Abstract
Background:
The epidemic of opioid abuse is increasing, and the number of deaths secondary to opioid overdose is also increasing. Recent attention has focused on opioid prescribing and management of chronic pain. However, opioid use in perioperative and periprocedural patients, whether they have chronic pain or exhibit new persistent opioid abuse after a procedure, has received little attention.
Methods:
We present an evidence-based technique that combines subanesthetic infusions of lidocaine and dexmedetomidine supplemented with other intravenous agents and a low dose of inhaled anesthetic.
Results:
Based on evidence of drug action and interaction, an opioid-free anesthetic can be delivered successfully. We present the cases of 2 patients in whom the opioid-free anesthetic technique was used with a successful outcome, adequate pain management, and avoidance of opioid drugs.
Conclusion:
This anesthetic prescription can be useful for opioid-naïve patients as well as for patients with chronic pain that is managed with opioids.
Keywords: Anesthesia, balanced anesthesia, dexmedetomidine, lidocaine, opioid-related disorders, perioperative care
INTRODUCTION
Deaths from opioid overdose continue to rise in the United States. The number of prescriptions for opioids quadrupled between 1999 and 2015, and access to illegal synthetic opioids such as fentanyl has also.1 The problem has become so severe on US college campuses that some schools have begun stocking naloxone to reverse opioid-induced respiratory depression in emergency situations.2 A study published in 2008 of 12,739 surgical inpatients noted a correlation between persistent perioperative opioid use following surgical procedures and mortality.3 In 2017, Brummett et al reported data on 36,177 surgical patients in whom new persistent opioid use—defined as an opioid prescription fulfillment 90-180 days after the surgical procedure—was identified after both major and minor surgical procedures, and the rate of new persistent opioid abuse of approximately 6% was similar between the 2 groups.4
In 2016, the Centers for Disease Control and Prevention released guidelines for prescribing opioids to treat chronic pain.5 However, focus on opioid prescribing for perioperative pain has been lacking, leading Birkmeyer to conclude, “Given the declining rates of morbidity and mortality following common elective procedures, new persistent opioid use represents an important, common, and unrecognized complication of perioperative care.”6
We present an evidence-based opioid-free anesthetic technique employing subanesthetic doses of intravenous anesthetic agents that are already in use but not specifically used in this combination. In addition, we present 2 cases that illustrate the use of this anesthetic regimen.
INTRAVENOUS ANESTHETIC AGENTS
The introduction of intravenous anesthetic agents is of historic interest. John Lundy of Mayo Clinic published an initial report in Minnesota Medicine in 1926;7 a full report followed in the Journal of the American Medical Association in 1931.8 At that time, intravenous access was not standard practice. Lundy introduced the term balanced anesthesia and suggested that using lower doses of more than one anesthetic agent improved outcomes. This thought process led to the adoption of the so-called nitrous oxide narcotic anesthetic: nitrous oxide combined with oxygen in a 60%:40% ratio supplemented with intermittent intravenous dosing of morphine sulfate.
In 1993, Kehlet and Dahl introduced the term multimodal analgesia for management of postoperative pain, along with the term balanced analgesia,9 and Woolf and Chong introduced the term preemptive analgesia.10 Thus, the confluence of volatile anesthetic agents, intravenous anesthetic agents, and regional and neuraxial anesthetic techniques gained favor among clinicians for improved pain management. Who first proposed continuous infusion of lidocaine for anesthesia and analgesia is not clear, but both surgical and medical literature report the use of the technique resulting in a more rapid return of bowel function, improved postoperative analgesia, and shorter length of stay for surgical patients compared to a control group of surgical patients.11,12 Continuous infusion of anesthetic and analgesic agents became more popular as drugs with a shorter half-life (t ½ β) than other intravenous anesthetic agents and a predictable volume of distribution (VD) were developed.13
OPIOID-FREE ANESTHETIC AGENTS
The anesthetic technique we propose is based on a multimodal approach using specific agents that have anesthetic or analgesic properties, administered in subanesthetic doses to achieve the desired effect. Continuous infusions of lidocaine and dexmedetomidine are supplemented with a low dose of a volatile anesthetic agent and intermittent dosing of acetaminophen, ketamine, ibuprofen, and ketorolac.
