Abstract
Vitiligo repigmentation is a complex process in which the melanocyte-depleted interfollicular epidermis (IE) is repopulated by melanocyte precursors from hair follicle (HF) bulge that proliferate, migrate, and differentiate into mature melanocytes on their way to the epidermis. The strongest stimulus for vitiligo repigmentation is narrow band UVB (NBUVB), but how the HF melanocyte precursors are activated by UV light has not been extensively studied. To better understand this process, we developed an application that combined laser capture microdissection and subsequent whole transcriptome RNA sequencing of HF bulge melanocyte precursors, and compared their gene signature to that of regenerated mature epidermal melanocytes from the NBUVB-treated vitiligo skin. Using this strategy, we found upregulation of Tenascin C (TNC), Gap junction beta-6 protein (GJB6) and Thrombospondin 1 (THBS1) in the HF bulge melanocytes and of Tyrosinase (TYR) in the epidermal melanocytes of the NBUVB-treated vitiligo skin. We validated these results by qRT-PCR using NBUVB-treated vitiligo skin and untreated normal skin. We also identified that GLI1, a candidate stem cell-associated gene, is significantly upregulated in the melanocytes captured from NBUVB-treated vitiligo bulge, as compared to untreated vitiligo bulge. The above signals are potential key players in the activation of bulge melanocyte precursors during vitiligo repigmentation.
Keywords: Vitiligo, Repigmentation, Laser capture microdissection, RNA sequencing, Melanocyte, Regeneration
INTRODUCTION
Vitiligo is a common depigmention disorder, affecting 0.3–0.5% of the population worldwide. It is characterized by white patches of the skin, due to autoimmune destruction of epidermal melanocytes by melanocyte-reactive cytotoxic T cells (Spritz 2013; Eby et al., 2014; Rashighi and Harris, 2017). Vitiligo repigmentation is characterized by repopulation of melanocyte-depleted interfollicular epidermis (IE) with melanocyte precursors, mostly originating from the hair follicle (HF) (Birlea et al., 2016). The strongest stimulus for the activation of melanocyte precursors is narrow band UVB (NBUVB), but this process has not yet been adequately studied in the HF. Our recent immunostaining study, using key-functional markers, identified that the HF bulge of depigmented vitiligo skin is inhabited by melanocyte stem cells (DCT(+)/C-KIT(−)) and melanoblasts (DCT(+)/C-KIT(+)), which proliferate, migrate and differentiate during NBUVB treatment on their way to epidermis (Goldstein et al., 2015). The proportion of melanocyte precursor populations slightly increased in the HF bulge after NBUVB treatment, indicative of melanocyte activation. To more thoroughly characterize the repigmentation process, we developed an application, which, to our knowledge was previously unreported, that combined laser capture microdissection of skin cells isolated from vitiligo patients with RNA-sequencing. With this application, we tested the gene signature of the HF bulge (the source of melanocyte precursors) and of the IE (the repopulated site) in the NBUVB-treated vitiligo skin. Our strategy consisted of a rapid immunostaining protocol, followed by fluorescent laser capture microdissection (F-LCM) of melanocytes and separately of keratinocytes from the HF bulge and epidermal basal layer, followed by RNA isolation, whole transcriptome RNA sequencing (RNA-Seq), and gene expression analysis. Our RNA-seq analysis identified the Integrin pathway as the top pathway activated in the bulge melanocytes of NBUVB-treated vitiligo skin, as compared with NBUVB-treated vitiligo epidermis, and β-catenin as top upstream transcription regulator. We also found upregulation of Tenascin C (TNC), Gap junction beta-6 protein (GJB6) and Thrombospondin1 (THBS1) in the bulge melanocytes and of Tyrosinase (TYR) in the epidermal melanocytes of NBUVB-treated vitiligo skin, results validated by qRT-PCR. We also identified that GLI1, a candidate stem cell-associated gene, is significantly upregulated in melanocytes captured from the NBUVB-treated vitiligo bulge as compared to the untreated vitiligo bulge. The above signals and pathway may have regulatory roles in the activation of bulge melanocyte precursors during vitiligo repigmentation process.
RESULTS
We used frozen biopsies from untreated and NBUVB-treated vitiligo patients to perform bulge mapping, rapid immunostaining, F-LCM of specific skin cells, and RNA-seq analysis.
Alignment and mapping of RNA sequencing reads
Whole transcriptome RNA sequencing (RNA-Seq), performed with an Illumina HiSeq 2000 sequencer, generated on average 3.2 and 3.6 million single read sequences per sample from each studied site (HF bulge and IE respectively), with an average length of 250 bp without adaptors and of 325 bp with adaptors. The median total raw reads for the bulge and IE samples were ~ 5.3 million and 5.9 million respectively, and the median passing rates for the bulge and epidermis were 84.6% and 85.6% respectively, indicating good data quality. We discarded samples that did not meet the control criteria due to low quality, presence of contaminants formed by adapter-adapter ligation, and presence of reads without insert tags.
RNA-Seq gene expression profiles show cell-specific and site-specific F-LCM capture differences
We isolated RNA from both melanocyte and keratinocyte samples. The purpose of including the keratinocytes was to demonstrate the specificity of our capture. After the validation step, we focused the current study on the melanocyte material of the NBUVB-treated vitiligo skin. The total RNA from melanocyte samples and separately from keratinocyte samples was isolated by F-LCM from 6 vitiligo NBUVB-treated patients, and subjected to RNA-seq followed by gene expression analysis. From the principal component analysis (PCA), we observed a good segregation of gene expression values between: 1) the melanocytes captured from the NBUVB-treated vitiligo bulge versus the melanocytes from the NBUVB-treated vitiligo IE (Fig. S1a); 2) the keratinocytes captured from the NBUVB-treated vitiligo bulge versus keratinocytes from the treated vitiligo IE (Fig. S1b); 3) the melanocytes versus keratinocytes captured from the NBUVB-treated vitiligo IE (Fig. S1c); 4) the melanocytes versus keratinocytes captured from the NBUVB-treated vitiligo bulge (Fig. S1d). Additionally, we examined the expression of melanocyte-specific genes and keratinocyte-specific genes in our samples to determine cell-specificity of capture (Fig. 1, Panels A–D). We found significant enrichment of the melanocyte-specific genes in melanocytes compared to keratinocytes and significant enrichment of the keratinocyte-specific genes in keratinocytes compared to melanocytes in both NBUVB-treated vitiligo epidermis (Panel C) and NBUVB-treated vitiligo bulge (Panel D). Based on the results of PCA and cell-type-specific gene expression analysis, we concluded that our F-LCM method is effective and accurate for selectively isolating RNA material from distinct populations of melanocytes and adjacent keratinocytes.
Fig. 1. Heatmaps of melanocyte (MC)-and keratinocyte (KC)-specific genes in the RNA-seq data.
