Abstract
The bottlenose dolphin, genus Tursiops is one of the best studied of all the Cetacea with a minimum of two species widely recognised. Common bottlenose dolphins (T. truncatus), are the cetacean species most frequently held in captivity and are known to hybridize with species from at least 6 different genera. In this study, we document several intra-generic hybridization events between T. truncatus and T. aduncus held in captivity. We demonstrate that the F1 hybrids are fertile and can backcross producing apparently healthy offspring, thereby showing introgressive inter-specific hybridization within the genus. We document that female F1 hybrids can reach sexual maturity at 4 yr and 3 mo of age, and can become pregnant and give birth before being fully weaned. The information presented has implications for understanding hybrid reticulation among cetacean species and practical implications for captive facilities housing either Tursiops species or hybrids thereof.
Introduction
It is becoming increasingly clear that reticulation among species lineages is common [1], and can even support the establishment of new species radiations [2]. In her 2009 review of hybridization events in marine mammals, Bérubé [3], summarises that 53 putative hybridization events have been reported within Cetacea, of which 28 hybrids have been identified within captive facilities. The evolutionary significance of hybridisation among cetacean species is not yet clear [4], however a better understanding of this process can be facilitated through investigations of hybridisation events in captivity.
The bottlenose dolphin (Tursiops spp.) is one of the best studied of all the cetaceans. However, there remains continued debate surrounding the number of Tursiops species recognised and the phylogenetic relationships between populations from which we have genetic information. In the past as many as 20 different Tursiops species were identified ([5] cited in [6]). In 1990, Ross and Cockcroft [6] re-assessed the genus Tursiops and recognised only T. truncatus, with high degrees of morphological variation linked to clines in sea surface temperate. More recently, genetic techniques in concert with morphological and osteological data, have helped to document variation in the genus at the species and population level (e.g. [7–10]). A minimum of two bottlenose dolphin species; the common bottlenose dolphin T. truncatus and Indo-Pacific bottlenose dolphin T. aduncus, are now widely accepted [11]. A third species, the Burrunan dolphin, T. australis has recently been proposed [12] and a subspecies T. truncatus ponticus is recognised from the Black Sea [13].
Hybridisation in Tursiops has been investigated in areas where the species ranges overlap. An early study in Taiwan based on mtDNA sequences found no evidence for introgression [8] between T. aduncus and T. truncatus, while a later study in that region using bi-parentally inherited nuclear DNA markers (20 microsatellite DNA loci) also found no evidence for admixture between the two species [14]. Off Australia, mtDNA lineages were distinct [12, 15] and there was no evidence for admixture between T. truncatus and T. aduncus lineages even when sympatric in coastal waters [16]. Although the Austral-Asian lineage of T. aduncus shows reciprocal monophyly with the South African T. aduncus lineage, they are both in the same lineage separate from T. truncatus based on a mitogenome phylogeny [9]. Estimated divergence time between T. truncatus and T. aduncus lineages was 790Ka, while the divergence between the two T. aduncus lineages was 327Ka. The divergence between these two species is relatively old within the wider delphinid radiation and while various studies have suggested polyphyly with this genus [9, 17–20], this is likely not fully resolved.
All captive hybrids are within the odontocete suborder [3]. Common bottlenose dolphins (T. truncatus) are the most frequent cetacean to be housed in captive facilities and have hybridized with species from 6 genera, including the rough toothed dolphin (Steno bredanensis), Guiana dolphin (Sotalia guianensis), Risso’s dolphin (Grampus griseus) and false killer whale (Pseudorca crassidens) [3, 21–23]. Such events may reflect naturally occurring hybridization in areas where species distributions overlap, and there is strong evidence across a range of odontocete and mystecete cetaceans for such hybridization events in the wild [17, 24–28]. However, documentation of intra-generic hybridization events in captive or free-ranging Tursiops are rare, possibly due to prior confusion over the taxonomic status of this genus, difficulties in identifying hybrids in the wild using morphological features, or lack of overlap in species ranges limiting opportunities for mating. Alternatively, mechanisms of reproductive isolation may be in place which actively reduce the occurrence of hybridization events within Tursiops.
Studies of free-ranging cetaceans have found compelling evidence that F1 female hybrids can be fertile and can both backcross (e.g. common minke whale x Antarctic minke whale [29], blue whale x fin whale [30]) and interbreed (e.g. Clymene dolphin [4]), which has important implications for introgressive gene flow and species evolution [1]. However, assessing the viability of F1 hybrids has largely been based on molecular work [31], inferred from pregnant F1 hybrids [29, 30] or been based on observations of F1 hybrids with neonatal calves [25]. Miralles et al., [31] identified the first hybridization event in pilot whales, between Globicephala melas × G. macrorhynchus, and provide evidence for intra-generic introgression through molecular identification of adult hybrids [32]. Interbreeding of hybrids may be responsible for the reticulate evolution of new species such as the Clymene dolphin (Stenella clymene) which displays a mitochondrial genome closely related to S. coeruleoalba and a nuclear genome closely related to S. longirostris [4]. Studies in captivity where animals can be closely observed provide a good opportunity to document the reproductive potential of hybrids. However, there is only one published account of a backcross being fertile. Here a T. truncatus × Delphinus capensis hybrid back-crossed with T. truncatus and the resulting calf died shortly after birth [33].
Before the taxonomic definitions of the Tursiops genus were clarified, hybridization between T. t. gilli (now regarded as T. truncatus) and T. t. aduncus (now T. aduncus) was documented [21]. The F1 offspring survived 5+ years in good health in Okinawa Expo Memorial Park Aquarium, Japan. More recently, Martien et al., [34] found molecular evidence for a T. aduncus × T. truncatus hybridization event from samples of wild animals collected near Hawaii, with STRUCTURE [35–37] analysis suggesting the sampled animal had T. aduncus ancestors at least two generations past. However, as this study was based on molecular sampling from wild animals, no mating history was available to confirm the hybrid status of the sampled individual.
Our study documents several hybridization events between T. truncatus and T. aduncus held in a single captive facility in Durban, South Africa. Best [38] provides a short description of the captive colony of T. truncatus, T. aduncus and hybrids of the two species housed in this facility. The F1 hybrids can be identified by their external morphological characteristics [38], however the differences are subtle. Data from this captive setting are used to unambiguously demonstrate the ability for F1 hybrids to produce healthy backcross hybrid offspring that live into adulthood. The results have implications for understanding the evolution of cetacean species as well as practical implications for captive facilities housing either species or hybrids.