Lidocaine
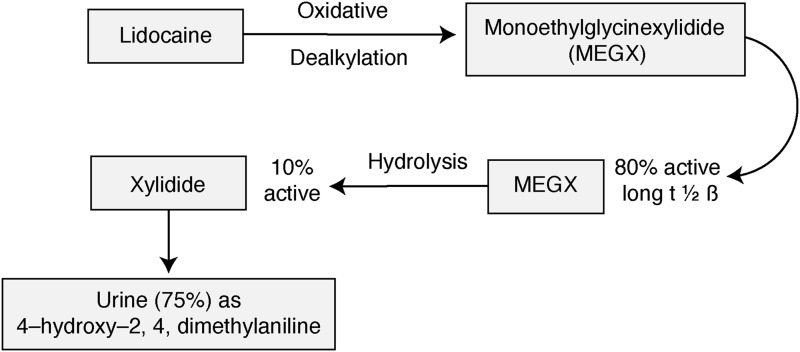
Lidocaine has analgesic,14-16 antiinflammatory,16-18 and antihyperalgesic properties.19,20 Because lidocaine infusion has been used to treat cardiac arrhythmias, its plasma levels for therapeutic effect and specific organ toxicity are well established (Table). Lidocaine has 2 active metabolites: monoethylglycinexylidide (MEGX) that is further metabolized to xylidine. MEGX is 80% as active as lidocaine, and xylidide is 10% as active as lidocaine (Figure).14 The t ½ β of MEGX is 2.5-3.0 hours, whereas the t ½ β of lidocaine is 1.5-2.0 hours.14 Lidocaine is metabolized in the liver and excreted by the kidneys.21 Subanesthetic doses of lidocaine, the stated goal for opioid-sparing, block ectopic nerve discharge but not conduction. Because of its pharmacokinetic profile, the drug should be used with caution in the elderly, in patients with known hepatic or renal dysfunction or heart failure, or in patients taking drugs that interfere with the cytochrome P450 enzyme. A major concern is combining intravenous lidocaine with concomitant injection of liposomal bupivacaine into abdominal fascial planes for pain control.13 The 2 drugs are amide local anesthetics with a similar structure. The transverse abdominis plane block is performed with a large volume of bupivacaine, up to 50 cc. Elevated plasma levels of both drugs may lead to immediate cardiac toxicity.
Table. Dose-Dependent Effects of Lidocaine.
| Plasma Level, μg/mL | Effect |
|---|---|
| 1-5 | Analgesia |
| >5-10 | Circumoral numbness |
| Tinnitus | |
| Skeletal muscle twitching | |
| Hypotension | |
| Myocardial depression | |
| >10-15 | Unconsciousness |
| Seizures | |
| >15-25 | Apnea |
| Coma | |
| >25 | Cardiovascular collapse |
Figure. The diagram shows the metabolism of lidocaine and the comparative potency of active metabolites.
Dexmedetomidine
Dexmedetomidine is an alpha-2 agonist that has hypnotic, sedative, and analgesic properties22 and is estimated to be 7-10 times more potent than clonidine.23 Compared to clonidine, dexmedetomidine is a full, rather than a partial, agonist and highly selective and specific for the alpha receptor. The drug is protein bound, and like lidocaine, is metabolized in the liver with subsequent renal excretion.23 Usually administered by continuous infusion, dexmedetomidine provides hypnosis and sedation and has analgesic properties. Pretreatment with dexmedetomidine attenuates the adrenergic response to tracheal intubation24 and decreases plasma catechol concentrations during anesthesia; infusing dexmedetomidine results in a decrease in minimum alveolar concentration (MAC) for volatile anesthetic agents and has an opioid-sparing effect.22,25 The analgesic effect is dose-dependent with little or no depression of ventilation. Loading doses of dexmedetomidine to rapidly reach the desired therapeutic effect may cause hypotension.26 Dexmedetomidine attenuates the cardiostimulatory effects and postanesthetic delirium induced by ketamine.27
Ketamine
Ketamine is a phencyclidine that was originally demonstrated to have a specific effect on N-methyl-D-aspartate receptors in the brain, a mechanism of action quite different than other anesthetic agents.28 The drug has since been demonstrated to have a more distributive effect in other parts of the brain (thalamus and limbic systems) and analgesic effects that are mediated at the level of the spinal cord.28 Ketamine is an amnesic and provides intense analgesia.28 The analgesia is profound even at subanesthetic doses; at higher doses, ketamine is a complete anesthetic. Subanesthetic doses of ketamine reverse tolerance to opioids.29 The drug exhibits extreme lipid solubility, rapid transfer across the blood-brain barrier, and a large VD. The t ½ β is 2-3 hours, but even so the drug has been successfully employed by continuous infusion.28
Acetaminophen (Paracetamol)