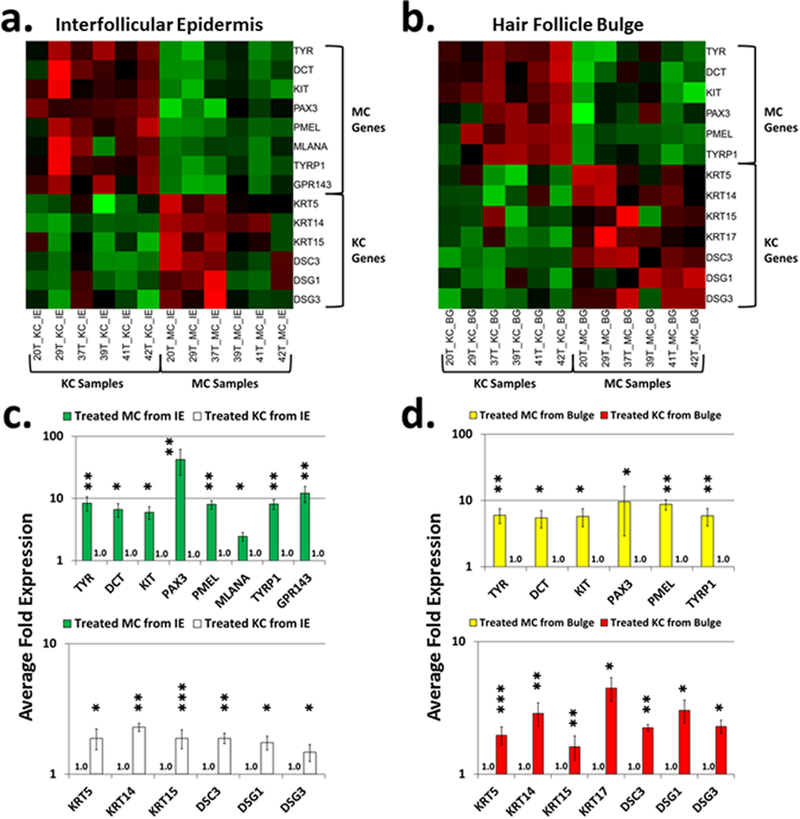
Heatmaps of RNA expression levels of melanocyte-specific and keratinocyte-specific genes in the vitiligo skin of NBUVB-treated IE (Panel a) and of NBUVB-treated bulge (Panel b) (N=6). We found upregulation of melanocyte-specific genes in melanocyte samples (green squares, right-sided upper corner) and keratinocyte-specific genes in keratinocyte samples (green squares, left-sided lower corner). The red squares represent downregulated melanocyte-specific genes. Bar plots represent the gene enrichment in the interfollicular epidermis (Panel c) and bulge (Panel d) +/− standard error of the mean (SEM) (N=6). In both NBUVB-treated IE and NBUVB-treated bulge we found a significant enrichment of melanocyte-specific genes in the melanocyte samples [upper figures of Panel c (green bars) and of Panel d (yellow bars), with expression values set to 1-fold in keratinocytes] and a significant enrichment of keratinocyte-specific genes in the keratinocytes samples [lower figures of Panel c (white bars), and Panel d (red bars), with expression values set to 1-fold in melanocytes]. (*P <0.05; **P <0.01; ***P <0.001). Each bar represents mean and standard deviation (n=3).
RNA-Seq reveals differentially expressed genes in the melanocyte samples of the HF bulge as compared with epidermis of the NBUVB-treated vitiligo skin
Next, we focused our analysis on characterization of melanocyte samples captured from different anatomic regions (IE and HF bulge) of 6 NBUVB-treated vitiligo patients. Of the total 54,009 genes assessed by RNA-Seq, 39 (0.07%) were differentially expressed in the melanocyte samples from NBUVB-treated vitiligo bulge as compared to the patient-matched melanocyte samples from NBUVB-treated vitiligo IE, including 27 significantly upregulated genes (0.05%) and 12 significantly downregulated genes (0.02%) [False Discovery Rate (FDR)-adjusted Q-value≤0.05)]. The top 10 differentially expressed protein-coding genes in the melanocyte samples are summarized in Table 1. We selected for qRT-PCR validation the top 5 differentially expressed genes, among which TNC (Q=2.E-02, fold change (FC)=22.7), THBS1 (Q=2.7E-02, FC=20.2), TM9SF3 (Q=2.7E–02, FC=2.5) and GJB6 (Q=2.9E-05, FC=27.7) were upregulated in the melanocytes of the NBUVB-treated vitiligo bulge, while TYR (Q=2.7E–02, FC=9.1) was upregulated in the melanocytes of the NBUVB-treated vitiligo IE. We confirmed that TNC (Padjusted=7.1E–03; FC=65.8), THBS1 (Padjusted=1.7E-02; only amplified in melanocyte samples from the bulge), and GJB6 (Padjusted=4.9E–02; FC=185.3) were upregulated in bulge melanocytes of NBUVB-treated vitiligo skin, and that TYR (Padjusted=2.0E–03; FC=65.0) was upregulated in epidermal melanocytes of NBUVB-treated vitiligo skin (Fig. 2a). In the IE melanocytes, we could not detect THBS1 transcript amplification in 4 NBUVB-treated patients, or GJB6 transcript amplification in 2 of the 4 NBUVB-treated patients.
Table 1. Top genes differentially expressed in the RNA-Seq study.
Top 10 protein encoding genes differentially expressed in the melanocyte samples from the NBUVB-treated vitiligo bulge as compared with melanocyte samples from the NBUVB-treated interfollicular epidermis. Fold changes, P-values, FDR-adjusted P-values (Q-values), and gene functions are provided.
| Gene | Fold Change Bulge |
P-value | Q-value | Known Function | Reference |
|---|---|---|---|---|---|
| TNC | 22.7 | 4.0E-06 | 0.020 |
Promotes β-catenin-mediated transcription in the presence of Wnt3a Highly expressed during embryonic development of humans, mouse |
Hendaoui et al., 2014 Imanaka-Yoshida and Aoki, 2014 |
| THBS1 | 20.2 | 1.4E-05 | 0.027 |
Downstream effector of p53, which coordinates UV-induced
pigmentation; highly expressed in embryonic stem cells |
Sundaram et al., 2011 Liu et al., 2006 |
| TYR | −9.1 | 1.7E-05 | 0.027 |
Involved in melanin biosynthesis; highly expressed by regenerated
epidermal melanocytes in the NBUVB-treated vitiligo |
Goldstein et al., 2015 |
| TM9SF3 | 2.5 | 2.2E-05 | 0.027 | Marker of tumor invasion | Oo et al., 2014 |
| GJB6 | 27.7 | 3.3E-05 | 0.029 |
Mutated in Clouston syndrome, which is associated with impairment
of hair growth; expressed in human skin and mouse embryo |
Lamartine et al., 2000 Fujimoto et al., 2013 |
| SAMD5 | 72.6 | 3.7E-05 | 0.029 | Stem cell gene in the mouse bulge | Kadaja et al. 2014 |
| CTNND2 | 188.1 | 5.6E-05 | 0.029 |
Promotes disruption of adherens junction by E-cadherin, inducing
cell migration |
Zhang et al., 2009 |
| TRPS1 | 12.4 | 5.8E-05 | 0.029 | Regulates epithelial proliferation in the mouse embryo | Fantauzzo et al., 2012 |
| LAMB4 | −26.9 | 6.1E-05 | 0.029 | Identified overexpressed in malignant melanoma | Liu et al., 2013 |
| SOX9 | 18.9 | 6.2E-05 | 0.029 |
Stem cell marker detected in >80% of malignant melanomas Stem cell gene in the human bulge |
Jo et al., 2014 Goldstein et al., 2016 |
Fig. 2. Expression of top genes differentially expressed in the NBUVB-treated vitiligo bulge melanocytes and NBUVB-treated epidermis melanocytes.
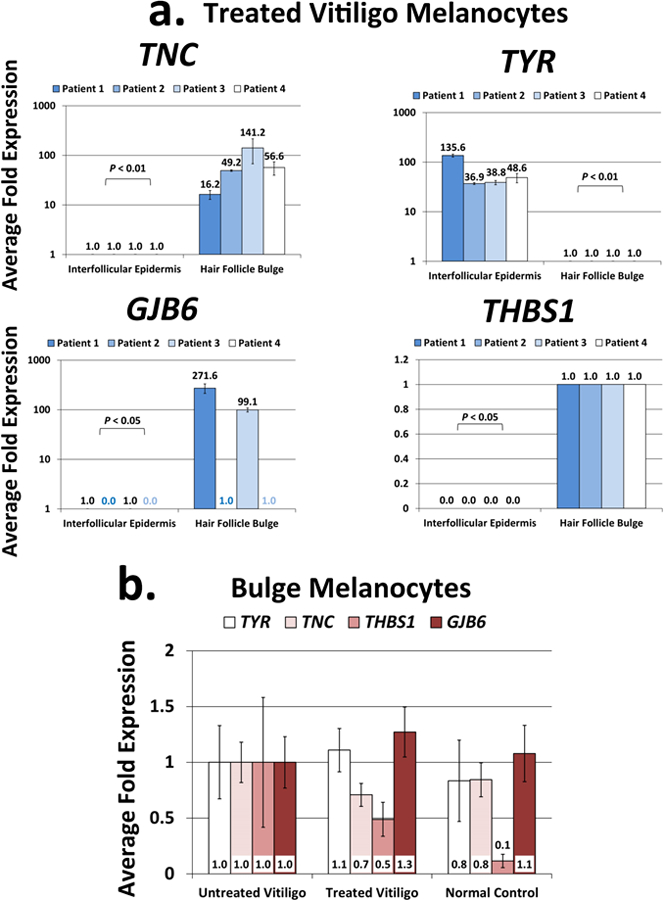
Fig.2a. qRT-PCR confirmation of the top differentially expressed genes resulted from the RNA-seq study: TNC, GJB6 and THBS1 were significantly upregulated in the melanocyte samples from the NBUVB-treated bulge, while TYR was significantly upregulated in the melanocyte samples from the NBUVB-treated IE (Paired T-test, Padjusted<0.05) (N=4 NBUVB-treated vitiligo patients). Each bar represents mean and standard deviation (n=3). Expression values were set to 1-fold in the IE for TNC and THBS1, and in the bulge for TYR and GJB6.
