ, Fallat RW, Millett F, Gartside P, Elston RC, Go RC. Familial hyper-alpha-lipoproteinemia: studies in eighteen kindreds. Metabolism. 1975;24(11):1243–1265. doi: 10.1016/0026-0495(75)90063-3. [DOI] [PubMed] [Google Scholar]
- 19.Segrest JP, Li L, Anantharamaiah GM, Harvey SC, Liadaki KN, Zannis V. Structure and function of apolipoprotein A-I and high-density lipoprotein. Curr Opin Lipidol. 2000;11(2):105–115. doi: 10.1097/00041433-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Umemoto T, Han CY, Mitra P, et al. Apolipoprotein AI and high-density lipoprotein have anti-inflammatory effects on adipocytes via cholesterol transporters: ATP-binding cassette A-1, ATP-binding cassette G-1, and scavenger receptor B-1. Circ Res. 2013;112(10):1345–1354. doi: 10.1161/CIRCRESAHA.111.300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro MD. Rare genetic disorders altering lipoproteins. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext [Internet] South Dartmouth, MA: MDText.com, Inc; 2000. [Last accessed September 4, 2018]. http://www.ncbi.nlm.nih.gov/books/NBK326744. [Google Scholar]
- 22.Ooi EM, Barrett PH, Chan DC, Watts GF. Apolipoprotein C-III: understanding an emerging cardiovascular risk factor. Clin Sci (Lond) 2008;114(10):611–624. doi: 10.1042/CS20070308. [DOI] [PubMed] [Google Scholar]
- 23.Miller M, Rhyne J, Hamlette S, Birnbaum J, Rodriguez A. Genetics of HDL regulation in humans. Curr Opin Lipidol. 2003;14(3):273–279. doi: 10.1097/00041433-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Luo M, Peng D. The emerging role of apolipoprotein C-III: beyond effects on triglyceride metabolism. Lipids Health Dis. 2016;15(1):184. doi: 10.1186/s12944-016-0352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollin TI, Damcott CM, Shen H, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322(5908):1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Crosby J, Peloso GM, Auer PL, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371(1):22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tall AR. Plasma cholesteryl ester transfer protein. J Lipid Res. 1993;34(8):1255–1274. [PubMed] [Google Scholar]
- 28.Yamashita S, Matsuzawa Y, Okazaki M, et al. Small polydisperse low density lipoproteins in familial hyperalphalipoproteinemia with complete deficiency of cholesteryl ester transfer activity. Atherosclerosis. 1988;70(1–2):7–12. doi: 10.1016/0021-9150(88)90094-9. [DOI] [PubMed] [Google Scholar]
- 29.Koizumi J, Mabuchi H, Yoshimura A, et al. Deficiency of serum cholesteryl-ester transfer activity in patients with familial hyperalphalipoproteinaemia. Atherosclerosis. 1985;58(1–3):175–186. doi: 10.1016/0021-9150(85)90064-4. [DOI] [PubMed] [Google Scholar]
- 30.Maruyama T, Sakai N, Ishigami M, et al. Prevalence and phenotypic spectrum of cholesteryl ester transfer protein gene mutations in Japanese hyperalphalipoproteinemia. Atherosclerosis. 2003;166(1):177–185. doi: 10.1016/S0021-9150(02)00327-1. [DOI] [PubMed] [Google Scholar]
- 31.de Grooth GJ, Klerkx AH, Stroes ES, Stalenhoef AF, Kastelein JJ, Kuivenhoven JA. A review of CETP and its relation to atherosclerosis. J Lipid Res. 2004;45(11):1967–1974. doi: 10.1194/jlr.R400007-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Boekholdt SM, Thompson JF. Natural genetic variation as a tool in understanding the role of CETP in lipid levels and disease. J Lipid Res. 2003;44(6):1080–1093. doi: 10.1194/jlr.R200018-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Hirano KI, Nagasaka H, Kobayashi K, et al. Disease-associated marked hyperalphalipoproteinemia. Mol Genet Metab Rep. 2014;1:264–268. doi: 10.1016/j.ymgmr.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosmas CE, DeJesus E, Rosario D, Vittorio TJ. CETP Inhibition: past failures and future hopes. Clin Med Insights Cardiol. 2016;10:37–42. doi: 10.4137/CMC.S32667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tall AR, Rader DJ. Trials and tribulations of CETP inhibitors. Circ Res. 2018;122(1):106–112. doi: 10.1161/CIRCRESAHA.117.311978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Li W, Hao L, et al. The therapeutic potential of CETP inhibitors: a patent review. Expert Opin Ther Pat. 2018;28(4):331–340. doi: 10.1080/13543776.2018.1439476. [DOI] [PubMed] [Google Scholar]