Acetaminophen has a different mechanism of action than other analgesic drugs30 but also has demonstrated analgesic and opioid-sparing properties.31 In adults, the appropriate dose for preemptive analgesia is 1,000 mg administered intravenously for approximately 20 minutes.30 The analgesic onset is rapid, within 5 minutes, but the peak concentration in plasma occurs at approximately 1 hour.32 The duration of effect is 4-6 hours, and the t ½ β is 2.0-2.5 hours.32 Acetaminophen undergoes metabolism in the liver by conjugation with glucuronic and sulfuric acids; these conjugates are excreted in the urine.32 Surgeons have embraced the use of intravenous acetaminophen, starting with a preoperative dose and repeating doses at 6-hour intervals.31 Acetaminophen has been recommended in perioperative pediatrics for both analgesic and opioid-sparing effects.33,34
Other Intravenous Analgesic Agents
Ketorolac is a nonsalicylate analgesic agent and nonsteroidal antiinflammatory drug (NSAID) that has desirable characteristics in managing postoperative pain.35 Ketorolac mean plasma concentrations, VD, and excretion are identical in healthy adults whether the dose is given by the intramuscular, intravenous, or oral route.36 The pharmacokinetic profile of ketorolac in children is the same as in adults; the VD and plasma clearance are identical.37 The COX-1 and COX-2 mechanisms of action may play a role in inhibition of prostaglandin synthesis, resulting in anticoagulation at higher doses. For adult postoperative patients, a 15-30 mg intravenous dose of ketorolac, repeated once, has been shown to be safe and effective, and for pediatric patients, ketorolac in a dose of 0.5 mg/kg (maximum dose 30 mg) is similarly safe and effective.37
Ibuprofen, another NSAID with established analgesic and antipyretic properties, is approved by the US Food and Drug Administration for intravenous administration.38 Doses of 400 mg and 800 mg have been shown to have an analgesic and opioid-sparing effect in postoperative patients.39 Smith and Voss have shown that shortening the recommended 30-minute infusion time is both safe and effective.39
AN OPIOID-FREE ANESTHETIC PRESCRIPTION
Based on evidence of drug action and interaction, an opioid-free anesthetic can be delivered with infusions of lidocaine (0.03 mg/kg/min), dexmedetomidine (0.5 mcg/kg/hr), and 0.5 MAC isoflurane. For procedures of less than 2 hours’ duration, a bolus dose of lidocaine and dexmedetomidine can be considered. For procedures greater than 2 hours, no bolus dose is necessary.
Preoperatively
Administer acetaminophen 1,000 mg intravenously over 10-20 minutes. Give midazolam 1-2 mg intravenously as needed for anxiolysis.
Anesthetic Induction
Begin intravenous lidocaine infusion (0.03 mg/kg/min) and dexmedetomidine infusion (0.5 mcg/kg/hr) after monitors are in place. Induce anesthesia with propofol (1-2 mg/kg) given intravenously for 2 minutes and add 10-25 mg of intravenous ketamine.
Anesthetic Maintenance
Begin inhalational anesthetic (0.8-1.0 MAC) and titrate to a lower dose (0.5 MAC) as tolerated. Maintain the infusion of lidocaine (0.03 mg/kg/hr) and dexmedetomidine (0.5 mcg/kg/hr). Reduce the infusion of dexmedetomidine to 0.25 mcg/kg/hr at 2 hours and discontinue 30 minutes prior to anesthetic emergence. At any time during anesthesia, but prior to emergence, ondansetron and dexamethasone can be given intravenously to prevent postoperative nausea.
Anesthetic Emergence
Decrease lidocaine infusion to 0.02 mg/kg/min at the time of emergence and to 0.015 mg/kg/min at the time of admission to the postanesthesia care unit (PACU). Use intravenous NSAIDs as the first choice for analgesia in recovery.
An Intralipid infusion should be immediately available for use in the operating room and the PACU if local anesthetic toxicity is suspected.40-42
OPIOID-FREE ANESTHETIC CASES
We present a perioperative surgical patient and a periprocedural patient who underwent the opioid-free anesthetic technique with a successful outcome, adequate pain management, and avoidance of opioid drugs.