Fig.2b. qRT-PCR gene expression analysis comparing the expression values of TNC, GJB6, THBS1 and TYR in the bulge melanocyte samples collected from NBUVB-treated vitiligo patients (N=6), untreated vitligo patients (N=4), and normal control subjects (N=6). The expression values of the 4 genes did not vary significantly between the groups tested (one-way ANOVA, Tukey’s Post Hoc Test: adjusted P>0.05 for all pairwise comparisons). Expression values were set to 1-fold in the untreated vitiligo samples. Each bar represents mean and standard deviation (n=3).
qRT-PCR reveals differentially expressed genes in the melanocyte samples from the bulge versus epidermis of normal untreated control skin
We tested the expression of top genes (that were found by the RNA-seq study and were validated by qRT-PCR) in melanocytes captured from normal untreated skin of 6 healthy controls. We identified a similar expression trend with that observed in the NBUVB-treated vitiligo skin: upregulation in bulge melanocytes of TNC (Padjusted=2.4E-02; FC=11.79), GJB6 (Padjusted=1.0E-03; FC=30.48), and THBS1 (Padjusted=0.15; only amplified in 4/6 bulge samples), and upregulation in epidermal melanocytes of TYR (Padjusted=7.0E-04; FC=13.44) (Fig. S2).
Next, we tested whether the expression of TNC, GJB6, THBS1, and TYR varied in the bulge melanocytes captured from untreated vitligo skin (N=4), NBUVB-treated vitligo skin (N=6), and normal skin (N=6). We did not find significant variation either among the three groups tested or among any of the paired comparisons (Fig. 2b).
Pathway analysis, upstream regulators and disease functions
To better understand the biology and functional relationship among differentially expressed genes in our RNA-Seq data, we performed pathway analysis using the Ingenuity pathway analysis (IPA) tool and a combined melanocyte and keratinocyte data set of 1873 differentially expressed genes (P<0.05), comparing gene expression values between samples captured from the NBUVB-treated vitiligo bulge and samples captured from the NBUVB-treated vitiligo IE. We found 15 canonical pathways activated and 1 downregulated in the bulge compared to the IE (Table 2). The top activated pathway in the bulge (with the highest Z-score=3.16 and the lowest P-value=1.0E-11) was Integrin signaling, and its component genes that were differentially expressed (P<0.05) are provided in Table S2. The top putative upstream regulator in the bulge was CTNNB1 encoding β-catenin (Z-score=3.61; P-value=2.0E-16) (Table S3), and the top activated cellular function was cellular movement (Z-score=3.30; P-value=1.4E-42) (Table S4).
Table 2. Top canonical pathways in the RNA-Seq study.
Top canonical pathways generated by combined datasets of melanocyte and keratinocyte samples from the NBUVB-treated vitiligo bulge versus NBUVB-treated vitiligo interfollicular epidermis (differentially expressed genes, P<0.05). P-values, Z-scores, the number of upregulated or down regulated genes in each pathway, gene functions, and reference articles are provided.
| Ingenuity Canonical Pathways | P-value | Z-score | Down | Up | Function | Reference |
|---|---|---|---|---|---|---|
| Integrin Signaling | 1.0E-10 | 3.162 | 8/201 (4%) | 37/201 (18%) |
Melanoma progression and metastasis |
Kuphal et. al, 2005 |
| Paxillin Signaling | 2.1E-09 | 2.600 | 4/101 (4%) | 24/101 (24%) |
Decreased paxillin impairs adequate generation of pro-melanoma signals |
Velasco-Velázquez et al., 2008 |
| ILK Signaling | 3.6E-08 | 3.569 | 7/186 (4%) | 31/186 (17%) |
Pro-proliferative roles on several types
of cancers, including melanoma |
Dai et al., 2003 |
|
Mouse Embryonic Stem Cell
Pluripotency |
2.0E-07 | 2.858 | 4/95 (4%) | 20/95 (21%) | Pro-proliferative roles | Niwa et al., 2007 |
|
Regulation of eIF4 and p70S6K
Signaling |
2.2E-07 | 2.138 | 4/145 (3%) | 27/145 (19%) | Pro-proliferative roles | Flynn et al., 1996 |
| Rac Signaling | 3.2E-07 | 2.449 | 7/104 (7%) | 18/104 (17%) | Controls melanocyte dendricity | Scott et al., 2003 |
| Signaling by Rho Family GTPases | 2.2E-06 | 2.667 | 10/234 (4%) | 30/234 (13%) | Controls melanocyte dendricity | Scott et al., 2003 |
| Calcium Signaling | 2.7E-06 | 2.132 | 7/178 (4%) | 26/178 (15%) |
Regulator of keratinocyte
differentiation |
Bikle et al., 2012 |
| EIF2 Signaling | 5.9E-06 | 3.638 | 2/184 (1%) | 31/184 (17%) |
Key component of the translation
initiation system in living cells |
Stolboushkina and Garber, 2011 |
|
Role of NANOG in Mammalian
Embryonic Stem Cell Pluripotency |
1.3E-05 | 2.646 | 6/111 (5%) | 17/111 (15%) |
NANOG functions as trigger for the
reprogramming process in human cells |
Silva et al., 2009 |
|
Melanocyte Development and
Pigmentation Signaling |
2.0E-05 | 2.065 | 5/84 (6%) | 14/84 (17%) |
Melanocyte proliferation and
differentiation |
Costin and Hearing 2007 |
| PAK Signaling | 4.8E-05 | 2.357 | 3/89 (3%) | 16/89 (18%) |
Promotes α-MSH/UVB-induced
melanogenesis |
Yun et al., 2015 |
| Wnt/Ca+ pathway | 7.8E-05 | 2.887 | 2/56 (4%) | 12/56 (21%) |
Putatively involved in the invasion
and metastasis of melanoma |
Yang et al., 2014 |
| IGF-1 Signaling | 1.6E-04 | 2.496 | 5/97 (5%) | 14/97 (14%) |
Regulates keratinocyte shape and
migration |
Haase et al., 2003 |
| FGF Signaling | 8.1E-04 | 2.500 | 3/85 (4%) | 13/85 (15%) |
Regulates cellular proliferation,
survival, migration and differentiation |
Turner and Grose 2010 |
| RhoGDI Signaling | 1.1E-05 | −2.191 | 9/173 (5%) | 22/173 (13%) |
Rho family GTPase inhibitor;
suppresses melanoma cell growth |
Wang et al., 2011 |
Candidate stem cell-associated gene expression analysis
To describe the stem cell gene signature in the bulge, we examined the RNA-Seq expression of 189 genes from a published stem cell differentiation panel (nCounter® Virtual Stem Cell Gene Set, Nanostring Technologies). The top differentially expressed genes in melanocytes from the NBUVB-treated bulge compared with the NBUVB-treated epidermis were FZD7 (P=4.1E-04; Q-value=0.05; FC=46.9) and GLI1 (P=7.7E-04; Q-value=0.06; FC=49.6) (Fig. 3a). We further validated these results by qRT-PCR using new laser capture rounds of melanocytes from NBUVB-treated vitiligo patients (N=7) [FZD7 (Padjusted=4.0E-03; FC=39.5); GLI1 (Padjusted=9.7E-04; only amplified in bulge samples)] (Fig. 3b). Next, we examined whether NBUVB modulates the expression of FZD7 and GLI1 transcripts in the bulge melanocytes isolated from NBUVB-treated vitiligo skin (N=7), untreated vitiligo skin (N=6), or control skin (N=6). We found that GLI1 was significantly upregulated in the bulge melanocytes of NBUVB-treated vitiligo skin, compared to untreated vitiligo skin (Padjusted=2.7E-03; FC=9.2), but did not significantly vary in other comparisons (Fig. 3c). In addition, FZD7 transcript was not significantly modulated by NBUVB after multiple testing correction (Padjusted=0.5; FC=1.5) (Fig. 3c).