Methods
This study focuses on a captive colony of T. truncatus, T. aduncus and T. aduncus × T. truncatus hybrids held at uShaka Sea World (Durban, South Africa). The colony was established in 1976 within the Durban Sea World dolphinarium (a division of the South African Association for Marine Biological Research, SAAMBR). It moved to new facilities in 2004 under the name uShaka Sea World. For simplicity, we will use the current name (uShaka Sea World) to refer to the dolphinarium throughout time. It is currently the only captive facility housing dolphins in South Africa. The enclosure, some 7200 m3, encompasses an indoor and external holding facility and a large 3800m3 presentation pool. Although the seven pools in the holding facility can be separated by physical barriers, they allow visual and acoustic contact between groups. Configuration of the social groups has changed over time, and during the principle time of data collection in November 2016 the dolphins were held in three social groups, with most adult males and females held separately in two same-sex groups, and a mature T. truncatus and T. aduncus (Tt1 and Ta1) held together.
We here provide details on the breeding history, morphological characteristics (length, weight, ventral colouration pattern) and health status of this captive colony, detailing the existence of viable F1 Tursiops hybrids and a healthy backcross adult offspring. This study utilises historical medical and husbandry data collected through routine veterinary procedures and training records for the dolphins collated in November 2016. Photographs were taken in 2014 and November 2016. Updated length-weight data are summarised from March 2018, with length-weight data from the T. aduncus parent population included for comparison. No comparable length-weight data are available for the parent T. truncatus population.
Species assignment of the T. aduncus dam (Ta1) and T. truncatus sire (Tt1) of the first generation hybrids residing in uShaka Sea World was confirmed by phylogenetic analysis. DNA was extracted from blood samples preserved in 20% DMSO saturated with NaCl using a standard phenol chloroform method (after [39]). A 932bp fragment of the mtDNA control region was amplified using the forward 5' TTC TAC ATA AAC TAT TCC 3' primer and the reverse 5' ATT TTC AGT GTC TTG CTT T 3'. PCR reactions were carried out in 25μl containing 10mM Tris-HCL (pH 8.3), 50 mM KCL, 1.5 mM MgCL2, 0.2 mM dNTP, 2 mM of each primer, 10-15ng template DNA, and 0.625 U DNA Taq Polymerase (New England Biolabs, USA). The PCR cycle was 2 min at 95°C followed by 35 cycles of 40s at 95°C, 40s at 44°C, 45s at 72°C and a final extension for 10 min at 72°C. PCR products were then cleaned using the PureLink PCR Micro Kit (Invitrogen, USA). Sequencing was on an ABI 3730 and resulting sequences were analysed using Chromas 2.6.5 (https://technelysium.com.au/wp/chromas/). A neighbour joining tree was constructed using MEGA 5.2 with the Tamura-Nei evolution model (suitable given the rate variation observed across the control region) and 1000 bootstrap replications. Reference sequences were from Genbank including T. truncatus samples from the North Atlantic [40] and T. aduncus samples from South Africa and the tree was constructed using 488bp overlapping sequence from the control region Hypervariable Region 1. The outgroup chosen was Stenella attenuata (from [41]).
Ethics statement
Dolphins are kept under human care under a South African Department of Environmental Affairs permit (DEA permit number withheld for confidentiality purposes). Blood samples for genetic analysis were collected during routine veterinary supervised preventative health screening procedures, performed in compliance with accredited best international welfare standards and conventions. They were collected in a voluntary manner during routine husbandry training. Other data are purely descriptive and therefore no ethics clearance was necessary. All data generated or analysed during this study are included in this published article, or are available on Genbank.
Results
The captive colony of Tursiops held at uShaka Sea World Durban includes wild stock of T. truncatus and T. aduncus captured in the southern African sub-region in the 1970s and early 1980's and their offspring born at the facility since this time (see Fig 1 and Table 1 for details). Captures of T. truncatus took place in 1976 and 1983 in Walvis Bay, Namibia (22°57’S, 14°30’E) of which Tt1 (male) is the only surviving animal. A further two pure bred T. truncatus are held: Tt3 (male) born in captivity of a pregnant wild caught dam (Tt2, now deceased) and a wild sire, and Tt5 (female) the offspring of Tt1 and the female Tt4 (now deceased). The only pure bred T. aduncus (Ta1, female) was captured from the waters of Umhlanga (South Africa) in 1979. Species confirmation of Ta1 and Tt1 was confirmed by lineage assignment in the mtDNA control region phylogeny (Fig 2).
Fig 1. Family tree of the Tursiops held in the uShaka Sea World, Durban South Africa.
Table 1. Background information on each bottlenose dolphin held at the uShaka Sea World.
| Code | Species | Sex | Date of Capture | Date of Birth | Current status (age on 1st November 2016 or age at death) |
|---|---|---|---|---|---|
| Ta1 | Ta | F | 26/06/1979 | ≤ 26/06/1974* | Alive (42y, 4m) |
| Tt1 | Tt | M | 08/12/1976 | ≤ 08/12/1971* | Alive (44y, 10m) |
| Tt2 | Tt | F | 20/10/1983 | 15/06/1973* | Deceased (12y, 7m,) |
| Tt3 | Tt | M | Captive born | 22/01/1984 | Alive (32y, 9m) |
| Tt4 | Tt | F | 20/10/1983 | 20/10/1978* | Deceased (17y, 11m) |
| Tt5 | Tt | F | Captive born | 12/05/1995 | Alive (21y, 5m) |
| Ta-t1 | F1 Ta×Tt | F | Captive born | 23/04/1986 | Deceased (9y, 1m) |
| Ta-t2 | F1 Ta×Tt | M | Captive born | 28/07/1990 | Deceased (24y, 9m) |
| Ta-t3 | F1 Ta×Tt | F | Captive born | 23/05/1993 | Alive (23y, 5m) |
| Ta-t4 | F1 Ta×Tt | M | Captive born | 07/09/1995 | Alive (21y, 1m) |
| Ta-t5 | F1 Ta×Tt | F | Captive born | 09/12/1998 | Alive (17y, 10m) |
| Ta-t6 | F1 Ta×Tt | M | Captive born | 22/05/2004 | Alive (12y, 5m) |
| Ta-t7 | F1 Ta×Tt | F | Captive born | 25/11/2008 | Alive (7y, 11m) |
| BC1 | Ta-t × Tt | F | Captive born | 17/07/1993 | Alive (23y, 3m) |
| BC2 | Ta-t × Tt | M | Unborn | - | Deceased (>8 m in utero) |
| BC3 | Ta-t × Tt | F | Captive born | 09/02/2014 | Deceased (9d) |
* Estimated from age at capture.