Patient 1
A 43-year-old female underwent bariatric surgery outside of the United States. Within 2 weeks after her return home, she presented to her primary care physician who diagnosed small bowel obstruction. She was referred for exploratory laparotomy, resulting in resection of dead bowel, and she was taken to the surgical intensive care unit with an open abdomen, the bowel in discontinuity, and an ileostomy. She subsequently became septic and developed acute respiratory distress syndrome. She required an extended period of mechanical ventilation, antibiotic therapy, and hemodynamic support. After 2 months in the hospital, she was transferred to a local long-term acute care facility for further recovery.
The patient presented 6 months later for takedown of the ileostomy and bowel anastomosis. During the preoperative interview, she requested that no narcotics be administered during or after her surgery because of her struggle to be opioid-free following her recent episode. We implemented the opioid-free anesthetic plan to honor the patient's request.
The surgical procedure was more difficult than anticipated because of adhesions, and the anesthetic time was 4.1 hours. The patient was extubated in the operating room.
At the time of admission to the PACU, the patient's lidocaine infusion at 0.015 mg/kg/hr was maintained; she appeared sedated but responded to commands and was oriented. On examination, she was warm and dry, with excellent pulses and good peripheral perfusion. Vital signs were normal except for blood pressure of 98/60 mmHg (her preoperative blood pressure was 132/80 mmHg). Other vital signs were pulse of 68 bpm, respiratory rate of 23 breaths per min, and oxygen saturation of 98% on room air. With fluid resuscitation, her blood pressure increased to 108/62 mmHg. She was discharged from the PACU after a 4-hour stay with vital signs within normal limits.
During her stay in the PACU, the patient was given one 30-mg dose of intravenous ketorolac for pain, and she was discharged with a visual analog pain score of 3/10. The lidocaine infusion was discontinued at the time of PACU discharge. Bowel sounds returned on the afternoon of the first postoperative day, and the patient began a clear liquid diet and was mobilized. She was discharged on the second postoperative day on oral acetaminophen and ibuprofen. She received no narcotics during her hospital stay.43
Patient 2
A 44-year-old male with a diagnosis of chronic pancreatitis presented to the emergency department with acute on chronic pancreatitis and intractable abdominal pain, with little response to large doses of narcotics. The plan was to perform endoscopic retrograde cholangiopancreatography (ERCP) with possible stent placement. The patient was brought to the holding area, routine monitors were placed, and a lidocaine infusion was begun at 0.03 mg/kg/min following a 1.5 mg/kg bolus. Dexmedetomidine infusion of 0.5 mcg/kg/hr was also started with no initial bolus. After 2 hours, the patient's pain began to subside. At 4 hours, anesthesia was induced with intravenous propofol 2 mg/kg and 25 mg ketamine intravenously; anesthesia was maintained with the infusions of lidocaine and dexmedetomidine and inhaled isoflurane. A stent was successfully positioned during ERCP. Dexmedetomidine was discontinued prior to anesthetic emergence. The patient was successfully extubated; the lidocaine infusion was decreased to 0.015 mg/kg/hr and continued for 24 hours. Postprocedure, the patient received 1,000 mg of intravenous acetaminophen; further scheduled doses of acetaminophen were given by mouth (1,000 mg every 6 hours). He was discharged on the second postprocedural day, on no narcotics, with resolution of his pain postprocedure.
CONCLUSION
The opioid epidemic in the United States is a complex issue with biochemical, sociologic, and behavioral factors. The availability of synthetic opioids and the increase in illegal distribution must be addressed. Prescribing habits must be analyzed and altered to balance the obligation to adequately treat pain without subjecting patients to the possibility of addiction because of overprescribing.
We know that the incidence of new persistent opioid use following surgery and medical procedures is contributing to addiction. As an alternative to opioid anesthetic regimens, we present a multimodal approach to anesthetic care that works through the interaction of subanesthetic doses of intravenous agents; the mainstay of our anesthetic technique is infusing lidocaine and dexmedetomidine.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
A report on these data was presented at the International Anesthesia Research Society Annual Meeting on May 8, 2017, in Washington, DC.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Practice-Based Learning and Improvement.
REFERENCES
- 1.Centers for Disease Control and Prevention. Drug overdose deaths in the United States continue to increase in 2015. www.cdc.gov/drugoverdose/epidemic/index.html. Accessed August3, 2017.
