Abstract
Introduction
Inadequate management of patients with atrial fibrillation (AF) has been reported in China for anticoagulation therapy and treatment for concomitant diseases. An effective quality improvement programme has been lacking to promote the use of evidence-based treatments and improve outcome in patients with AF.
Methods and analysis
The Improving Care for Cardiovascular Disease in China-AF programme is a collaboration of the American Heart Association and the Chinese Society of Cardiology. This programme is designed to promote adherence to AF guideline recommendations and outcomes for inpatients with AF. Launched in February 2015, 150 hospitals are recruited by geographic-economic regions across 30 provinces in China. Each month, 10–20 inpatients with AF are enrolled in each hospital. A web-based data collection platform is used to collect clinical information for patients with AF, including patients’ demographics, admission information, medical history, in-hospital care and outcomes, and discharge medications for managing AF. The quality improvement initiative includes monthly benchmarked reports on hospital quality, training sessions, regular webinars and recognitions of hospital quality achievement. Primary analyses will include adherence to performance measures and guidelines. To address intrahospital correlation, generalised estimating equation models will be applied. As of March 2017, 28 801 AF inpatients have been enrolled.
Ethics and dissemination
This study protocol was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University. Results will be published in peer-reviewed medical journals.
Trial registration number
Keywords: atrial fibrillation, quality improvement, healthcare, registry study
Strengths and limitations of this study.
Improving Care for Cardiovascular Disease in China-atrial fibrillation (CCC-AF) is a nationwide quality improvement project with tools including monthly benchmarked reports on hospital quality, training sessions, regular webinars and recognition for quality achievement.
Data accuracy and completeness of this study is ensured by training sessions, standardised web-based data collection tool, onsite quality control and monitoring of data completeness.
Experience from this programme will help to guide development of the national health quality improvement system.
Participation is voluntary and only tertiary hospitals are recruited in the CCC-AF programme, which may not be able to represent the care quality for China overall.
Introduction
As the most common sustained arrhythmia, atrial fibrillation (AF) is responsible for major morbidity and healthcare costs. It is associated with a fourfold to fivefold increased risk for stroke, a 40%–90% increased risk for overall mortality and impaired quality of life.1 2 The prevalence of AF is estimated to be 0.77% in China, and >5 million Chinese adults aged >35 years currently have AF.3–6 There is wide availability of evidence-based guidelines with effective treatments for improving outcomes of AF.7–9 However, poor compliance with evidence-based therapies has been reported in China, including assessment of thromboembolic risk, anticoagulation therapy, heart rate control, rhythm control and adequate treatment of concomitant diseases.10 11 Several studies with diversified methodologies have reported that >60% of patients with AF that are eligible for anticoagulant treatment receive no risk stratification for stroke or therapy.10–13
Although previous registries have expanded understanding of clinical practice of AF in China, they have been limited in the representativeness of hospitals and lack of quality improvement components.10 12 13 To promote the use of guidelines of recommended therapies and to assess the performance of quality improvement efforts, the Chinese Society of Cardiology (CSC) and the American Heart Association (AHA) initiated the Improving Care for Cardiovascular Disease in China (CCC) project-AF programme. The CCC-AF is on the Get With The Guidelines initiative of AHA.14 As a quality improvement initiative with timely feedback for care of patients with AF, CCC-AF will enhance the quality of care for AF through furnishing specially designed tools for quality improvement.
This paper aims to present the objectives, organisational framework and governance, hospital and patient enrolment, data collection, quality improvement tools for AF care and current progress of the CCC-AF programme.
Methods
Study objectives
The CCC-AF programme aims to promote implementation of guideline-recommended therapies for patients with AF using rapid cycle of data collection, analysis, feedback and process improvement. The objectives of the CCC-AF programme include understand the current situation and main problems for management of AF inpatients, assess the performance of the current strategy on quality improvement for AF management, as well as explore and refine the optimal approach to improve clinical management of AF. The timing and dynamic monitoring of AF management will provide an opportunity to identify the potential needs of further evidence in management of AF. Experience from this programme may also help to guide development of the national health quality improvement system and provide a blueprint for adaptation to other regions of the world.