Fig 2. Neighbour-joining phylogeny illustrating the relationships between Ta1 and Tt1 to T. aduncus and T. truncatus specimens (NCBI accession numbers given at terminal nodes).
Bootstrap values are shown based on 1000 replications.
Periodically, since the inception of the dolphin programme, uShaka Sea World has allowed controlled breeding events to occur in the facility. In total, seven F1 hybrids and two backcross progeny have been born at the Sea World facilities. Of these, all the F1 hybrids and one calf from a backcross (paternal T. truncatus) have survived to adulthood. All F1 T. aduncus × T. truncatus hybrids held at the facility are the offspring of Ta1 and Tt1. Five out of the seven F1 hybrids were sired before 2000, when T. truncatus and T. aduncus were considered to be the same taxonomic species [6]. Tt1 and Ta1 are strongly bonded (as demonstrated by consistent affiliative behaviour, authors and trainers observations) and throughout time have been held together with their dependent offspring.
Two backcross progeny have been born at uShaka Sea World, with a third pregnancy documented. The first backcross hybrid offspring; BC1, is a female and was born on the 17th of July, 1993 to Ta-t1 (dam now deceased) with Tt3 the sire. The dam was an estimated 6 years and 3 months at the time of conception, based on back calculations from the date of birth (DOB) of BC1, using a gestation length of 12 months [42]. The BC1 adult is currently housed at uShaka Sea World, attaining an age of 23 years in 2016 and currently (2018) weighing 240.5 kg (Fig 3). Regular veterinary monitoring demonstrates that BC1 is a healthy individual and ultrasound examinations indicate normal ovulation activity in this female.
Fig 3. Image of BC1—an apparently healthy backcross hybrid at age 23 yrs.
A second pregnancy was documented in Ta-t1 (foetus hereby referred to as BC2), representing another backcross event with Tt3. Of note is that Ta-t1 was lactating at the time of conception, with BC1 who was two years old during this time period observed suckling. However, Ta-t1 died on the 30th of May 1995 (at age 9 years) whilst pregnant with the unborn male calf in utero. She was estimated to be in the third trimester of pregnancy at the time of her death. The cause of death for Ta-t1 and associated unborn calf (BC2) was a peracute infection, possibly caused by the bacterium Clostridium chauvoei, resulting in toxaemia. The autopsy report states that the foetus and amniotic fluid appeared normal.
The second backcross (BC3) offspring born at uShaka Sea World was born to Ta-t7 on the 9th of February 2014. Ta-t7 is estimated to have been 4 years and 3 months old at the time of conception (again back calculated from the DOB of BC3) and demonstrated no obvious behaviour or physical signs to demonstrate reproductive receptivity. At the time of conception she was physically small, weighing around 222 kg (weight as of February 2013) and had no clear pattern of ventral speckling—a sign of physical maturation in some Tursiops species [6, 43]. Although fed on a diet of fish and squid from April 2009 onwards, she continued to suckle milk from her mother. As such, she was housed in a social unit consisting of Ta1 and Tt1, her biological mother and father. Copulation was not observed but as they were housed together, it is most likely that Tt1 sired BC3, as all other males were held together in adjacent pools, with no free intermixing between groups taking place. Pregnancy was confirmed in Ta-t7 during a routine ultra sound examination on the 14th June 2013 and she was carefully monitored thereafter. Body length measured around this time in 2014 was estimated at 2.65 m i.e. longer than her thoroughbred mother (Ta1) but shorter than the adult hybrids. Ta-t7 continued to grow by an est. 26 cm in the following years, attaining an adult length of 2.91 m in 2018 (Fig 3).
No abnormal behaviour or physical symptoms were demonstrated during Ta-t7's pregnancy. When born, BC3 was closely observed and appeared healthy, although for managerial reasons no individual medical examinations were conducted with BC3. In the days following birth, BC3 suckled from both her mother (Ta-t7) and maternal grandmother (Ta1). BC3 died on the 18th of February at 9 days old. Post mortem examinations revealed BC3 suffered nutritional complications, most likely resulting from a lack of sufficient colostrum intake in the days following birth and an associated undetermined infection.
The length-weight relationships of the hybrid and backcross offspring fall between the parent species (Fig 4). The first generation hybrid offspring (i.e. all Ta-t) have a length of 2.89 to 3.02 m. (mean 2.95 m) and weigh between 231 to 273 kg (mean 247 kg), with BC1 falling within this range (2.99 m and 241 kg). The two pure bred male T. truncatus held at uShaka Sea World are considerably larger (for instance Tt1 is 3.55 m in length and weighs 470 kgs). However, the pure bred female T. truncatus (Tt5) is unusual in this sample, by having a comparatively small length and weight for the species, attributed to premature maternal separation and restricted development (authors observations). All hybrids are longer and weigh more than Ta1 and the largest T. aduncus specimens measured from the wild parent population where Ta1 originates (Fig 4.).
Fig 4. Body length-weight relationship for dolphins housed at uShaka Sea World, as well as examples from the parent T. aduncus population.
Data from three T. aduncus from KwaZulu Natal are by-caught specimens and the largest examples in the data-set from this region [44]. Growth curves for each species calculated by Best (2007) from 16 common bottlenose (Weight = 11.32 x Length2.9869) and 41 Indo-Pacific bottlenose dolphin (Weight = 12.365 x Length2.9495) necropsies of animals within the study area.
Some T. aduncus populations exhibit ventral speckling [43, 45], the degree of which increases with age and may indicate sexual maturation. We inspected the ventral surfaces of all dolphins within uShaka Sea World to determine the degree of ventral speckling. Ventral speckling was absent in Ta-t7 before conception and in 2016 (at age 7 yrs 11 mo) Ta-t7 still did not exhibit significant ventral speckling (Fig 5A and 5B). In 2016, some ventral speckling was present on the older hybrids held at uShaka Sea World (Fig 5D), although visual assessment indicated a much lesser degree of speckling than considered normal for mature individuals from the parent T. aduncus or Shark Bay Tursiops spp. species [6, 43] (compare Fig 5C and 5D). On the adult hybrids held at uShaka Sea World, the ventral speckles are faint and coverage of the ventral area is sparse (Fig 5D).