- 2.Korn M, Kamp J. Fatal student opioid overdoses prompt colleges to action. The Wall Street Journal. https://www.wsj.com/articles/colleges-take-action-on-opioid-epidemic-1494158403. Published May 7, 2017. Accessed November15, 2017.
- 3.Fecho K, Lunney AT, Boysen PG, Rock P, Norfleet EA. Postoperative mortality after inpatient surgery: incidence and risk factors. Ther Clin Risk Manag. 2008. August;4(4):681-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brummett CM, Waljee JF, Goesling J et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017. June 21;152(6):e170504. doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016. April 19;315(15):1624-1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkmeyer JD. Progress and challenges in improving surgical outcomes. Br J Surg. 2012. November;99(11):1467-1469. doi: 10.1002/bjs.8933. [DOI] [PubMed] [Google Scholar]
- 7.Lundy JS. Balanced anesthesia. Minnesota Med. 1926;9:399-404. [Google Scholar]
- 8.Lundy JS. Useful anesthetic agents and methods. JAMA. 1931;97(1):25-31. doi: 10.1001/jama.1931.27310010003010. [DOI] [Google Scholar]
- 9.Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993. November;77(5):1048-1056. [DOI] [PubMed] [Google Scholar]
- 10.Woolf CJ, Chong MS. Preemptive analgesia—treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993. August;77(2):362-379. [DOI] [PubMed] [Google Scholar]
- 11.Groudine SB, Fisher HA, Kaufman RP Jr et al. Intravenous lidocaine speeds the return of bowel function, decreases postoperative pain, and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesth Analg. 1998. February;86(2):235-239. [DOI] [PubMed] [Google Scholar]
- 12.Herroeder S, Pecher S, Schönherr ME et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg. 2007. August;246(2):192-200. Erratum in: Ann Surg. 2009. April;249(4): 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butterworth JF 4th, Strichartz GR. Molecular mechanisms of local anesthesia: a review. Anesthesiology. 1990. April;72(4):711-734. [DOI] [PubMed] [Google Scholar]
- 14.Narang PK, Crouthamel WG, Carliner NH, Fisher ML. Lidocaine and its active metabolites. Clin Pharmacol Ther. 1978. December;24(6):654-662. [DOI] [PubMed] [Google Scholar]
- 15.Vigneault L, Turgeon AF, Côté D et al. Perioperative intravenous lidocaine infusion for postoperative pain control: a meta-analysis of randomized controlled trials. Can J Anaesth. 2011. January;58(1):22-37. doi: 10.1007/s12630-010-9407-0. [DOI] [PubMed] [Google Scholar]
- 16.Yardeni IZ, Beilin B, Mayburd E, Levinson Y, Bessler H. The effect of perioperative intravenous lidocaine on postoperative pain and immune function. Anesth Analg. 2009. November;109(5):1464-1469. doi: 10.1213/ANE.0b013e3181bab1bd. [DOI] [PubMed] [Google Scholar]
- 17.Okada S, Hagan JB, Kato M et al. Lidocaine and its analogues inhibit IL-5-mediated survival and activation of human eosinophils. J Immunol. 1998. April 15;160(8):4010-4017. [PubMed] [Google Scholar]
- 18.MacGregor RR, Thorner RE, Wright DM. Lidocaine inhibits granulocyte adherence and prevents granulocyte delivery to inflammatory sites. Blood. 1980. August;56(2):203-209. [PubMed] [Google Scholar]
- 19.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006. March;104(3):570-587. [DOI] [PubMed] [Google Scholar]
- 20.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011. Mar-Apr;14(2):145-161. [PubMed] [Google Scholar]
- 21.Peat MA, Deyman ME, Crouch DJ, Margot P, Finkle BS. Concentrations of lidocaine and monoethylglycylxylidide (MEGX) in lidocaine associated deaths. J Forensic Sci. 1985. October;30(4):1048-1057. [PubMed] [Google Scholar]
- 22.Kamibayashi T, Maze M. Clinical uses of alpha 2-adrenergic agonists. Anesthesiology. 2000. November;93(5):1345-1349. [DOI] [PubMed] [Google Scholar]
- 23.Sandler AN. The role of clonidine and alpha 2-agonists for postoperative analgesia. Can J Anaesth. 1996. December;43(12):1191-1194. [DOI] [PubMed] [Google Scholar]
- 24.Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004. September;101(3):787-790. [DOI] [PubMed] [Google Scholar]