Organisational framework and governance
As a collaborative project of the CSC and the AHA, the CCC-AF is conducted by the Beijing Institute of Heart, Lung and Blood Vessel Diseases. AHA secured the initial project funding and CSC provided hospital network for CCC-AF. As a continuous quality improvement programme launched in February 2015, CCC-AF has secured its funding up to December 2018. This programme consists of a senior management group (SMG) and a project management group (online figure s1). Six senior clinician volunteers from AHA (SCS, GCS and KAT) and CSC (YH, JG and CM) in the SMG communicate frequently via teleconferences, emails and face-to-face meetings to ensure the scientific integrity and supervise the implementation of the CCC-AF programme. Under the leadership of the international and national directors (LM and DZ), the project management group oversees the operation of the CCC-AF project. The project coordinator (JL) supervises for functional groups, namely daily routine management (JL and MZ), data (YH and YG), and advisory and education groups. Data collection and analysis are managed by daily routine management group and data group, with guidance from SMG. Researchers from SMG, daily routine management group and participating hospitals have the access to analysis the data for publications.
bmjopen-2017-020968supp001.pdf (1.1MB, pdf)
Hospital recruitment
Hospitals were recruited by geographic-economic regions,15 and the detailed hospital sampling frame is shown in supplementary table 1. A total of 150 hospitals were recruited, accounting for about 10% of the tertiary hospitals in China. Tertiary hospitals in the CCC- AF programme meet the following criteria: (1) the annual number of patients hospitalised with AF was >120 and (2) the director of the Cardiology Department agreed to join the programme. Traditional Chinese medicine hospitals and specialised hospitals without cardiology wards were not included in CCC-AF programme. Online supplementary tables 2 and 3 provide the information of the 150 CCC-AF hospitals recruited in phases I and II.
Patient recruitment
In each hospital, the first 10–20 hospitalised patients with AF are enrolled in a consecutive manner. Study inclusion of AF is based on ECG results, which are recorded by 12-lead ECG, 24 hours Holter ECG or other cardiac rhythm monitors. Patients with AF secondary to reversible condition (eg, untreated thyroid disease and pulmonary embolism) are excluded from the study.
Clinical data elements are collected referring to American College of Cardiology (ACC)/AHA recommendations on the data standards for clinical research of AF.16 17 As a quality improvement initiative, the CCC-AF programme has high priority for collection of data elements that are involved in assessment for quality of care. Moreover, additional data elements are gathered to facilitate in-depth analysis. Data elements include patients’ demographics, admission information, medical history, in-hospital care and outcomes, and discharge medications for management of AF, as presented in the case report form (online supplementary table 4). Collection of personal identifiers allows the potential for linking our dataset to other health records including death and hospitalisation data in the future. Eligible patients with AF are reported to the database using Oracle Clinical Remote Data Capture system (Oracle Corporation). Each participating centre assigns a data abstractor responsible for data collection. The data abstractor collects the clinical information from medical records and enter it into the online data reporting system before middle of the month after discharge of the patient.
Quality control of data collection
Similar to the CCC-Acute Coronary Syndrome (ACS) programme,18 four approaches are adopted to secure the accuracy and completeness of data in the CCC-AF programme. (1) Face-to-face training workshops are conducted prior to the data entry, with interpretations for each data item. (2) The standardised online reporting tool checks the data automatically for invalid values and sends the system-generated error messages to data abstractor for validation. (3) Onsite quality control from the third party is performed to ensure the data accuracy. Recruited cases are compared with the inpatient list to make sure that cases are reported in a consecutive manner, and 5% of the records are chosen at random for comparison with the medical charts. (4) Data completeness is calculated as number of data elements filled in the database divided by number of data elements that should be filled. Each month, data completeness is inspected and sent to participating sites as feedback in the monthly reports. For hospitals with data completeness <90%, local investigators are contacted to improve quality of data.
Performance measures
Primary and secondary performance measures are designed to evaluate the quality of care for patients hospitalised with AF in the CCC-AF programme. They are constructed referring to the ACC/AHA statements on AF performance measures19–21 integrating recommendations from the most updated ACC/AHA guideline and Chinese statement for AF.7 22 The six primary performance measures are assessment of thromboembolic risk, anticoagulant drug at discharge, prothrombin time (PT)/international normalised ratio (INR) planned follow-up, ACE inhibitor (ACEI)/angiotensin receptor blocker (ARB), statins and beta-blockers at discharge in AF inpatients with indications (table 1). The full measure specifications are shown in online supplemental table 5. Eight secondary performance measures are shown in table 2 with their full measure specifications shown in online supplemental table 6. For each performance measure, specialised inclusion and exclusion criteria are used and only appropriate tolerable patients with no contraindications are counted as denominators.