Fig. 3. Candidate stem cell associated gene expression analysis in the RNA-Seq data and GLI1 confirmation in the hair follicle bulge of vitiligo skin.
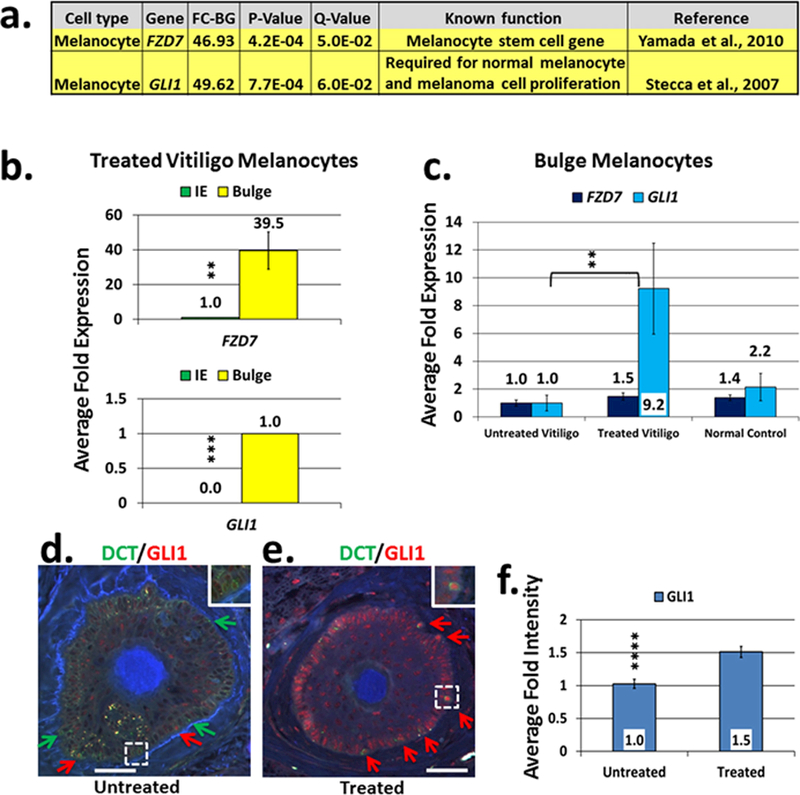
Fig.3a. Candidate stem cell associated gene expression analysis of the RNA-seq data: FZD7 and GLI1 were the top candidate stem cell-associated genes differentially expressed in the melanocyte samples from the bulge versus interfollicular epidermis collected from NBUVB-treated vitiligo patients (N=6).
Fig.3b. qRT-PCR confirmation study of FZD7 and GLI1 following the RNA-seq study: both genes were significantly upregulated in the melanocyte samples from the bulge compared to the interfollicular epidermis (IE) of NBUVB-treated vitiligo patients (N=6) (**Padjusted <0.01; ***Padjusted <0.001). RNA analyzed was extracted from melanocyte samples after new rounds of laser capture microdissection. Expression values were set to 1-fold in the IE for FZD7 and in the bulge for GLI1.
Fig.3c. Analysis of NBUVB effects on FZD7 and GLI1 in the human HF bulge of vitiligo skin: Expression analysis of FZD7 and GLI1 in melanocyte samples from the bulge of NBUVB-treated vitiligo skin (N=7), untreated vitiligo skin (N=6) and normal control skin (N=6). GLI1 expression showed significant upregulation in the bulge of NBUVB-treated vs untreated vitiligo samples (**Padjusted<0.01), and did not show significant variation in the other comparisons. Expression values were set to 1-fold in the untreated vitiligo samples. Each bar represents mean and standard deviation (n=3).
Fig.3d–f. Immunostaining analysis of GLI1 localization in the hair follicle bulge.
Figs. 3d and 3e. Double fluorescent immunostaining of FFPE transverse sections using an anti-GLI1 antibody (red) in combination with anti-DCT antibody (green, labels melanocytes) in the untreated vitiligo bulge (3d) and NBUVB-treated vitiligo bulge (3e). White scale bars: 50 μm. Red arrows indicate DCT(+)/GLI1(+) cells; green arrows indicate DCT(+)/GLI1(−) cells. White dotted lines indicate area of higher magnification (inset). GLI1 is also expressed in the bulge keratinocytes which are DCT(−)/GLI1(+) cells.
Fig. 3f. Signal intensity analysis for the anti-GLI1 antibody. The average intensity of the anti-GLI1 antibody signal is significantly higher in the bulge melanocytes of the NBUVB-treated vitiligo skin (N=7) as compared with untreated vitiligo skin (N=7) (****P=4.4E-04; +1.5-fold).
Next, to validate the expression of GLI1 in the bulge melanocytes in response to NBUVB, we performed immunohistochemistry using an anti-GLI1 antibody combined with anti-DCT antibody (melanocyte-specific) using skin from new NBUVB-treated vitiligo patients (N=6) and untreated patients (N=6). GLI1 protein was expressed in the bulge melanocytes of untreated vitligo skin, carrying the DCT(+)/GLI1(+) phenotype, and in the bulge keratinocytes of untreated vitligo skin, carrying the DCT(−)/GLI1(+) phenotype (Fig. 3d); GLI1 was also expressed in the bulge melanocytes and keratinocytes of NBUVB-treated vitiligo skin (Fig. 3e), and in the melanocytes and keratinocytes of NBUVB-treated and untreated epidermis (not shown). We observed that GLI1(−) melanocytes, carrying the DCT(+)/GLI1(−) phenotype, and GLI1(−) keratinocytes, carrying the DCT(−)/GLI1(−) phenotype were more numerous in the untreated bulge (Fig. 3d), while GLI1(+) melanocytes DCT(+)/GLI1(+) and GLI1(+) keratinocytes DCT(−)/GLI1(+) were more numerous in the NBUVB-treated bulge (Fig. 3e). Intensity analysis of microscopic images revealed a stronger anti-GLI1 antibody signal in the bulge melanocytes of NBUVB-treated vitiligo skin as compared to the signal in untreated vitiligo skin (P=4.4E-04; FC=1.5) (Fig. 3f).
We further examined in the RNA-seq data the expression of two gene targets that directly interact with GLI1: SOX9 (encoding SOX9) (Deng et al., 2015) and CTNNB1 (encoding β-catenin) (Liao et al., 2009), together with other key-components of Wnt/β-catenin pathway (FZD8, SFRP1, WIF1, and FZD8) (Fig. S3). We found that the expression values of SOX9 transcript (Q=2.0E-02;P=6.2E-05;FC=18.9) and SFRP1 transcript (Q=3.0E-02;P=8.3E-05; FC=43.8), were significantly upregulated in the melanocyte samples of NBUVB-treated vitiligo bulge as compared to those of NBUVB-treated vitiligo epidermis, while expression differences of CTNNB1, FZD8, and WIF1 did not surpass the FDR adjustment threshold (Q≤0.05) (Fig. S3).