- 37.Hoekstra M, Van Berkel TJ, Van Eck M. Scavenger receptor BI: a multi-purpose player in cholesterol and steroid metabolism. World J Gastroenterol. 2010;16(47):5916–5924. doi: 10.3748/wjg.v16.i47.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rigotti A, Miettinen HE, Krieger M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr Rev. 2003;24(3):357–387. doi: 10.1210/er.2001-0037. [DOI] [PubMed] [Google Scholar]
- 39.Constantineau J, Greason E, West M, et al. A synonymous variant in scavenger receptor, class B, type I gene is associated with lower SR-BI protein expression and function. Atherosclerosis. 2010;210(1):177–182. doi: 10.1016/j.atherosclerosis.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanoni P, Khetarpal SA, Larach DB, et al. CHD Exome+ Consortium; CARDIoGRAM Exome Consortium; Global Lipids Genetics Consortium. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351(6278):1166–1171. doi: 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson J, Rotondo D. Scavenger receptor B1 mutation, elevated HDL cholesterol and a paradoxical increase in atherosclerosis. Curr Opin Lipidol. 2016;27(5):541–542. doi: 10.1097/MOL.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 42.Vergeer M, Korporaal SJ, Franssen R, et al. Genetic variant of the scavenger receptor BI in humans. N Engl J Med. 2011;364(2):136–145. doi: 10.1056/NEJMoa0907687. [DOI] [PubMed] [Google Scholar]
- 43.Jaye M, Lynch KJ, Krawiec J, et al. A novel endothelial-derived lipase that modulates HDL metabolism. Nat Genet. 1999;21(4):424–428. doi: 10.1038/7766. [DOI] [PubMed] [Google Scholar]
- 44.Strauss JG, Zimmermann R, Hrzenjak A, et al. Endothelial cell-derived lipase mediates uptake and binding of high-density lipoprotein (HDL) particles and the selective uptake of HDL-associated cholesterol esters independent of its enzymic activity. Biochem J. 2002;368(Pt 1):69–79. doi: 10.1042/BJ20020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riederer M, Köfeler H, Lechleitner M, Tritscher M, Frank S. Impact of endothelial lipase on cellular lipid composition. Biochim Biophys Acta. 2012;1821(7):1003–1011. doi: 10.1016/j.bbalip.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96(12):1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 47.Edmondson AC, Brown RJ, Kathiresan S, et al. Loss-of-function variants in endothelial lipase are a cause of elevated HDL cholesterol in humans. J Clin Invest. 2009;119(4):1042–1050. doi: 10.1172/JCI37176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singaraja RR, Sivapalaratnam S, Hovingh K, et al. The impact of partial and complete loss-of-function mutations in endothelial lipase on high-density lipoprotein levels and functionality in humans. Circ Cardiovasc Genet. 2013;6(1):54–62. doi: 10.1161/CIRCGENETICS.111.962613. [DOI] [PubMed] [Google Scholar]
- 49.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rader DJ, deGoma EM. Approach to the patient with extremely low HDL-cholesterol. J Clin Endocrinol Metab. 2012;97(10):3399–3407. doi: 10.1210/jc.2012-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaefer EJ, Heaton WH, Wetzel MG, Brewer HB., Jr Plasma apolipoprotein A-1 absence associated with a marked reduction of high density lipoproteins and premature coronary artery disease. Arteriosclerosis. 1982;2(1):16–26. doi: 10.1161/01.atv.2.1.16. [DOI] [PubMed] [Google Scholar]
- 52.Ng DS, Leiter LA, Vezina C, Connelly PW, Hegele RA. Apolipoprotein A-I Q[-2]X causing isolated apolipoprotein A-I deficiency in a family with analphalipoproteinemia. J Clin Invest. 1994;93(1):223–229. doi: 10.1172/JCI116949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santos RD, Schaefer EJ, Asztalos BF, et al. Characterization of high density lipoprotein particles in familial apolipoprotein A-I deficiency. J Lipid Res. 2008;49(2):349–357. doi: 10.1194/jlr.M700362-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Santos RD, Asztalos BF, Martinez LR, Miname MH, Polisecki E, Schaefer EJ. Clinical presentation, laboratory values, and coronary heart disease risk in marked high-density lipoprotein-deficiency states. J Clin Lipidol. 2008;2(4):237–247. doi: 10.1016/j.jacl.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaefer EJ, Santos RD, Asztalos BF. Marked HDL deficiency and premature coronary heart disease. Curr Opin Lipidol. 2010;21(4):289–297. doi: 10.1097/MOL.0b013e32833c1ef6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franceschini G, Sirtori CR, Capurso A, 2nd, Weisgraber KH, Mahley RW. A-IMilano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J Clin Invest. 1980;66(5):892–900. doi: 10.1172/JCI109956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sirtori CR, Calabresi L, Franceschini G, et al. Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: the Limone sul Garda study. Circulation. 2001;103(15):1949–1954. doi: 10.1161/01.cir.103.15.1949. [DOI] [PubMed] [Google Scholar]