Table 1.
Primary performance measures for the Improving Care for Cardiovascular Disease in China-atrial fibrillation project
| Reference | Title of performance measure | Proportion, % (numerator/denominator) |
| 7, 22 | Proportion of patients with non-valvular AF in whom assessment of thromboembolic risk | 23.6 (5384/22 864) |
| 7, 22 | Proportion of AF patients with indication prescribed an anticoagulant drug at discharge* | 42.3 (6413/15 150) |
| 7, 22 | Proportion of patients discharged on warfarin who have PT/INR follow-up planned at discharge | 87.2 (7721/8857) |
| 7, 22 | Proportion of AF patients with indications receiving ACEI/ARB at discharge† | 53.1 (1794/3382) |
| 7, 22 | Proportion of AF patients with indication prescribed a beta-blocker at discharge‡ | 57.0 (1245/2184) |
| 7 | Proportion of AF patients with indication prescribed a statin at discharge§ | 61.2 (8524/13 925) |
| – | Composite scores of primary performance measures | 46.8 (31 081/66 362) |
*Indications refer to non-valvular AF patients with CHA2DS2-VASc≥2.
†Indications refer to AF patients with acute myocardial infarction; or coronary heart disease with comorbidity of hypertension, diabetes mellitus or chronic kidney disease; or left ventricular ejection fraction <40% according to the case records.
‡Indications refer to AF patients with heart failure.
§Indications refer to AF patients with coronary heart disease, ischaemic stroke/transient ischaemic attack, peripheral vascular disease or diabetes mellitus.
ACEI, ACE inhibitor; ARB, angiotensin receptor blocker.; INR, international normalised ratio; PT, prothrombin time.
Table 2.
Secondary performance measures for the Improving Care for Cardiovascular Disease in China-atrial fibrillation project
| Reference | Title of performance measure | Proportion, % (numerator/denominator) |
| 22 | Proportion of non-valvular AF patients who had a CHADS2 score reported | 15.0 (3422/22 864) |
| 7, 22 | Proportion of non-valvular AF patients who had a CHA2DS2-VASc score reported | 19.2 (4397/22 864) |
| 7, 22 | Proportion of AF patients who have a documented resting heart rate of <80 bpm closest to discharge | 65.0 (7140/10 989) |
| 7, 22 | Proportion of AF patients receiving anticoagulation therapy education | 89.4 (10 941/12 243) |
| 7, 22 | Proportion of AF patients receiving conventional medical education | 89.3 (25 547/28 615) |
| 20 | Proportion of AF patients with indication prescribed aldosterone antagonist at discharge* | 72.2 (888/1230) |
| 7, 22 | Proportion valvular AF patients prescribed warfarin at discharge | 52.4 (2010/3838) |
| 20 | Proportion of AF patients who are given smoking cessation advice or counselling | 22.5 (1263/5623) |
| Composite scores of secondary performance measures | 51.4 (55 608/108 266) |
*Indications refer to acute myocardial infarction patients with left ventricular ejection fraction <40% or heart failure or diabetes mellitus; or the heart failure patients with left ventricular ejection fraction <35%.
The hospital composite scores of primary performance measure are constructed using an opportunity-based method. These scores are defined as the total number of treatments correctly given divided by the sum of eligible opportunity of treatments among the six primary performance measures.23 Patients at a CCC-AF hospital contribute eligible opportunities for care to the composite performance scores of this hospital. In the same way, the composite score for secondary performance measures is constructed using the eight secondary performance measures.
The CCC team will update the performance measures and keep them aligned with the new or updated clinical guidelines for management of AF when necessary. After the new or updated AF guidelines are released, clinical experts will evaluate whether applying these changes in guidelines to CCC-AF performance measures and data elements associated with them in the case report form is necessary. When changes are adopted in the performance measures, hospitals have a transition period that allows them to have flexibility in meeting recognition criteria for the update.
Quality improvement tools
Several quality improvement tools are designed to promote the adherence to AF guidelines including monthly hospital quality report, annual hospital recognition, training session, as well as online educational materials.