Fig 5. Ventral speckling is a sign of physical maturation in some populations of T. aduncus.
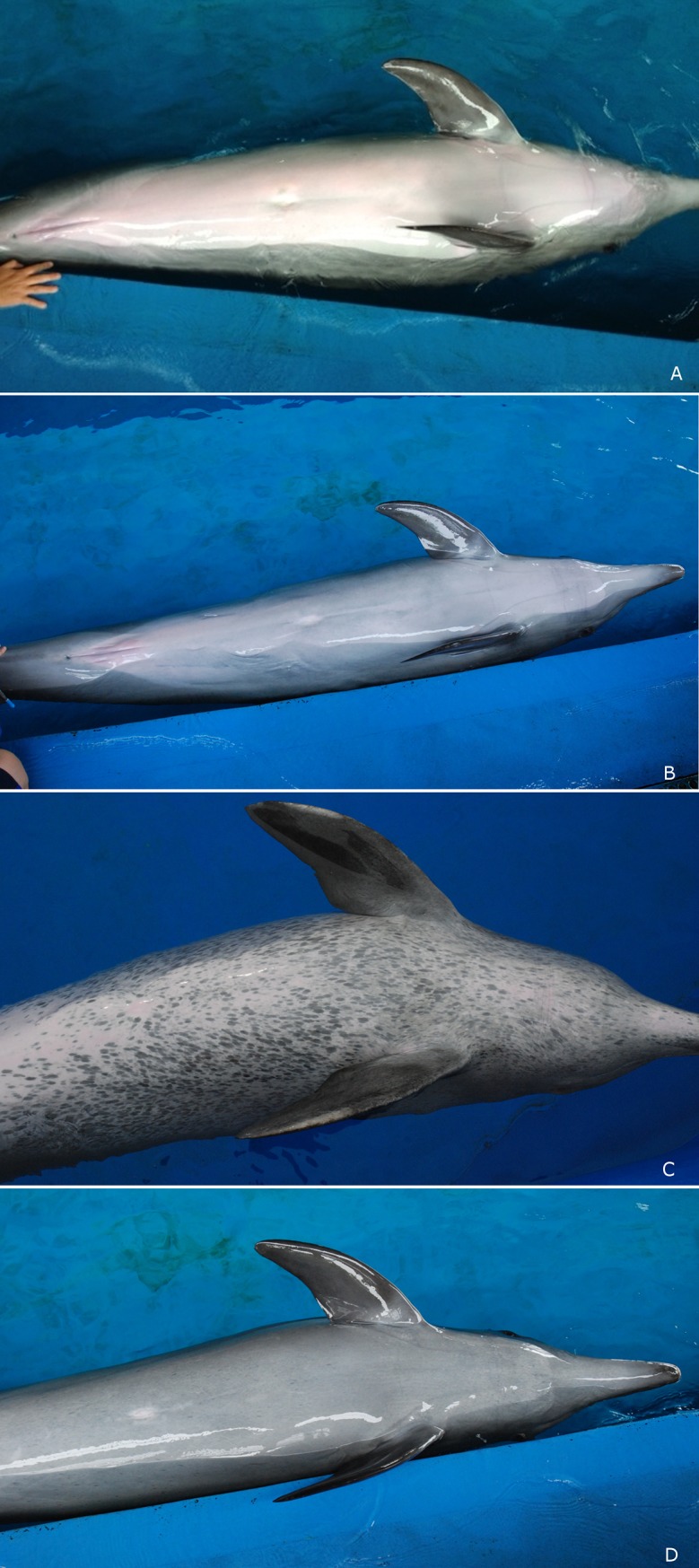
Comparisons of the ventral surfaces of Ta-t7, Ta1 and Ta-t3 demonstrating degree of ventral speckling or lack thereof. A) Ta-t7 aged 4 yrs i.e. before conception, (photo credit S. Pillay), B) Ta-t7 aged 7 yrs (i.e. following conception), C) The ventral surface of Ta1 the T. aduncus dam of Ta-t7 at age 42 yrs, D). The oldest Ta-t female hybrid at uShaka Sea World displays low levels of ventral speckling at age 23 yrs.
Observation and training with the F1 hybrids and the surviving backcross hybrid (BC1) is ongoing at uShaka Sea World. In all cases, the hybrids are fully incorporated into the daily activities of the facility and demonstrate social and cognitive functions, such as response rates during training for veterinary procedures and strong social bonding, similar to the thoroughbred dolphins housed at the same facility.
Discussion
To date, most hybridization events in wild cetaceans have been identified through morphological descriptions (e.g. [46, 47]) with the recent application of molecular techniques (e.g. [17, 28, 30, 48, 49]) used to identify hybrids and their parent species. Reports from captive facilities enable the tracking of breeding history (e.g. [50]), and as in our case, can provide important information on the breeding capabilities of dolphin species. Of the odontocetes, the common bottlenose dolphin is the species recorded most frequently to hybridize in captivity [3]. Although there are exceptions [33], the majority of hybrid offspring born in captivity do not survive [3, 21]. Here we demonstrate that F1 T. aduncus and T. truncatus can survive to adulthood, are healthy and can produce healthy backcross hybrid offspring in cases where the dam is the F1 hybrid and the sire is T. truncatus.
The longevity of the hybrid offspring and most notably the BC1 hybrid at uShaka Sea World is unusual amongst captive facilities [3, 21]. This may be explained by the closer taxonomic relationship between Tursiops species compared to species involved in inter-generic hybridization events, perhaps facilitating genetic compatibility. Breeding success may also be a reflection of good animal husbandry at the uShaka Sea World captive facility. The apparently normal ovulatory behaviour of the surviving backcross hybrid adult, suggests that subsequent generational hybrids may also be reproductively viable, though the lack of a test for F2 compatibility of hybrids is a limitation, especially since it is often the heterogametic sex (males) that shows hybrid sterility ('Haldane's rule' [51]).
Although rare, there are documented cases of inter-generic hybridization involving T. truncatus, resulting in fertile hybrids which have subsequently backcrossed with the parent T. truncatus species. For example, Duffield [52] report that an F1 T. truncatus x P. crassidens hybrid backcrossed with T. truncatus on two occasions. In another example, an F1 T. truncatus x D. capensis hybrid backcrossed with T. truncatus, however the calf died shortly after birth [33]. Both examples demonstrate the capability for T. truncatus to hybridize and for the hybrids to backcross. Here we describe in some detail multiple intra-generic hybridization events between T. truncatus and T. aduncus and a successful backcross, supporting the potential for this type of reticulation in this genus and the consequent influence on evolution in the wild. We document backcross mating by two parental configurations, and so too few to draw any strong conclusions. We can note however that the parents were unrelated for the offspring that survived (Tt3 with Ta-t1, see Fig 1), while the offspring from the inbred mating (Tt1 with his daughter Ta-t7) did not.