DISCUSSION
In this study, we report an application (which, to our knowledge, is previously unreported), consisting of rapid immunostaining combined with F-LCM [to isolate RNA from specific cells (melanocytes), located at specific sites (bulge and epidermis)], and with RNA sequencing. In a previous study, we showed that the RNA captured from the HF bulge and epidermal melanocytes of the NBUVB-treated vitiligo and normal skin is of satisfactory quality for qRT-PCR analysis (Goldstein et al., 2016). Our current application offers a better characterization of melanocyte populations in the regenerated epidermis and the bulge of the NBUVB-treated vitiligo skin, because it uses RNA-Seq technology that has deeper coverage and higher sensitivity than qRT-PCR. We showed that the resulting RNA from the enriched melanocyte or keratinocyte samples is of satisfactory quality and reliability for RNA-Seq based on: i). high rate of passed sequences in all RNA-Seq samples; ii). significant enrichment of melanocyte-specific genes in the melanocyte samples and of keratinocyte-specific genes in the keratinocyte samples, in both epidermis and bulge of NBUVB-treated vitiligo skin (Fig. 1a–d); iii). good segregation of gene signatures between the mature epidermal reservoir and the bulge stem cell reservoir of the NBUVB-treated vitiligo skin, in both melanocyte and keratinocyte samples (Fig. S1a,b); iv). good segregation of gene signatures between the melanocyte and keratinocyte samples, in both epidermis and bulge of the NBUVB-treated skin (Fig. S1c,d).
We found by RNA-Seq analysis and confirmed by qRT-PCR that TNC, GJB6, and THBS1 were significantly upregulated in the melanocyte samples from the NBUVB-treated vitiligo bulge, while TYR was significantly upregulated in the melanocyte samples of the NBUVB-treated epidermis (Fig. 2a). We observed similar expression trends in the samples of untreated normal skin (Fig. S2). All of these data indicate that we have successfully isolated RNA from stem-like melanocytic cells in the bulge that are TNC(+), GJB6(+) and THBS1(+) and from differentiated melanocytes that are TYR(+) in the IE. TNC encodes an extracellular matrix protein that promotes CTNNB1 transcription in the presence of Wnt3a, which is required for Wnt/β-catenin signaling in the mouse HF bulge (Hendaoui et al., 2014). GJB6 encodes a gap junction protein responsible for Clouston syndrome, which is associated with hair growth impairment (Lamartine et al., 2000). THBS1 is a downstream effector of p53 (Sundaram et al., 2011), which controls pigmentation in response to UV (Birlea et al., 2016). Interestingly, TNC, GJB6 and THBS1 in the bulge were not upregulated by the NBUVB treatment. It is possible that NBUVB can exert short-lived effects on these genes, which should be the subject of more comprehensive future study at different time points.
We found that TYR was the top gene significantly upregulated in the epidermal melanocytes of NBUVB-treated vitiligo skin, providing further validation of our method. TYR encodes tyrosinase, the major enzyme of melanin biosynthesis, and is highly expressed in melanocytes undergoing differentiation (Cichorek et al., 2013), e.g. epidermal melanocytes. Using immunostaining and qRT-PCR, we previously identified TYR upregulation in the epidermal melanocytes of NBUVB-treated vitiligo skin and unremarkable TYR expression in the bulge melanocyte precursors (Goldstein et al., 2015; 2016). Indeed, our RNA-seq study confirmed the low expression of TYR in the NBUVB-treated vitiligo bulge melanocytes (Fig. 2a), with no significant increase in response to NBUVB (Fig. 2b), compared to untreated vitiligo. While the epidermal melanocytes in this study are presumed to be fully differentiated in contrast to those in the bulge, it is likely that a subpopulation of epidermal melanocytes in treated skin could be only partially differentiated, and thus share expression of some precursor genes with the bulge melanocytes. Thus, it is possible that some melanocyte stem cell markers are present in both populations. In future studies, we might compare the expression values from epidermal melanocytes in NBUVB-treated skin with values in the NBUVB-treated non-lesional vitiligo skin, assuming that these melanocytes are enriched in genes associated with regeneration process, having pro-migratory, pro-proliferative and pro-differentiation roles. Another useful comparison is with the expression values in the epidermal melanocytes of healthy normal skin, assuming that these melanocytes are fully differentiated.
In addition, our application identified the Integrin pathway as the top pathway upregulated in the NBUVB-treated bulge melanocytes, along with 14 other significant pathways (Table 1), many of which have been previously associated with either melanoma or with melanocyte/keratinocyte proliferation, migration, differentiation and stemness control.
We found by RNA-Seq (Fig. 3a) and confirmed by qRT-PCR (Fig. 3b) that GLI1, a candidate stem cell-associated gene, was significantly upregulated in the melanocyte precursors of the NBUVB-treated vitiligo bulge as compared to the melanocytes of NBUVB-treated vitiligo epidermis. Also using qRT-PCR (Fig. 3c) and immunostaining (Fig. 3d), we identified that GLI1 was significantly modulated by NBUVB in the bulge, with higher transcript and protein expression observed in the melanocyte samples from the NBUVB-treated vitiligo bulge as compared to melanocyte samples from the untreated vitiligo bulge. Interestingly, our immunostaining study revealed that the GLI1 was upregulated in both melanocytes and keratinocytes of NBUVB-treated and untreated vitiligo bulge, indicating that this may be an important bulge-associated gene involved in the response to the NBUVB in the HF niche. GLI1, an effector of the Sonic Hedgehog (Shh) pathway, is required for melanocyte proliferation and for melanoma growth and metastasis (Barakat et al., 2013; Santiago-Walker and Herlyn, 2010). Interestingly, the Shh and Wnt/β-catenin pathways can interact through GLI1’s regulation of both nuclear localization and transcriptional activity of β-catenin (Liao et al., 2009). In this study, β-catenin was identified as the top upstream regulator in the melanocyte precursors of NBUVB-treated vitiligo bulge (Table S3). This suggests that in vitiligo patients, NBUVB can act as an essential co-activator of CTNNB1 transcription in bulge melanocytes, which interact with GLI1 to induce melanocyte proliferation, migration and differentiation (Fig. 4a–c).
Fig. 4. Hypothetical model of the effects of NBUVB on the bulge and epidermis of human vitiligo skin during repigmentation.