Monthly hospital quality report
Each month, hospitals receive site-specific feedback reports on quality of care for AF through the CCC website. The content of hospital quality report includes individual and composite scores for primary and secondary performance measures, as well as data completeness of the reported AF cases. External and internal benchmarks are provided and compared with the hospital-specific data in the report. External benchmarks are designed to present rational nation level of performance thresholds and help to find out the areas for further enhancement of performance. The monthly hospital quality report provides two external benchmarks: the nation-level benchmark and the attainable benchmark of performance describing the composite performance of care delivered by the 15% hospitals with top performing. Internal benchmark presents the time trend of performance measures using hospital specific data. These benchmarking quality reports help hospitals to identify areas for improvement and refine treatment processes to ensure they are in line with the guidelines. Frequencies of website visit and download are recorded to assess involvement of these participating hospitals.
Regional workshop
Regional workshops in line with CSC meetings occur once or twice each year to outline the project update, exchange experience, consult the obstacles confronted and share the most recent advances in therapies of AF. These face-to-face meetings serve as venues where the knowledge are shared, with discussion forums enabling delegates to identify personal and local actions needed to improve clinical practice for AF. Recourses for education of AF healthcare professionals, including handout, bulletin, pocket guideline and booklet, are delivered to attendees.
Hospital recognition
Participating hospitals with best practice are awarded during the annual scientific sessions of CSC. Six types of awards are issued each year, including gold, silver and bronze prizes for AF performance measures, and recognitions for active participation, progress and data quality. Recognition criteria are the same as those in the CCC-ACS programme.18 Hospitals with best practice share their processes behind the achievement of good clinical practice for AF.
Online educational materials
The CCC website provides a variety of online educational materials in the education source centre for healthcare professionals in the participating hospitals to view and download. These web-based training materials include updated clinical guidelines and scientific statements for AF and webinars. Webinars are specially designed by clinical experts, focusing on the areas with gaps between clinical practice and guideline recommendations identified in the programme.
Data management
Data reported by hospitals are reserved at the central office in solidly protected computer systems. Data managers perform regular data cleaning inspecting for the potential illogical and invalid values. Invalid values are defined as outliers in numeric variables and unexpected values in character variables. Once the data manager detect the illogical and invalid values, they will review the related observations and trace to solve the potential errors.
Statistical considerations
Recruitment of 1500 patients (10 in each of the 150 hospitals) with AF per month will detect an improvement in the primary composite score from 45% at baseline to an expected score of 51%, with 91% power in a two-sided test with a significant level of 0.05. The projected 6% improvement (from 45% to 51%) in primary composite score means that >6% guideline recommended treatments will be correctly given for patients with AF. Researchers from SMG, project management group and participating hospitals have the access to analyse the data in a de-identified way, in both hospital level and patient level. Categorical variables are presented as frequencies and percentages and continuous variables are as means and SD or medians and IQRs. Missing data are managed based on the purpose of the study and analysis method used. The χ2 trend test is performed to evaluate the temporal trend of performance measures. Univariate and multivariable analysis are performed to recognise the element related with outcomes concerned, for example, the implement of specific treatments and in-hospital events. To address intrahospital correlation, generalised estimating equation models will be applied. All statistical analyses are performed by SAS V.9.2 (SAS Institute). Two-sided p values <0.05 are considered statistically significant.
Progress to date
A total of 150 tertiary hospitals were recruited into the programme in two phases, with 96 (64%) sites in municipalities or provincial capitals, and 54 (36%) in regional cities. The locations of participating centres are displayed in figure 1. A total of 28 801 AF inpatients were recruited from February 2015 to March 2017. The demographic and clinical information of the overall population is presented in table 3. The mean age of the population was 68.6 years and 15 738 (54.6%) were men. These patients had high prevalence of the concomitant diseases, including hypertension (65.4%), coronary artery disease (31.4%), heart failure (20.1%) and diabetes mellitus (17.9%).
Figure 1.
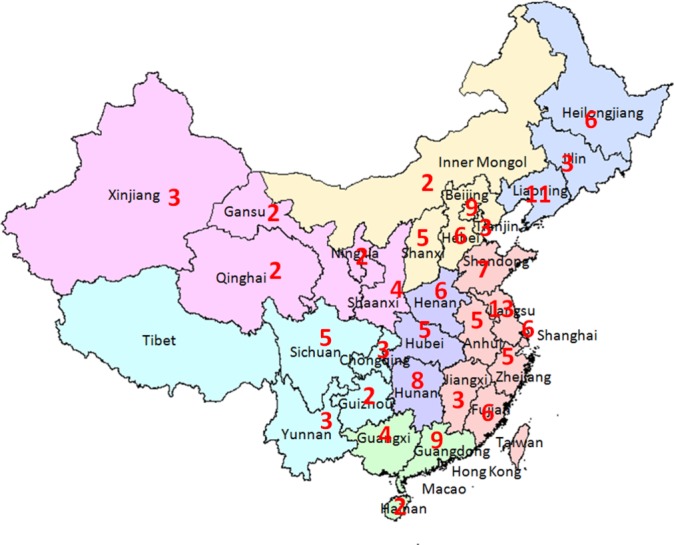
Distribution of hospitals for the Improving Care for Cardiovascular Disease in China-atrial fibrillation programme. Numerals on the map indicate the number of hospitals in the area. From Hao et al.18
Table 3.