Data on age at sexual maturity in female T. aduncus are sparse. Sexual maturity occurs before physical maturity, and earlier in females than males [6, 53]. Timing of maturity may also differ between captive and wild born animals [54] and between geographically separated populations [55], further complicating assessments of reproductive age. For example, mean ovulation age in captive killer whales (Orcinus orca) is 7.5 years and age at first conception 9.8 years, compared to the average first conception age of 12.1 years in wild, free ranging populations [56]. In the wild, ovulation in female T. aduncus from South African waters is reported to take place between 9.5 and 11 years of age [6]. However, reports of a stranded female from an earlier study suggest that sexual maturity can be attained under 9 yrs of age, and possibly as early as 6 yrs [42]. There are reports of sexual maturity as early as 3.5 years in Tursiops from Japan [57]. However, these data are derived from the examination of deceased dolphins, and it is unclear whether this minimum age is based on the occurrence of corpora lutea in the ovaries or observed pregnancies in animals of this young age (or both), with no further data on whether the outcome of pregnancy was a viable offspring [57]. Data from free ranging T. truncatus from Namibia are similarly sparse, although there is evidence from this population that first conception can take place around 5.5 years of age [58] and at approximately 2.8 m total length [38]. There are few data on the age at maturity of hybrids and whether, like other morphological [22, 25, 33, 38] and behavioural [25] characteristics, it is intermediate between that of the parent species. Zornetzer and Duffield [33], for example report the birth of a calf to a hybrid T.truncatus x D.capensis, born when the dam was 7.5 yrs and presumably conceived around 6.5 yrs of age. Our data on pregnancy in F1 T. aduncus x T. truncatus hybrids demonstrates that these animals can become pregnant early in life compared to the parent species. The estimated age of conception of 4 years and 3 months reported here for Ta-t7 may therefore be the youngest known viable pregnancy for either parent Tursiops species or hybrid thereof.
That Ta-t7 was still observed nursing during the period of conception is also of interest. Bottlenose dolphins can begin ingesting solid food between 4 and 11 months of age [59], with a combined solid and milk diet thereafter. At uShaka Sea World, Ta-t7 began eating solids from 4.5 months onwards. Bottlenose dolphins and other odontocetes are known to have prolonged lactation [59] and in South African T. aduncus milk remains have been documented in the stomachs of calves up to three years of age [60]. Although the majority of calves from bottlenose dolphins from Shark Bay, Western Australia were weaned before four years, some continued to suckle after this, with one animal only weaned at eight years of age [61]. Lactation in mammals, including dolphins, relies on close proximity and physical stimulation of the mammary area [62–64]. Captive studies have demonstrated that persistent suckling attempts can induce lactation when orphaned calves are held in close proximity to previously non-lactating Tursiops females [65]. In the wild, pre-weaned animals maintain a close association with their mother, with weaning initiated during the females' next pregnancy [61]. Therefore, the close association of mother and calf in the captive facility may have prolonged the lactation period of Ta1 to four years of age and beyond.
Morphological characteristics of hybrid cetacean offspring appear intermediate to the parent species [3, 33]. In the wild T. aduncus are smaller in length and estimated weight compared to T. truncatus [38]. Although limited, our length-weight data indicate that the size of hybrid offspring is intermediate to the biological parents, indicating it falls intermediate between the parent species (Fig 3). This observation might help identification of hybrids in the wild, however a greater sample size including unrelated individuals would clarify this relationship. The coloration patterns of hybrids can also differ from parent species, usually being somewhat intermediate [22, 33, 38]. Ventral speckling is absent in T. truncatus but is prominent in some populations of T. aduncus and the Tursiops spp. population found in Shark Bay, Western Australia which have had an uncertain taxonomic status but speckling patterns similar to T. aduncus [6, 43]. In the latter population, speckling develops with age, first appearing around the genital area around 10 years of age, but can occur as early as 7 years. The age of speckle onset around the genitalia usually correlates with the age of first parturition and is considered an honest sign of sexual maturation in the Shark Bay population [43]. The development of speckling has not yet been determined in hybrid Tursiops dolphins. Our observations indicate that the onset or degree of ventral speckling is not a reliable indicator of sexual maturity in F1 Tursiops hybrids.
Karyological similarity within the Cetacea (most have the same number of chromosomes: 2n = 44 [3]) has been proposed as one explanation for the apparent ease with which distinctly related cetacean species hybridize [66]. Where their distributions overlap, new cetacean species can originate through hybridization, as demonstrated for the Clymene's dolphin [4] and environmental pressures such as climate change may increase the frequency of introgressive hybridization, as recently suggested for pilot whales, genus Globicephala [32]. The distribution of T. aduncus and T. truncatus occur in parapatry throughout the Indo-Pacific region, with sympatric distributions in some areas such as the waters off South East China [8]. Given that we have demonstrated several hybridization events, it is somewhat surprising that other hybridization events have not been documented in wild populations and the genetic integrity of the parent species remains intact in areas where their distributions overlap such as in the Taiwan Strait [8, 67] and Australia [16]. Indeed, relatively high levels of genetic isolation have been documented in such areas [67]. Behavioural isolation mechanisms may be operating in the wild to reduce hybridization events. For example, T. aduncus and T. truncatus produce acoustic communication signals (whistles) with distinguishable frequency compositions [68, 69], which could assist in inter-species recognition thereby reducing intra-generic mating attempts.
Conclusion
We have demonstrated that T. aduncus x T. truncatus F1 hybrids can survive to adulthood, are healthy and can produce healthy backcross hybrid offspring. The documented hybridization in captivity may be an artefact of the close proximity and the limited mating opportunities afforded by captive situations, limiting mate choice and assortative mating. However, low levels of intra-generic hybridization in Tursiops may well be taking place in the wild [34], and may be revealed following more extensive molecular screening in the relevant geographic regions.