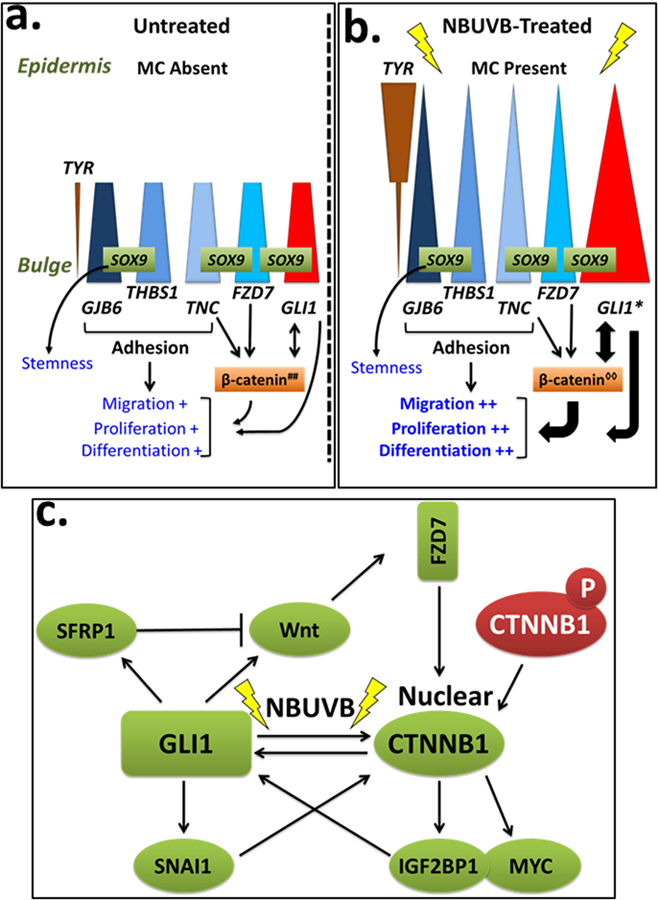
Fig 4a, b. Scheme of melanocyte-induced proliferation, migration and differentiation through GLI1 activation. In the human vitiligo skin, bulge-specific genes GJB6, THBS1, TNC, and FZD7 (blue shapes) are expressed in the melanocyte precursors in the bulge at similar levels before (Fig. 4a) and after NBUVB-treatment (Fig. 4b). These genes are proposed to be involved in cellular adhesion and in maintaining stemness in the bulge melanocyte precursors through β-catenin signaling. Epidermal melanocytes are absent in the interfollicular epidermis (IE) of untreated vitiligo skin, illustrated by the decreased height of all triangles, and their absence in the IE in Fig. 4a. In contrast, TYR (brown triangles) is expressed at low levels in both untreated and NBUVB-treated bulge melanocyte precursors, and is expressed at significantly higher levels in the regenerated epidermal melanocytes of NBUVB-treated vitiligo skin, indicating active melanogenesis. GLI1 expression (red triangles) is significantly higher in the NBUVB-treated bulge melanocytes of vitiligo skin compared to untreated vitiligo skin, suggesting a role for GLI1 in the repigmentation process. We identified SOX9 constitutively expressed by the melanocytes precursors in the human bulge of normal and vitiligo skin (Goldstein et al, 2016). Activation of FZD7 (a Wnt receptor) was associated with reduced phosphorylation of β-catenin and its nuclear accumulation (Merle et al., 2005). Gli1 can induce the accumulation of transcriptionally active β-catenin in cell nuclei (Li et al., 2007). β-catenin can enhance the transcriptional activity of GLI1 (Maeda et al., 2006; Varnat et al., 2010; Song et al., 2015). GLI1 induction in the bulge melanocyte and keratinocyte precursors by NBUVB is supposedly influenced by nuclear translocation of β-catenin, and by interaction with SOX9, with a shift in the balance of maintaining stemness to greater differentiation, proliferation, and migration.
Fig. 4c. Cross-talk activation between β-catenin and GLI1 under NBUVB. β-catenin can enhance the transcriptional activity of GLI1 (Maeda et al., 2006) likely through regulation of the β-catenin key downstream targets, IGF2BP1 (CRD-BP) and myc (Noubissi et al. 2006). Once activated by β-catenin, IGF2BP1 and myc can activate GLI1 likely by binding to the GLI1 mRNA coding region (Varnat at et al., 2010; Noubissi et al, 2009). GLI1 also regulates β-catenin at the transcription level, through its targets, SNAIL, WNT, and SFRP1 (Song et al., 2015). GLI1 can induce SNAIL expression which then interacts with β-catenin and stimulates its expression activity at transcription level (Song et al., 2015), and at protein level (Li et al., 2007). SFRP1 and Wnt have opposite effects on β-catenin signaling, as a negative feedback, or as a control mechanism after GLI1 induction, to prevent over-activation of WNT/β-catenin pathway. SFRP1 is a melanocyte stem gene that we identified upregulated in the bulge of NBVUB-treated vitiligo skin and normal skin (Goldstein et al., 2016).
Experimental evidence used for this figure was generated in either human models, or Gli1 transgenic mice, or on in vitro experiments performed on rat kidney cells, murine LLC-11 hepatocellular carcinoma cells, human stomach, colon, and lung cancer cells.
##, cytoplasmic β-catenin; ◊◊, nuclear β-catenin
Moreover, our RNA-Seq analysis shows that the increased expression of GLI1 was associated with increased expression of SOX9 (Fig. S3) in the melanocyte samples from the NBUVB-treated vitiligo bulge as compared with NBUVB-treated vitiligo epidermis. This supports our previous qRT-PCR study that identified SOX9 as a stem cell gene in the bulge of untreated normal skin and NBUVB-treated vitiligo skin (Goldstein et al., 2016). The above findings suggest that GLI1 and SOX9 may interact in the melanocyte precursors of the human HF bulge to influence proliferation and/or stemness. Interestingly, a SOX9-GLI1 functional relationship has been previously reported: SOX9 was involved in the maintenance of GLI1 in RK3E cells, while repression of SOX9 in PDA cells resulted in the reduction of endogenous GLI1 levels. Furthermore, mRNA analysis of Panc-1 cells transfected with SOX9-siRNA revealed synchronous loss of both SOX9 and GLI1 signals (Deng et al., 2015), while ectopic Sox9 expression induced the expression of Gli1 in maturing chondrocytes of chick embryos (Zeng et al., 2002).
In summary, we present here a work model on the effects of NBUVB in the human vitiligo bulge (Fig. 4a,b). Under NBUVB, the intercellular adhesion molecules (TNC, GJB6 and THBS1) and FZD7 work together with GLI1, through β-catenin nuclear translocation and SOX9 constitutive expression in the bulge, to modulate the balance between stemness and activation of melanocyte precursors in the bulge of vitiligo skin. Further, the GLI1 activation process is followed by melanocyte proliferation, migration and differentiation (Fig. 4b). Our previous comprehensive reivews have summarized essential signals and pathways that promote epidermal melanocyte regeneration by follicular melanocytes, and that regulate the balance between stemness and differentiation states of melanocytes and keratinocytes (Birlea et al 2016; 2017) including in vitro studies and studies on animal and human models of repigmentation. These genes and pathways include: p53 and Wnt/β-catenin pathways; integrins, cadherins, tetraspanins, and metalloproteinases; TGF-β and its effector PAX3. Future functional studies focused on the bulge stem cell genes identified in this study (Fig. 4) will examine their molecular interaction with the pathways and signals already known to be involved in the repigmentation process.
Our long-term goal is to improve the treatment outcome for vitiligo through identification of molecules that activate melanocyte precursors in the HF bulge. Since existing F-LCM techniques were not adequate for these studies (Xu et al., 2003; Ohyama et al., 2006; Amoh et al., 2012), we developed this application (which, to our knowledge, is previously unreported), to isolate and characterize melanocyte populations in the human HF and epidermis. There are several important characteristics of this model: i. the ability to perform a rapid immunostaining protocol that minimizes RNA degradation instead of the usual overnight antibody incubation; ii. the ability to capture RNA from specific cells located in specific anatomic sites; iii. the ability to capture the cells from their natural microenvironment. Even so, there are limitations of our method (modest RNA contamination from neighbor cells, small number of cells captured, and RNA degradation), which we have tried to minimize. Contamination with RNA material of neighboring cells was minimized by using a small diameter laser pulse (of~16 μM). The small number of cells harvested was not a major liability in previous studies that successfully analyzed RNA after F-LCM (Dolter et al., 2001; Nakamura et al., 2007; Vandewoestyne et al., 2013). We decreased RNA degradation by optimizing the rapid immunostaininig protocol, using the Illumina HiSeq 2000 sequencer which processed only short RNA fragments (150–350 bp), and designing short amplicons (≤150 bp in our case) for qRT-PCR confirmation (Vandewoestyne et al., 2013).
Taken together, our data suggest that our F-LCM samples are enriched in target cells that are anatomically distinct and that we can successfully use RNA-Seq to analyze changes of melanocyte signals associated with NBUVB-induced repigmentation in human vitiligo. The HF bulge signals and pathways activated in the melanocyte precursors presented here (Fig. 4a,b) are, to our knowledge, previously unreported in the HF bulge of human vitiligo or in the untreated normal skin. Most of them have been associated with cell proliferation and migration in different malignancies or in the in utero organ formation, suggesting that repigmentation initiation in the bulge shares common aspects with carcinogenesis and embryonic development.