Characteristics of enrolled patients with atrial fibrillation (AF) enrolled from February 2015 to March 2017
| Men (n=15 738) | Women (n=13 063) | Overall (n=28 801) | |
| Age | |||
| Mean (SD), years | 67.0 (12.7) | 70.6 (11.2) | 68.6 (12.1) |
| Age group, n (%) | |||
| <65 years | 6528 (41.5) | 3823 (29.3) | 10 351 (35.9) |
| 65–74 years | 4512 (28.7) | 4079 (31.2) | 8591 (29.8) |
| ≥75 years | 4698 (29.9) | 5161 (39.5) | 9859 (34.2) |
| Healthcare insurance, n (%) | |||
| Urban employees—basic insurance | 6823 (43.4) | 5045 (38.6) | 11 868 (41.2) |
| Urban residents—basic insurance | 2817 (17.9) | 2782 (21.3) | 5599 (19.4) |
| New rural cooperative insurance | 2696 (17.1) | 2830 (21.7) | 5526 (19.2) |
| Self-paying | 1654 (10.5) | 1186 (9.1) | 2840 (9.9) |
| Others | 1748 (11.1) | 1220 (9.3) | 2968 (10.3) |
| Medical history, n (%) | |||
| Hypertension | 9973 (63.4) | 8874 (67.9) | 18 847 (65.4) |
| CAD | 5050 (32.1) | 3991 (30.6) | 9041 (31.4) |
| Heart failure | 2994 (19.0) | 2788 (21.3) | 5782 (20.1) |
| Diabetes mellitus | 2694 (17.1) | 2473 (18.9) | 5167 (17.9) |
| Stroke/TIA | 2187 (13.9) | 1843 (14.1) | 4030(14) |
| PAD | 1647 (10.5) | 915 (7.0) | 2562 (8.9) |
| Myocardial infarction | 1191 (7.6) | 551 (4.2) | 1742 (6.0) |
| Previous bleeding | 96 (0.6) | 74 (0.6) | 170 (0.6) |
| Current smoker | 5241 (33.3) | 413 (3.2) | 5654 (19.6) |
| AF type | |||
| Newly diagnosed | 1742 (11.1) | 1330 (10.2) | 3072 (10.7) |
| Paroxysmal | 6143 (39.0) | 5053 (38.7) | 11 196 (38.9) |
| Persistent | 4256 (27.0) | 3405 (26.1) | 7661 (26.6) |
| Permanent | 2447 (15.5) | 2327 (17.8) | 4774 (16.6) |
| Unknown | 1150 (7.3) | 948 (7.3) | 2098 (7.3) |
CAD, coronary artery disease; PAD, peripheral artery disease; TIA, transient ischaemic attack.