Acknowledgments
The authors would like to thank everyone involved in helping collect the data at uShaka Sea World, and in particular Ms Sarah Pillay, Ms Kelly de Klerk, Tracy Shaw, Ada Natoli and Caryl Knox. We would like to thank Dr Larry Oellermann, Mr Gavin Drysdale, and Mr Tony McEwan for their support in publishing this work. We gratefully acknowledge the support of Assoc. Prof Res Alwegg from the University of Cape Town and the Sea Search Research and Conservation NPC group of scientists and students. We also thank the input of two anonymous reviewers for their constructive comments.
Data Availability
All relevant data are within the paper. The mtDNA sequences of Ta1 and Tt1 are available on GenBank, accession numbers MH733901 and MH733902 respectively.
Funding Statement
This research was supported in part by a Claude Leon Post Doctoral Fellowship to TG (www.leonfoundation.co.za) and National ResearchFoundation Fellowship to SE (www.nrf.ac.za/). Sea Search Africa Pty provided travel funding to TG (www.seasearch.co.za). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.The author(s) GH and FL received no specific funding for this work. The funder provided support in the form of salaries for authors as follows: Claude Leon Post Doctoral Fellowship to TG, National Research Foundation Fellowship to SE. Salaries from SAAMBR uShaka Sea World to GH and FL, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Arnold ML. Divergence with Genetic Exchange Oxford: University Press; 2015. 272 p. [Google Scholar]
- 2.Seehausen O. Hybridization and adaptive radiation. Trends Ecol Evol. 2004;19(4):198–207. 10.1016/j.tree.2004.01.003 [DOI] [PubMed] [Google Scholar]
- 3.Bérubé M. Hybridism 2009. In: Encyclopedia of marine mammals [Internet]. New York: Academic Press. 2nd. [588–91]. [Google Scholar]
- 4.Amaral AR, Lovewell G, Coelho MM, Amato G, Rosenbaum HC. Hybrid Speciation in a Marine Mammal: The Clymene Dolphin (Stenella clymene). PLoS One. 2014;9(1):e83645 10.1371/journal.pone.0083645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hershkovitz P. A catalog of living whales. United States National Museum Bulletin. 1966;246:1–259. [Google Scholar]
- 6.Ross GJB, Cockcroft VG. Comments on Australian bottlenose dolphins and the taxonomic status of Tursiops aduncus (Ehrenberg,1832) In: Leatherwood S, Reeves RR, editors. The Bottlenose Dolphin. San Diego: Academic Press; 1990. p. 101–28. [Google Scholar]
- 7.Natoli A, Peddemors VM, Hoelzel AR. Population structure and speciation in the genus Tursiops based on microsatellite and mitochondrial DNA analyses. Journal of Evolutionary Biology. 2004;17(2):363–75. PubMed PMID: ISI:000188990500015. [DOI] [PubMed] [Google Scholar]
- 8.Wang JY, Chou LS, White BN. Mitochondrial DNA analysis of sympatric morphotypes of bottlenose dolphins (genus: Tursiops) in Chinese waters. Molecular Ecology. 1999;8(10):1603–12. PubMed PMID: ISI:000083466800005. [DOI] [PubMed] [Google Scholar]
- 9.Moura AE, Nielsen SC, Vilstrup JT, Moreno-Mayar JV, Gilbert MT, Gray HW, et al. Recent diversification of a marine genus (Tursiops spp.) tracks habitat preference and environmental change. Syst Biol. 2013;62(6):865–77. 10.1093/sysbio/syt051 [DOI] [PubMed] [Google Scholar]
- 10.Wang JY, Chou LS, White BN. Differences in the external morphology of two sympatric species of bottlenose dolphins (genus Tursiops) in the waters of China. Journal of Mammalogy. 2000;81(4):1157–65. PubMed PMID: ISI:000165637900024. [Google Scholar]
- 11.Rice DW. Marine mammals of the world: systematics and distribution. Society for Marine Mammalogy. 1998;Special Publication 4:1–231. [Google Scholar]
- 12.Charlton-Robb K, Gershwin L-A, Thompson R, Austin J, Owen K, McKechnie S. A new dolphin species, the burrunan dolphin Tursiops australis sp. nov., endemic to southern Australian coastal waters. PLoS One. 2011;6(9):E24047 10.1371/journal.pone.0024047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viaud-Martinez KA, Brownell RL, Komnenou A, Bohonak AJ. Genetic isolation and morphological divergence of Black Sea bottlenose dolphins. Biological Conservation. 2008;141(6):1600–11. 10.1016/j.biocon.2008.04.004 PubMed PMID: ISI:000257536100015. [DOI] [Google Scholar]
- 14.Chen I, Nishida S, Yang W-C, Isobe T, Tajima Y, Hoelzel AR. Genetic diversity of bottlenose dolphin (Tursiops sp.) populations in the western North Pacific and the conservation implications. Marine Biology. 2017;164(10):202 10.1007/s00227-017-3232-8 PubMed PMID: PMC5592193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlton K, Taylor AC, McKechnie SW. A note on divergent mtDNA lineages of bottlenose dolphins from coastal waters of Southern Australia. Journal of cetacean research management. 2006;8(2):173–9. [Google Scholar]
- 16.Allen SJ, Bryant KA, Kraus RH, Loneragan NR, Kopps AM, Brown AM, et al. Genetic isolation between coastal and fishery-impacted, offshore bottlenose dolphin (Tursiops spp.) populations. Mol Ecol. 2016;25(12):2735–53. 10.1111/mec.13622 [DOI] [PubMed] [Google Scholar]
- 17.Kingston SE, Adams LD, Rosel PE. Testing mitochondrial sequences and anonymous nuclear markers for phylogeny reconstruction in a rapidly radiating group: molecular systematics of the Delphininae (Cetacea: Odontoceti: Delphinidae). Bmc Evolutionary Biology. 2009;9:19. doi: 245 10.1186/1471-2148-9-19 PubMed PMID: ISI:000271888200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeDuc RG, Perrin WF, Dizon AE. Phylogenetic relationships among the delphinid cetaceans based on full cytochrome B sequences. Marine Mammal Science. 1999;15(3):619–48. PubMed PMID: ISI:000080863700001. [Google Scholar]