MATERIALS AND METHODS
Tissue sample collection and processing for RNA studies
Skin biopsies from the untreated depigmented skin of 6 vitiligo patients, and from the repigmented skin of 10 vitiligo patients treated with NBUVB were collected in the Dermatology Clinic at Colorado University (CU) Hospital (Table S5). The study was approved by the Human Subjects Committee at the CU and written informed consent was obtained from all subjects. Untreated normal human skin from 6 control subjects was either purchased from the National Disease Resource Interchange or obtained from the Skin Cancer Biorepository at the CU.
Hair follicle bulge mapping
To locate the HF bulge in our samples, frozen or formalin-fixed paraffin-embedded (FFPE) transverse sections were immunostained with a combination of anti-K15/anti-Desmin antibodies every 10th slide (Goldstein et al., 2016).
Rapid fluorescent immunostaining and fluorescent laser capture microdissection (F-LCM)
Frozen sections were immunostained using the NKI-beteb antibody. F-LCM was performed with an infrared laser microdissection system, under direct fluorescent microscopic visualization (Goldstein et al., 2016). From each sample, we captured 100 NKI-beteb (+) melanocytes from the epidermal basal layer, 100 NKI-beteb (+) cells from the bulge-outer root sheath (ORS), and 100 adjacent keratinocytes (NKI-beteb (−)) from each of these two regions.
RNA extraction, amplification, whole transcriptome RNA sequencing and data analysis
We analyzed the RNA isolated from melanocyte and separately keratinocyte samples laser captured from the repigmented skin of 6 vitiligo patients treated with NBUVB 3–4 months. Total RNA extracted was amplified. Good RNA quality required an RNA integrity number (RIN) ≥6.5 and a concentration of >500 pg/μl. About >1 ng total RNA was used for the final libraries and was sequenced with Illumina HiSeq 2000. The RNA-Seq reads from each library were aligned to the human genome (hg19). Transcripts were assembled from the aligned reads, and gene expression levels were estimated. From the global transcription, we tested the accuracy of our data by principal component analysis (PCA), which assessed the overall similarity between samples. We used a paired t-test in R to analyze the genes differentially expressed in the melanocytes captured from the NBUVB-treated bulge as compared with the NBUVB-treated IE. To correct for multiple testing, we used FDR with a significance threshold of Q≤0.05. From the differential gene expression, we identified the significantly modulated canonical pathways, the upstream regulators and the genes functions that were critical in the NBUVB-treated bulge compared to the NBUVB-treated IE using the IPA tool (http://www.ingenuity.com/products/ipa).
qRT-PCR validation of RNA-seq results
To confirm the top genes differentially expressed in the melanocytes captured from NBUVB-treated bulge and NBUVB-treated IE, we used biopsies from 4 NBUVB-treated vitiligo patients and performed new F-LCM sessions of melanocytes, followed by total RNA extraction and amplification (as described in Supplementary Material and Methods). The resulting cDNA was subjected to qRT-PCR, using primers that we designed (Table S1). For each sample, expression values were calculated using the standard delta Ct method normalized to B-Actin expression. Paired t-tests were used to compare the average delta Ct values for NBUVB-treated samples collected from IE versus bulge (P<0.05). Differences in gene expression in the bulge melanocyte capture from normal skin, untreated vitiligo skin, and NBUVB-treated vitiligo skin were analyzed with one-way ANOVA followed by Tukey’s test for multiple comparisons (Padjusted <0.05).
Immunohistochemistry
Vitiligo patients’ demographics and collection methods used for immunostaining are presented in Table S6.
The antibodies used for this study are listed in the Supplementary Material and Methods. Standard procedures were used for immunohistochemistry. To quantify the GLI1 marker’s expression, we measured the average signal intensity of the anti-GLI1 antibody in melanocytes for each patient sample. The differences in signal intensity between the bulge-ORS of NBUVB-treated vitiligo skin and the bulge-ORS of untreated vitiligo skin were compared with a one-tail unpaired t-test.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by a Research Scholar Award in Vitiligo from the American Skin Association to SAB and by UC Denver F-LCM Shared Resource funded by the NIH/NCATS Colorado CTSA Grant UL1 TR001082. We are grateful to our team in the Dermatology Clinic at UCH for sample collection, Mrs. Kathleen Ryan-Morgan and Mrs. Susan Chalmers. We thank Dr. Adriaan van Bokhoven, Zachary Grasmick and Nicole Spoelstra from the Laser Capture Microdissection Core for their valuable support with the laser capture machine. We are grateful to Drs. Manabu Ohyama, Randall Cohrs and Mark Burgoon for valuable advice on laser capture microdissection technique and qRT-PCR. We acknowledge the National Disease Research Interchange for providing human skin samples.
Footnotes
CONFLICT OF INTEREST
We state no conflict of interest.
REFERENCES
- Amoh Y, Aki R, Hamada Y, Niiyama S, Eshima K, Kawahara K, Sato Y, et al. Nestin-positive hair follicle pluripotent stem cells can promote regeneration of impinged peripheral nerve injury. J Dermatol 2012; 39:33–8. [DOI] [PubMed] [Google Scholar]
- Barakat B, Yu L, Lo C, Vu D, De Luca E, Cain JE, et al. Interaction of smoothened with integrin-linked kinase in primary cilia mediates Hedgehog signalling. EMBO Rep 2013;14:837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Xie Z, Tu CL. Calcium regulation of keratinocyte differentiation. Expert Rev Endocrinol Metab 2012; 7:461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birlea SA, Costin GE, Roop DR, Norris DA. Trends in Regenerative Medicine: Repigmentation in Vitiligo Through Melanocyte Stem Cell Mobilization. Med Res Rev 2016; [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Birlea SA, Goldstein NB, Norris DA. Repigmentation through melanocyte regeneration in vitiligo. Dermatol Clin 2017; 35:205–18. [DOI] [PubMed] [Google Scholar]
- Cichorek M, Wachulska M, Stasiewicz A, Tymińska A. Skin melanocytes: biology and development. Postepy Dermatol Alergol 2013; 30:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J 2007; 21:976–94. [DOI] [PubMed] [Google Scholar]
- Dai DL, Makretsov N, Campos EI, Huang C, Zhou Y, Huntsman D, et al. Increased expression of integrin-linked kinase is correlated with melanoma progression and poor patient survival. Clin Cancer Res 2003; 9:4409–14. [PubMed] [Google Scholar]
- Deng W, Vanderbilt DB, Lin CC, Martin KH, Brundage KM, Ruppert JM. SOX9 inhibits β-TrCP-mediated protein degradation to promote nuclear GLI1 expression and cancer stem cell properties. J Cell Sci 2015;128:1123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolter KE, Braman JC. Small-sample total RNA purification: laser capture microdissection and cultured cell applications. Biotechniques 2001; 30:1358–61. [DOI] [PubMed] [Google Scholar]
- Eby JM, Kang HK, Klarquist J, Chatterjee S, Mosenson JA, Nishimura MI, et al. Immune responses in a mouse model of vitiligo with spontaneous epidermal de- and repigmentation. Pigment Cell Melanoma Res 2014. ;27:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantauzzo KA, Kurban M, Levy B, Christiano AM. Trps1 and its target gene Sox9 regulate epithelial proliferation in the developing hair follicle and are associated with hypertrichosis. PLoS Genet 2012; 8:e1003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn A, Proud CG. The role of eIF4 in cell proliferation. Cancer Surv 1996; 27:293–310. [PubMed] [Google Scholar]
- Fujimoto A, Kurban M, Nakamura M, Farooq M, Fujikawa H, Kibbi AG, et al. GJB6, of which mutations underlie Clouston syndrome, is a potential direct target gene of p63. J Dermatol Sci 2013; 69:159–66. [DOI] [PubMed] [Google Scholar]