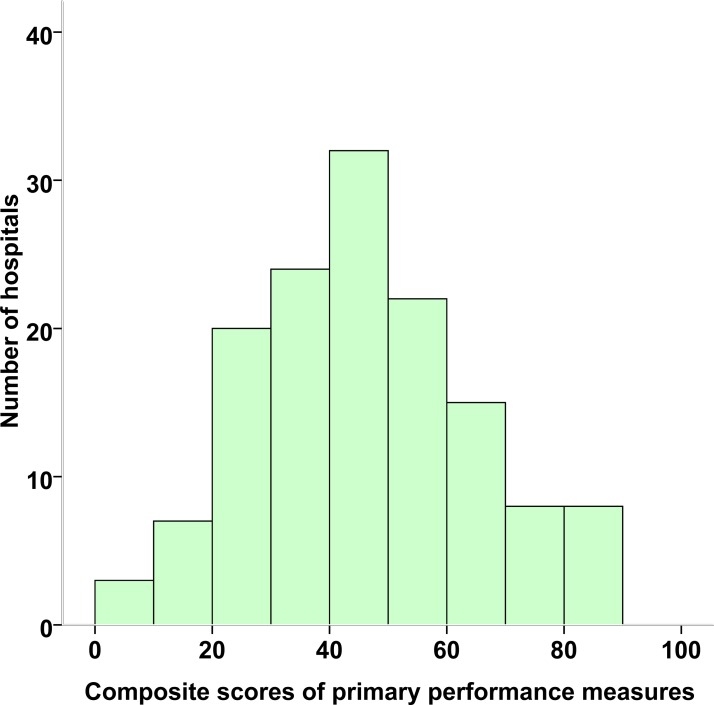
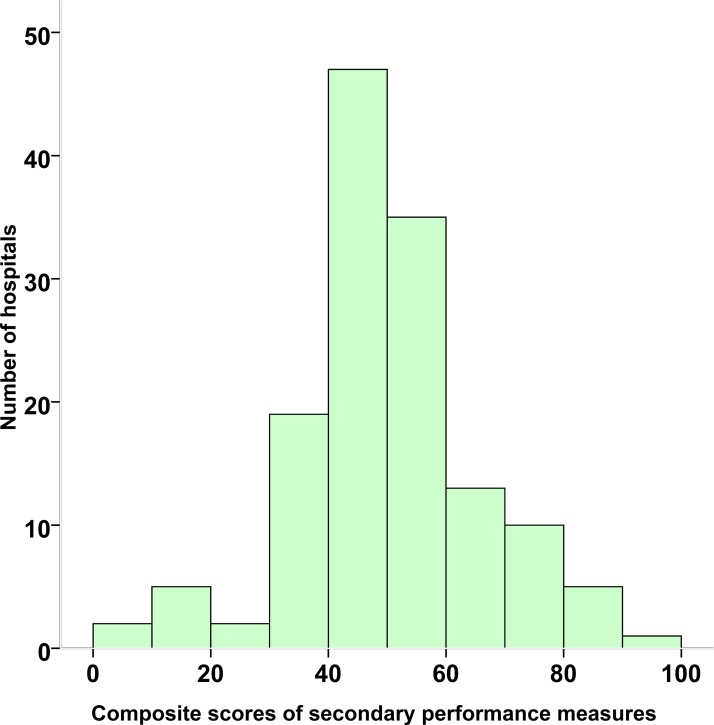
The composite score for primary performance measures was 46.8% in the patients with AF recruited (figure 2). There were remarkable variations in composite scores for AF across centres, ranging from 8.2% to 89.4%. The composite score of the AF secondary performance measures for all hospitals was 51.4%, with a wide range of 1.8%–96.2% (figure 3). Tables 1 and 2 present the adherence rates to each of the primary and secondary performance measures. Hospital-specific AF monthly quality reports between February 2015 and March 2017 are deposited on the website (http://www.ccc-heart.com).
Figure 2.
Distribution of Improving Care for Cardiovascular Disease in China-atrial fibrillation composite scores of primary performance measures across hospitals from February 2015 to March 2017. The composite scores were calculated based on the six primary performance measures, using the sum of total instances when a required measure was performed (correct care provided) divided by the total number of eligible opportunities.
Figure 3.
Distribution of Improving Care for Cardiovascular Disease in China-atrial fibrillation composite scores of secondary performance measures across hospitals from February 2015 to March 2017. The composite scores were calculated based on eight secondary performance measures, using the sum of total instances when a required measure was performed (correct care provided) divided by the total number of eligible opportunities.
Patient and public involvement
Public and patients have not been engaged in proposal of the research question, design, recruitment and implement of the study. The results will be dispersed to study participants by public reporting.
Discussion
The growing population with AF and expanded awareness of AF-related mortality, morbidity and impaired quality of life leads to the development of further therapies for AF. Currently, there are several evidence-based, highly effective, guidelines of recommended therapies that can significantly improve long-term care outcomes. The ACC, AHA and European Society of Cardiology have compiled these treatments into clinical practice guidelines.7 8 Although these guidelines are widely available, studies have suggested that the compliance with guideline-recommended treatments is low in China, even among tertiary hospitals.10 11 Reasons for low compliance to guideline-based therapies include a lack of timely feedback, poor communication and a lack of knowledge, financial resource or time.24 25
Accordingly, multiple strategies for improving adherence for clinical guideline are developed. Among them, three strategies are widely used, which are increasing reporting of the data, offering incentives to increase adherence for guideline and providing hospitals and healthcare professionals with framework and instruments which are essential for adherence improvement.26 In 2008, the National Health and Family Planning Commission of China (currently known as National Health Commission) launched a project for quality management of single diseases for heart failure, acute myocardial infarction and coronary artery bypass grafting. This project aimed to enhance the reporting of these diseases and promote improvement in quality.27 However, reporting without timely feedback and a lack of necessary infrastructure for quality improvement prevented quality management of this single diseases programme from effective improvement in care.