- 19.Vilstrup JT, Ho SY, Foote AD, Morin PA, Kreb D, Krutzen M, et al. Mitogenomic phylogenetic analyses of the Delphinidae with an emphasis on the Globicephalinae. BMC Evol Biol. 2011;11(65):1471–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGowen M, R.. Toward the resolution of an explosive radiation—A multilocus phylogeny of oceanic dolphins (Delphinidae). Molecular Phylogenetics and Evolution. 2011;60:345–57. 10.1016/j.ympev.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 21.Sylvestre J-P, Tasaka S. On the intergeneric hybrids in cetaceans. Aquatic Mammals. 1985;11(3):101–8. [Google Scholar]
- 22.Caballero S, Baker CS. Captive-born intergeneric hybrid of a Guiana and bottlenose dolphin: Sotalia guianensis×Tursiops truncatus. Zoo Biology. 2010;29(5):647–57. 10.1002/zoo.20299 [DOI] [PubMed] [Google Scholar]
- 23.Schaurich MdN Lopes FRV, de Oliveira LR. Hybridization phenomenon in cetacean and pinniped species. Neotropical Biology and Conservation. 2012;7(3):199–209. [Google Scholar]
- 24.Glover KA, Kanda N, Haug T, Pastene LA, Øien N, Goto M, et al. Migration of Antarctic minke whales to the Arctic. PLoS One. 2010;5(12):e15197 10.1371/journal.pone.0015197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willis PM, Crespi BJ, Dill LM, Baird RW, Hanson MB. Natural hybridization between Dall’s porpoises (Phocoenoides dalli) and harbour porpoises (Phocoena phocoena). Canadian Journal of Zoology. 2004;82:828–34. [Google Scholar]
- 26.Bérubé M, Aguilar A. A new hybrid between a blue whale, Balaenoptera musculus, and a fin whale, B. physalus: frequency and implications of hybridization. Marine Mammal Science. 1998;14(1):82–98. 10.1111/j.1748-7692.1998.tb00692.x [DOI] [Google Scholar]
- 27.Reyes JC. A possible case of hybridism in wild dolphins. Marine Mammal Science. 1996;12(2):301–7. 10.1111/j.1748-7692.1996.tb00581.x [DOI] [Google Scholar]
- 28.Brown AM, Kopps AM, Allen SJ, Bejder L, Littleford-Colquhoun B, Parra GJ, et al. Population Differentiation and Hybridisation of Australian Snubfin (Orcaella heinsohni) and Indo-Pacific Humpback (Sousa chinensis) Dolphins in North-Western Australia. PLoS One. 2014;9(7):e101427 10.1371/journal.pone.0101427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glover K, Kanda N, Haug T, Pastene L, Oien N, Seliussen B, et al. Hybrids between common and Antarctic minke whales are fertile and can back-cross. BMC Genetics. 2013;14(1):25 10.1186/1471-2156-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spilliaert R, Vikingsson G, Arnason U, Palsdottir A, Sigurjonsson J, Arnason A. Species hybridization between a female blue whale (Balaenoptera musculus) and a male fin whale (B. physalus): molecular and morphological documentation. J Hered. 1991;82(4):269–74. [DOI] [PubMed] [Google Scholar]
- 31.Miralles L, Lens S, Rodríguez-Folgar A, Carrillo M, Martín V, Mikkelsen B, et al. Interspecific Introgression in Cetaceans: DNA Markers Reveal Post-F1 Status of a Pilot Whale. PLoS One. 2013;8(8):e69511 10.1371/journal.pone.0069511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miralles L, Oremus M, Silva MA, Planes S, Garcia-Vazquez E. Interspecific Hybridization in Pilot Whales and Asymmetric Genetic Introgression in Northern Globicephala melas under the Scenario of Global Warming. PLoS One. 2016;11(8):e0160080 10.1371/journal.pone.0160080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zornetzer HR, Duffield DA. Captive-born bottlenose dolphin × common dolphin (Tursiops truncatus × Delphinus capensis) intergeneric hybrids. Canadian Journal of Zoology. 2003;81(10):1755–62. 10.1139/z03-150 [DOI] [Google Scholar]
- 34.Martien KK, Baird RW, Hedrick NM, Gorgone AM, Thieleking JL, McSweeney DJ, et al. Population structure of island-associated dolphins: Evidence from mitochondrial and microsatellite markers for common bottlenose dolphins (Tursiops truncatus) around the main Hawaiian Islands. Marine Mammal Science. 2012;28(3):E208–E32. Epub 2011. 10.1111/j.1748-7692.2011.00506.x [DOI] [Google Scholar]
- 35.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164(4):1567–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubisz MJ, Falush D, Stephens M, Pritchard JK. Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour. 2009;9(5):1322–32. 10.1111/j.1755-0998.2009.02591.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Best PB. Whales and dolphins of the Southern African Subregion Cambridge: Cambridge University Press; 2007. 338 p. [Google Scholar]
- 39.Hoelzel AR. Molecular Genetic Analysis of Populations; A Practical Approach Oxford: Oxford University Press; 1992. [Google Scholar]
- 40.Natoli A, Birkun A, Aguilar A, Lopez A, Hoelzel AR. Habitat structure and the dispersal of male and female bottlenose dolphins (Tursiops truncatus). Proc R Soc B-Biol Sci. 2005;272(1569):1217–26. PubMed PMID: ISI:000230563200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oremus M, Leqata J, Baker CS. Resumption of traditional drive hunting of dolphins in the Solomon Islands in 2013. Royal Society Open Science. 2015;2(5):140524 10.1098/rsos.140524 PubMed PMID: PMC4453245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross GJB. The taxonomy of bottlenosed dolphins Tursiops species in South African waters, with notes on their biology. Annals of the Cape Provincial Museum (Natural Histroy). 1977;11:135–94. [Google Scholar]
- 43.Krzyszczyk E, Mann J. Why become speckled? Ontogeny and function of speckling in Shark Bay bottlenose dolphins (Tursiops sp.)1. Marine Mammal Science. 2012;28(2):295–307. 10.1111/j.1748-7692.2011.00483.x [DOI] [Google Scholar]
- 44.Plön S, Albrecht K, Cliff G, Froneman PW. Organ weights of three dolphin species from South Africa-implications for ecological adaptation? 2012. 265–76 p. [Google Scholar]