- Goldstein NB, Koster MI, Hoaglin LG, Spoelstra NS, Kechris KJ, Robinson SE, et al. Narrow band ultraviolet B treatment for human vitiligo is associated with proliferation, migration, and differentiation of melanocyte precursors. J Invest Dermatol 2015;135:2068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein NB, Koster MI, Hoaglin LG, Wright MJ, Robinson SE, Robinson WA, et al. Isolating RNA from precursor and mature melanocytes from human vitiligo and normal skin using laser capture microdissection. Exp Dermatol 2016; 25:805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase I, Evans R, Pofahl R, Watt FM. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J Cell Sci 2003; 116 (Pt 15):3227–38. [DOI] [PubMed] [Google Scholar]
- Hendaoui I, Tucker RP, Zingg D, Bichet S, Schittny J, Chiquet-Ehrismann R. Tenascin-C is required for normal Wnt/β-catenin signaling in the whisker follicle stem cell niche. Matrix Biol 2014; 40:46–53. [DOI] [PubMed] [Google Scholar]
- Imanaka-Yoshida K, Aoki H.Tenascin-C and mechanotransduction in the development and diseases of cardiovascular system. Front Physiol 2014; 5:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo A, Denduluri S, Zhang B, Wang Z, Yin L, Yan Z,. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis 2014;1:149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaja M, Keyes BE, Lin M, Pasolli HA, Genander M, Polak L, et al. SOX9: a stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev 2014; 28:328–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuphal S, Bauer R, Bosserhoff AK. Integrin signaling in malignant melanoma. Cancer Metastasis Rev 2005;24:195–222. [DOI] [PubMed] [Google Scholar]
- Lamartine J, Munhoz Essenfelder G, Kibar Z, Lanneluc I, Callouet E, Laoudj D, et al. Mutations in GJB6 cause hidrotic ectodermal dysplasia. Nat Genet 2000; 26:142–4. [DOI] [PubMed] [Google Scholar]
- Li X, Deng W, Lobo-Ruppert SM, and Ruppert JM. Gli1 acts through Snail and E-cadherin to promote nuclear signaling by β-catenin. Oncogene 2007; 26: 4489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Siu MK, Au CW, Chan QK, Chan HY, Wong ES. Aberrant activation of hedgehog signaling pathway contributes to endometrial carcinogenesis through beta-catenin. Mod Pathol 2009; 22:839–47. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shin S, Zeng X, Zhan M, Gonzalez R, Mueller FJ, et al. Genome wide profiling of human embryonic stem cells (hESCs), their derivatives and embryonal carcinoma cells to develop base profiles of U.S. Federal government approved hESC lines. BMC Dev Biol 2006; 6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Peng Y, Tobin DJ. A new 12-gene diagnostic biomarker signature of melanoma revealed by integrated microarray analysis. PeerJ 2013;1:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda O, Kondo M, Fujita T, Usami N, Fukui T, Shimokata K, Ando T, Goto H, Sekido Y. Enhancement of GLI1-transcriptional activity by beta-catenin in human cancer cells. Oncol Rep 2006; 16:91–6. [PubMed] [Google Scholar]
- Merle P, Kim M, Herrmann M, Gupte A, Lefrançois L, Califano S, Trépo C, Tanaka S, Vitvitski L, de la Monte S, Wands JR. Oncogenic role of the frizzled-7/beta-catenin pathway in hepatocellular carcinoma. J Hepatol 2005;43:854–62. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Ruebel K, Jin L, Qian X, Zhang H, Lloyd RV. Laser capture microdissection for analysis of single cells. Methods Mol Med 2007; 132:11–8. [DOI] [PubMed] [Google Scholar]
- Niwa H How is pluripotency determined and maintained? Development 2007; 134:635–46. [DOI] [PubMed] [Google Scholar]
- Noubissi FK, Elcheva I, Bhatia N, Shakoori A, Ougolkov A, Liu J, Minamoto T, Ross J, Fuchs SY, Spiegelman VS. CRD-BP mediates stabilization of betaTrCP1 and c-myc mRNA in response to beta-catenin signalling. Nature 2006; 441: 898–901. [DOI] [PubMed] [Google Scholar]
- Noubissi FK, Goswami S, Sanek NA, Kawakami K, Minamoto T, Moser A, Grinblat Y, Spiegelman VS. Wnt signaling stimulates transcriptional outcome of the Hedgehog pathway by stabilizing GLI1 mRNA. Cancer Res 2009; 69:8572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest 2006; 116:249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo HZ, Sentani K, Sakamoto N, Anami K, Naito Y, Oshima T, et al. Identification of novel transmembrane proteins in scirrhous-type gastric cancer by the Escherichia coli ampicillin secretion trap (CAST) method: TM9SF3 participates in tumor invasion and serves as a prognostic factor. Pathobiology 2014; 81:138–48. [DOI] [PubMed] [Google Scholar]
- Rashighi M, Harris JE. Vitiligo Pathogenesis and Emerging Treatments. Dermatol Clin 2017; 35:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Walker A, Herlyn M. The ups and downs of transcription factors in melanoma. J Natl Cancer Inst 2010;102:1103–4. [DOI] [PubMed] [Google Scholar]
- Scott G, Leopardi S. The cAMP signaling pathway has opposing effects on Rac and Rho in B16F10 cells: implications for dendrite formation in melanocytic cells. Pigment Cell Res 2003;16:139–48. [DOI] [PubMed] [Google Scholar]
- Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, et al. Nanog is the gateway to the pluripotent ground state. Cell 2009;138:722–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Li ZY, Liu WP, Zhao MR. Crosstalk between Wnt/β-catenin and Hedgehog/ Gli signaling pathways in colon cancer and implications for therapy. Cancer Biol Ther 2015;16:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritz RA. Modern vitiligo genetics sheds new light on an ancient disease. J Dermatol 2013; 40:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolboushkina EA, Garber MB. Eukaryotic type translation initiation factor 2: structure- functional aspects. Biochemistry (Mosc) 2011;76:283–94. [DOI] [PubMed] [Google Scholar]
- Sundaram P, Hultine S, Smith LM, Dews M, Fox JL, Biyashev D, et al. p53-responsive miR-194 inhibits thrombospondin-1 and promotes angiogenesis in colon cancers. Cancer Res 2011; 71:7490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 2010; 10:116–29. [DOI] [PubMed] [Google Scholar]
- Vandewoestyne M, Goossens K, Burvenich C, Goossens K, Burvenich C, Van Soom A, et al. Laser capture microdissection: should an ultraviolet or infrared laser be used? Anal Biochem 2013; 439:88–98. [DOI] [PubMed] [Google Scholar]
- Varnat F, Siegl-Cachedenier I, Malerba M, Gervaz P, Ruiz i Altaba A. Loss of WNT TCF addiction and enhancement of HH GLI1 signalling define the metastatic transition of human colon carcinomas. EMBO Mol Med 2010; 2:440–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco-Velázquez MA, Salinas-Jazmín N, Mendoza-Patiño N, Mandoki JJ. Reduced paxillin expression contributes to the antimetastatic effect of 4-hydroxycoumarin on B16-F10 melanoma cells. Cancer Cell Int 2008;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Xu S, Wang Y, Wu P, Zhang J, Sato T, et al. GM3 suppresses anchorage-independent growth via Rho GDP dissociation inhibitor beta in melanoma B16 cells. Cancer Sci 2011;102:1476–85. [DOI] [PubMed] [Google Scholar]
- Xu X, Lyle S, Liu Y, Solky B, Cotsarelis G. Differential expression of cyclin D1 in the human hair follicle. Am J Pathol 2003;163:969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Qian Q. Wnt5a/Ca (2+) /calcineurin/nuclear factor of activated T signaling pathway as a potential marker of pediatric melanoma. J Cancer Res Ther 2014; 10 Suppl:C83–8. [DOI] [PubMed] [Google Scholar]
- Yun CY, You ST, Kim JH, Chung JH, Han SB, Shin EY, et al. p21-activated kinase 4 critically regulates melanogenesis via activation of the CREB/MITF and β- catenin/MITF pathways. J Invest Dermatol 2015;135:1385–94. [DOI] [PubMed] [Google Scholar]
- Zeng L, Kempf H, Murtaugh LC, Sato ME, Lassar AB. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev 2002;16:1990–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell 2009; 5:267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