Previous registry studies have provided information on clinical management of patients with AF in China (table 4)10 12 13 28–31 and worldwide.31–35 Zhang et al enrolled 2016 patients with AF in 20 hospitals from 2008 to 2011.10 Among patients with non-valvular AF, only 12.7% of those with a CHADS2 score of ≥2 received treatments with oral anticoagulants, which was much lower than that in developed countries reported by international registries.32 36–39 As an ongoing registry, the Chinese Atrial Fibrillation Registry was launched in 2011 with an enrolment goal of 20 000 patients with AF in Beijing from 32 hospitals, with a follow-up of every six months up to 2020.12 Although these registries have expanded understanding for clinical practice and long-term outcomes of AF, they have been limited in a finite number of hospitals and lack of quality improvement components.
Table 4.
Characteristics of atrial fibrillation registries involving sites in China
| Studies | Countries | Sites | Population | Sample size | QI measure | Follow-up | Study period |
| Sun et al 28 | China only | 50 | Outpatient | 3017 | No | No | 2012 |
| CAFR12 | China only | 32 | Outpatient/inpatient | 11 496 | No | Up to 2020 | 2011–present |
| GLORIA-AF II31 | 42 | 736 | Outpatient | 10 871 | No | 2 year | 2011–present |
| Nanchang AF Project13 | China only | 1 | Inpatient | 2442 | No | No | 2011–2013 |
| GARFIELD-AF30 | 30 | 858 | Outpatient/inpatient | 17 184 | No | 1 year | 2010–2013 |
| Chinese AF registry10 | China only | 20 | Emergency department | 2016 | No | 1 year | 2008–2011 |
| Sun et al 29 | China only | 18 | Inpatient | 3425 | No | No | 2000–2004 |
CAFR, Chinese Atrial Fibrillation Registry Study; GARFIELD-AF, Global Anticoagulant Registry in the FIELD-Atrial Fibrillation; GLORIA-AF, Global Registry on Long-term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation; QI, quality improvement.
In 2014, the AHA and CSC launched the CCC-AF programme, aiming to promote the quality of care for AF using timely feedback and quality improvement tools. The CCC-AF programme is similar to the CCC-ACS programme.18 This programme will contribute essential nationwide information regarding characteristic, management and in-hospital outcomes of AF inpatients. It supports hospitals for improvement in use of guideline-recommended treatments with providing hospital-specific monthly quality reports. Targeted tools, including regional workshops and webinars, can further help to narrow the gaps identified between clinical practice and guideline recommendations. As an ongoing registry, the CCC-AF programme has the potential to track the expansion of new evidence-based therapies and highlight the fields calling for additional quality improvement efforts.
The CCC-AF programme aims to improve implement of guideline-recommended therapies for AF with multiple quality improvement tools. As shown in the improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF) study, educational intervention with integrative education increased the percentage of patients receiving oral anticoagulant treatments and can potentially improve stroke prevention for patients with AF.40 Currently, guideline-based therapies for AF mainly focus on antithrombotic management, rate and rhythm control, and therapy of concomitant cardiac diseases. For antithrombotic management, risk stratification is an essential step for recognising candidate patients for therapy and deciding which patients have sufficient risk to warrant oral anticoagulation. Quality improvement tools of the CCC-AF programme are designed to prompt assessment of thromboembolic risk based on easily used risk stratification scores, as well as the use of anticoagulant drugs at discharge, PT/INR planned follow-up, and therapy of concomitant cardiac diseases using ACEIs/ARBs, beta-blockers and statins at discharge in patients with AF with indications. Because patients with AF are usually admitted with concomitant cardiovascular diseases, careful consideration of co-prescription of anticoagulant and antiplatelet therapies is warranted, balancing the risk of stroke and bleeding. The CCC-AF programme will provide important insight for patterns of current clinical practice for patients with AF and concomitant cardiovascular diseases (eg, ACS) and procedures (eg, percutaneous coronary intervention).