- 58.Alexander ET, Tanaka M, Kono M, Saito H, Rader DJ, Phillips MC. Structural and functional consequences of the Milano mutation (R173C) in human apolipoprotein A-I. J Lipid Res. 2009;50(7):1409–1419. doi: 10.1194/jlr.M800578-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franceschini G, Calabresi L, Chiesa G, et al. Increased cholesterol efflux potential of sera from ApoA-IMilano carriers and transgenic mice. Arterioscler Thromb Vasc Biol. 1999;19(5):1257–1262. doi: 10.1161/01.atv.19.5.1257. [DOI] [PubMed] [Google Scholar]
- 60.Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290(17):2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 61.Kallend DG, Reijers JA, Bellibas SE, et al. A single infusion of MDCO-216 (ApoA-I Milano/POPC) increases ABCA1-mediated cholesterol efflux and pre-beta 1 HDL in healthy volunteers and patients with stable coronary artery disease. Eur Heart J Cardiovasc Pharmacother. 2016;2(1):23–29. doi: 10.1093/ehjcvp/pvv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gursky O, Jones MK, Mei X, Segrest JP, Atkinson D. Structural basis for distinct functions of the naturally occurring Cys mutants of human apolipoprotein A-I. J Lipid Res. 2013;54(12):3244–3257. doi: 10.1194/jlr.R037911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez-Mendez O, Bruckert E, Franceschini G, et al. Metabolism of apolipoproteins AI and AII in subjects carrying similar apoAI mutations, apoAI Milano and apoAI Paris. Atherosclerosis. 2000;148(2):317–325. doi: 10.1016/S0021-9150(99)00279-8. [DOI] [PubMed] [Google Scholar]
- 64.Bielicki JK, Oda MN. Apolipoprotein A-I(Milano) and apolipoprotein A-I(Paris) exhibit an antioxidant activity distinct from that of wild-type apolipoprotein A-I. Biochemistry. 2002;41(6):2089–2096. doi: 10.1021/bi011716p. [DOI] [PubMed] [Google Scholar]
- 65.Matsunaga A. Apolipoprotein A-I mutations and clinical evaluation. In: Komoda T, editor. The HDL Handbook 2nd ed: Biological Functions and Clinical Implications. Elsevier Inc; 2014. pp. 9–35. [DOI] [Google Scholar]
- 66.Calabresi L, Pisciotta L, Costantin A, et al. The molecular basis of lecithin:cholesterol acyltransferase deficiency syndromes: a comprehensive study of molecular and biochemical findings in 13 unrelated Italian families. Arterioscler Thromb Vasc Biol. 2005;25(9):1972–1978. doi: 10.1161/01.ATV.0000175751.30616.13. [DOI] [PubMed] [Google Scholar]
- 67.von Eckardstein A. Differential diagnosis of familial high-density lipoprotein deficiency syndromes. Atherosclerosis. 2006;186(2):231–239. doi: 10.1016/j.atherosclerosis.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 68.Asztalos BF, Schaefer EJ, Horvath KV, et al. Role of LCAT in HDL remodeling: investigation of LCAT deficiency states. J Lipid Res. 2007;48(3):592–599. doi: 10.1194/jlr.M600403-JLR200. [DOI] [PubMed] [Google Scholar]
- 69.Najafian B, Lusco MA, Finn LS, Alpers CE, Fogo AB. AJKD atlas of renal pathology: Lecithin-Cholesterol Acyltransferase (LCAT) deficiency. Am J Kidney Dis. 2017;70(1):e5–e6. doi: 10.1053/j.ajkd.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 70.Calabresi L, Simonelli S, Gomaraschi M, Franceschini G. Genetic lecithin:cholesterol acyltransferase deficiency and cardiovascular disease. Atherosclerosis. 2012;222(2):299–306. doi: 10.1016/j.atherosclerosis.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 71.Kunnen S, Van Eck M. Lecithin:cholesterol acyltransferase: old friend or foe in atherosclerosis? J Lipid Res. 2012;53(9):1783–1799. doi: 10.1194/jlr.R024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oldoni F, Baldassarre D, Castelnuovo S, et al. Complete and partial LCAT deficiency are differentially associated with atherosclerosis. Circulation. 2018 May 10; doi: 10.1161/CIRCULATIONAHA.118.034706. pii: CIRCULATIONAHA.118.034706 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 73.Fredrickson DS, Altrocchi PH, Avioli LV, Goodman DWS, Goodman HC. Tangier disease. Ann Intern Med. 1961;55(6):1016–1031. doi: 10.7326/0003-4819-55-6-1016. [DOI] [Google Scholar]