- 45.Amir OA, Jiddawi NS, Berggren P. The occurence and distribution of dolphins in Zanzibar, Tanzania, with comments on differences between two species of Tursiops. Western Indian Ocean Journal of Marine Science. 2005;4(1):85–93. [Google Scholar]
- 46.Hodgins NK, Dolman SJ, Weir CR. Potential hybridism between free-ranging Risso's dolphins (Grampus griseus) and bottlenose dolphins (Tursiops truncatus) off north-east Lewis (Hebrides, UK). Marine Biodiversity Records. 2014;7 10.1017/s175526721400089x [DOI] [Google Scholar]
- 47.Heide-Jørgensen MP, Reeves RR. Description of an anomalous monodontid skull from West Greenland: A possible hybrid? Marine Mammal Science. 1993;9(3):258–68. 10.1111/j.1748-7692.1993.tb00454.x [DOI] [Google Scholar]
- 48.Baird RW, Willis PM, Guenther TJ, Wilson PJ, White BN. An intergeneric hybrid in the family Phocoenidae. Canadian Journal of Zoology. 1998;76(1):198–204. 10.1139/z97-175a [DOI] [Google Scholar]
- 49.Arnason U, Spilliaert R, Palsdottir A, Arnason A. Molecular identification of hybrids between the two largest whale species, the blue whale (Balaenoptera musculus) and the fin whale (B. physalus). Hereditas. 1991;115(2):183–9. [DOI] [PubMed] [Google Scholar]
- 50.Dohl TP, Norris KS, Kang I. A Porpoise Hybrid: Tursiops × Steno. Journal of Mammalogy. 1974;55(1):217–21. 10.2307/1379276 [DOI] [PubMed] [Google Scholar]
- 51.Haldane JBS. Sex ratio and unisexual sterility in hybrid animals. Journal of Genetics. 1922;12(2):101–9. 10.1007/bf02983075 [DOI] [Google Scholar]
- 52.Duffield DA, editor Examples of captive hybridization and a genetic point of view. World Marine Mammal Science Conference; 1998; UK. [Google Scholar]
- 53.Kemper CM, Trentin E, Tomo I. Sexual maturity in male Indo-Pacific bottlenose dolphins (Tursiops aduncus): evidence for regressed/pathological adults. Journal of Mammalogy. 2014;95(2):357–68. 10.1644/13-mamm-a-007.1 [DOI] [Google Scholar]
- 54.O'Regan HJ, Kitchener AC. The effects of captivity on the morphology of captive, domesticated and feral mammals. Mammal Review. 2005;35(3–4):215–30. 10.1111/j.1365-2907.2005.00070.x [DOI] [Google Scholar]
- 55.Hale PT, Barreto AS, Ross GJB. Comparative morphology and distribution of the aduncus and truncatus forms of bottlenose dolphin Tursiops in the Indian and Western Pacific Oceans. Aquatic Mammals. 2000;26(2):101–10. [Google Scholar]
- 56.Robeck TR, Willis K, Scarpuzzi MR, O’Brien JK. Comparisons of life-history parameters between free-ranging and captive killer whale (Orcinus orca) populations for application toward species management. Journal of Mammalogy. 2015;96(5):1055–70. 10.1093/jmammal/gyv113 PubMed PMID: PMC4668992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kasuya T. Fishery-dolphin conflict in the Iki Island area of Japan 1985. In: Marine Mammals and Fisheries [Internet]. London: George Allen & Vnwin:; [253–72]. [Google Scholar]
- 58.Peddemors VM. Minimum age at sexual maturation of a female south east atlantic bottlenose dolphin Tursiops truncatus. South African Journal of Marine Science. 1989;(8):345–8. [Google Scholar]
- 59.Oftedal OT. Lactation in whales and dolphins: evidence of divergence between baleen- and toothed-species. J Mammary Gland Biol Neoplasia. 1997;2(3):205–30. [DOI] [PubMed] [Google Scholar]
- 60.Cockcroft VG, Ross GJB. Age, growth, and reproduction of bottle-nosed dolphins Tursiops-truncatus from the East Coast of Southern Africa. Fish Bull. 1990;88(2):289–302. PubMed PMID: ISI:A1990DX59600006. [Google Scholar]
- 61.Mann J, Connor RC, Barre LM, Heithaus MR. Female reproductive success in bottlenose dolphins (Tursiops sp.): life history, habitat, provisioning, and group-size effects. Behavioral Ecology. 2000;11(2):210–9. 10.1093/beheco/11.2.210 [DOI] [Google Scholar]
- 62.Peddemors VM, Fothergill M, Cockcroft VG. Feeding and growth in a captive-born bottlenose dolphin Tursiops truncatus. South African Journal of Zoology. 1992;27(2):74–80. 10.1080/02541858.1992.11448265 [DOI] [Google Scholar]
- 63.McClellan HL, Miller SJ, Hartmann PE. Evolution of lactation: nutrition v. protection with special reference to five mammalian species. Nutr Res Rev. 2008;21(2):97–116. 10.1017/S0954422408100749 [DOI] [PubMed] [Google Scholar]
- 64.Nowak R, Porter RH, Levy F, Orgeur P, Schaal B. Role of mother-young interactions in the survival of offspring in domestic mammals. Rev Reprod. 2000;5(3):153–63. [DOI] [PubMed] [Google Scholar]
- 65.Ridgway S, Kamolnick T, Reddy M, Curry C, Tarpley RJ. Orphan-induced lactation in Tursiops and analysis of collected milk. Marine Mammal Science. 1995;11(2):172–82. 10.1111/j.1748-7692.1995.tb00516.x [DOI] [Google Scholar]
- 66.Arnason U, Gullberg A. Comparison between the complete mtDNA sequences of the blue and the fin whale, two species that can hybridize in nature. J Mol Evol. 1993;37(4):312–22. [DOI] [PubMed] [Google Scholar]
- 67.Yang GA, Ji GQ, Ren WH, Zhou KY, Wei FW. Pattern of genetic variation of bottlenose dolphins in Chinese waters. Raffles Bull Zool. 2005;53(1):157–64. PubMed PMID: ISI:000231709900018. [Google Scholar]
- 68.Gridley T, Berggren P, Cockcroft VG, Janik VM. Whistle vocalizations of Indo-Pacific bottlenose dolphins (Tursiops aduncus) inhabiting the south-west Indian Ocean. Journal of the Acoustical Society of America. 2012;132(6):4032–40. 10.1121/1.4763990 [DOI] [PubMed] [Google Scholar]
- 69.Erbs F, Elwen SH, Gridley T. Automatic classification of whistles from coastal dolphins of the southern African subregion. The Journal of the Acoustical Society of America. 2017;141(4):2489–500. 10.1121/1.4978000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper. The mtDNA sequences of Ta1 and Tt1 are available on GenBank, accession numbers MH733901 and MH733902 respectively.