- 25.Aantaa R, Jaakola ML, Kallio A, Kanto J. Reduction of the minimum alveolar concentration of isoflurane by dexmedetomidine. Anesthesiology. 1997. May;86(5):1055-1060. [DOI] [PubMed] [Google Scholar]
- 26.Jalonen J, Hynynen M, Kuitunen A et al. Dexmedetomidine as an anesthetic adjunct in coronary artery bypass grafting. Anesthesiology. 1997. February;86(2):331-345. [DOI] [PubMed] [Google Scholar]
- 27.Levänen J, Mäkelä ML, Scheinin H. Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology. 1995. May;82(5):1117-1125. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Vlisides PE. Ketamine: 50 years of modulating the mind. Front Hum Neurosci. 2016. November 29;10:612. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guillou N, Tanguy M, Seguin P, Branger B, Campion JP, Mallédant Y. The effects of small-dose ketamine on morphine consumption in surgical intensive care unit patients after major abdominal surgery. Anesth Analg. 2003. September;97(3):843-847. [DOI] [PubMed] [Google Scholar]
- 30.Ward B, Alexander-Williams JM. Paracetamol revisited: a review of the pharmacokinetics and pharmacodynamics. Acute Pain. 1999. September;2(3):139-149. [Google Scholar]
- 31.Memis D, Inal MT, Kavalci G, Sezer A, Sut N. Intravenous paracetamol reduced the use of opioids, extubation time, and opioid-related adverse effects after major surgery in intensive care unit. J Crit Care. 2010. September;25(3):458-462. doi: 10.1016/j.jcrc.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Forrest JA, Clements JA, Prescott LF. Clinical pharmacokinetics of paracetamol. Clin Pharmacokinet. 1982. Mar-Apr;7(2):93-107. [DOI] [PubMed] [Google Scholar]
- 33.Anderson BJ. Paracetamol (acetaminophen): mechanisms of action. Paediatr Anaesth. 2008. October;18(10):915-921. doi: 10.1111/j.1460-9592.2008.02764.x. [DOI] [PubMed] [Google Scholar]
- 34.Wong I, St John-Green C, Walker SM. Opioid-sparing effects of perioperative paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) in children. Paediatr Anaesth. 2013. June;23(6):475-495. doi: 10.1111/pan.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Hara DA, Fragen RJ, Kinzer M, Pemberton D. Ketorolac tromethamine as compared with morphine sulfate for treatment of postoperative pain. Clin Pharmacol Ther. 1987. May;41(5):556-561. [DOI] [PubMed] [Google Scholar]
- 36.Jung D, Mroszczak E, Bynum L. Pharmacokinetics of ketorolac tromethamine in humans after intravenous, intramuscular and oral administration. Eur J Clin Pharmacol. 1988;35(4):423-425. [DOI] [PubMed] [Google Scholar]
- 37.Olkkola KT, Maunuksela EL. The pharmacokinetics of postoperative intravenous ketorolac tromethamine in children. Br J Clin Pharmacol. 1991. February;31(2):182-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bookstaver PB, Miller AD, Rudisill CN, Norris LB. Intravenous ibuprofen: the first injectable product for the treatment of pain and fever. J Pain Res. 2010. May 25;3:67-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith HS, Voss B. Pharmacokinetics of intravenous ibuprofen: implications of time of infusion in the treatment of pain and fever. Drugs. 2012. February 12;72(3):327-337. doi: 10.2165/11599230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Weinberg GL, Di Gregorio G, Ripper R et al. Resuscitation with lipid versus epinephrine in a rat model of bupivacaine overdose. Anesthesiology. 2008. May;108(5):907-913. doi: 10.1097/ALN.0b013e31816d91d2. [DOI] [PubMed] [Google Scholar]
- 41.Ciechanowicz S, Patil V. Lipid emulsion for local anesthetic systemic toxicity. Anesthesiol Res Pract. 2012;2012:131784. doi: 10.1155/2012/131784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinberg GL. Lipid infusion therapy: translation to clinical practice. Anesth Analg. 2008. May;106(5):1340-1342. doi: 10.1213/ane.0b013e31816a6c09. [DOI] [PubMed] [Google Scholar]
- 43.Paetzold J, Boysen PG. Opioid sparing anesthetic: a preliminary cost and feasibility. Anesth Analg. 2017. May;124 (5S Suppl):947-948. [Google Scholar]