- 74.Brooks-Wilson A, Marcil M, Clee SM, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22(4):336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 75.Weissglas-Volkov D, Pajukanta P. Genetic causes of high and low serum HDL-cholesterol. J Lipid Res. 2010;51(8):2032–2057. doi: 10.1194/jlr.R004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oram JF, Vaughan AM. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99(10):1031–1043. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- 77.Clee SM, Kastelein JJ, van Dam M, et al. Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J Clin Invest. 2000;106(10):1263–1270. doi: 10.1172/JCI10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chyu KY, Shah PK. HDL/ApoA-1 infusion and ApoA-1 gene therapy in atherosclerosis. Front Pharmacol. 2015;6:187. doi: 10.3389/fphar.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nicholls SJ, Puri R, Ballantyne CM, et al. Effect of infusion of high-density lipoprotein mimetic containing recombinant apolipoprotein A-I Milano on coronary disease in patients with an acute coronary syndrome in the MILANO-PILOT trial: a randomized clinical trial. JAMA Cardiol. 2018 Jul 25; doi: 10.1001/jamacardio.2018.2112. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nanjee MN, Crouse JR, King JM, et al. Effects of intravenous infusion of lipid-free apo A-I in humans. Arterioscler Thromb Vasc Biol. 1996;16(9):1203–1214. doi: 10.1161/01.atv.16.9.1203. [DOI] [PubMed] [Google Scholar]
- 81.Tardif JC, Grégoire J, L’Allier PL, et al. Effect of rHDL on Atherosclerosis-Safety and Efficacy (ERASE) Investigators. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297(15):1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 82.Gille A, Easton R, D’Andrea D, Wright SD, Shear CL. CSL112 enhances biomarkers of reverse cholesterol transport after single and multiple infusions in healthy subjects. Arterioscler Thromb Vasc Biol. 2014;34(9):2106–2114. doi: 10.1161/ATVBAHA.114.303720. [DOI] [PubMed] [Google Scholar]
- 83.Michael Gibson C, Korjian S, Tricoci P, et al. Safety and tolerability of CSL112, a reconstituted, infusible, plasma-derived apolipoprotein A-I, after acute myocardial infarction: the AEGIS-I trial (ApoA-I Event Reducing in Ischemic Syndromes I) Circulation. 2016;134(24):1918–1930. doi: 10.1161/CIRCULATIONAHA.116.025687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tardif JC, Ballantyne CM, Barter P, et al. Can HDL Infusions Significantly QUicken Atherosclerosis REgression (CHI-SQUARE) Investigators. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J. 2014;35(46):3277–3286. doi: 10.1093/eurheartj/ehu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kootte RS, Smits LP, van der Valk FM, et al. Effect of open-label infusion of an apoA-I-containing particle (CER-001) on RCT and artery wall thickness in patients with FHA. J Lipid Res. 2015;56(3):703–712. doi: 10.1194/jlr.M055665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Asokan A, Samulski RJ. An emerging adeno-associated viral vector pipeline for cardiac gene therapy. Hum Gene Ther. 2013;24(11):906–913. doi: 10.1089/hum.2013.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin XL, Hu HJ, Liu YB, et al. Allicin induces the upregulation of ABCA1 expression via PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells. Int J Mol Med. 2017;39(6):1452–1460. doi: 10.3892/ijmm.2017.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rousset X, Vaisman B, Auerbach B, et al. Effect of recombinant human lecithin cholesterol acyltransferase infusion on lipoprotein metabolism in mice. J Pharmacol Exp Ther. 2010;335(1):140–148. doi: 10.1124/jpet.110.169540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shamburek RD, Bakker-Arkema R, Shamburek AM, et al. Safety and tolerability of ACP-501, a recombinant human Lecithin:cholesterol acyltransferase, in a phase 1 single-dose escalation study. Circ Res. 2016;118(1):73–82. doi: 10.1161/CIRCRESAHA.115.306223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shamburek RD, Bakker-Arkema R, Auerbach BJ, et al. Familial lecithin:cholesterol acyltransferase deficiency: first-in-human treatment with enzyme replacement. J Clin Lipidol. 2016;10(2):356–367. doi: 10.1016/j.jacl.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]