The CCC-AF programme has several strengths, including involvement of international research team and nationwide network of tertiary hospitals. It helps to translate the research findings into action of quality improvement for care of AF. In China, tertiary hospitals deliver top-level healthcare and affect clinical practice in primary and secondary healthcare facilities. Quality improvement in these tertiary hospitals will lead to spreading the experience to healthcare facilities of other levels and promote the diffusion of guideline-recommended therapies. Moreover, data quality of our study is ensured by multiple strategies including training, standardised data collection platform, onsite quality control and monitoring of data completeness.
There are several limitations of CCC-AF programme that should be mentioned. Participation is voluntary and only tertiary hospitals are enrolled in this study. These centres tend to be bigger hospitals possessing more resources, which may overestimate the care quality. While participating centres are trained to report the eligible patients with AF consecutively, selection bias may exist as it is challenging to identify all patients with AF in an accurate and uniform manner. As clinical information is abstracted from inpatient records, quality of documentation has potential impact on the current study. Moreover, CCC-AF programme only collects information during hospitalisation, and future work tracking patients after discharge will promote further researches regarding effectiveness of specific treatments on long-term outcomes. Also, results presented here are preliminary and should be interpreted with caution.
As a nationwide quality improvement programme, CCC-AF aims to provide an opportunity to improve implement of guideline-recommended therapies in managing AF. This programme has diversified tools for quality improvement which could improve patients’ outcomes.
Ethics and dissemination
This study has been approved by the by the Ethics Committee of Beijing Anzhen Hospital, with the waiver of patient consent. In total, 111 hospitals accepted central ethics approval and the other 39 sites applied institutional review board approval from their own ethics committees. The protocol is listed on http://www.clinicaltrials.gov (NCT02309398). Results will be published in peer-reviewed medical journals.
Supplementary Material
Acknowledgments
The authors thank all the researchers from 150 participating centres.
Footnotes
Contributors: The manuscript was prepared on behalf of the CCC-AF Investigators. SCS, YH, GCF, JG, KAT, CM and DZ conceived the study idea. JL, JL, LM, YG and MZ made substantial contributions to the development of the study protocol. YH drafted the manuscript, and all authors contributed to critical revisions of the paper. This final manuscript was read and approved by all authors.
Funding: The CCC project is a collaborative programme of the AHA and the CSC. The AHA was funded by Pfizer for the quality improvement initiative through an independent grant for learning and change.
Disclaimer: Pfizer provided no oversight on the goals, execution or publication of the programme. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The Ethics Committee of Beijing Anzhen Hospital, Capital Medical University.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–8. 10.1161/01.STR.22.8.983 [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–52. 10.1161/01.CIR.98.10.946 [DOI] [PubMed] [Google Scholar]
- 3. Hu D, Sun Y. Epidemiology, risk factors for stroke, and management of atrial fibrillation in China. J Am Coll Cardiol 2008;52:865–8. 10.1016/j.jacc.2008.05.042 [DOI] [PubMed] [Google Scholar]
- 4. Zhou ZQ, Hu DY, Chen J, et al. [An epidemiological survey of atrial fibrillation in China]. Zhonghua Nei Ke Za Zhi 2004;43:491–4. [PubMed] [Google Scholar]
- 5. Li Y, Wu YF, Chen KP, et al. Prevalence of atrial fibrillation in China and its risk factors. Biomed Environ Sci 2013;26:709–16. 10.3967/0895-3988.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 6. Guo Y, Tian Y, Wang H, et al. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest 2015;147:109–19. 10.1378/chest.14-0321 [DOI] [PubMed] [Google Scholar]
- 7. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guidelineguideline for the managementmanagement of patientspatients withwith atrialatrial fibrillationfibrillation: executiveexecutive summarysummary: aa reportreport of the american college of cardiology/american heart association task force on practicepractice guidelinesguidelines and the heart rhythm society. Circulation 2014;130:2071–104. 10.1161/CIR.0000000000000040 [DOI] [PubMed] [Google Scholar]
- 8. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360–420. 10.1093/europace/euq350 [DOI] [PubMed] [Google Scholar]
- 9. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 10. Zhang H, Yang Y, Zhu J, et al. Baseline characteristics and management of patients with atrial fibrillation/flutter in the emergency department: results of a prospective, multicentre registry in China. Intern Med J 2014;44:742–8. 10.1111/imj.12487 [DOI] [PubMed] [Google Scholar]
- 11. Zhang S. Atrial fibrillation in mainland China: epidemiology and current management. Heart 2009;95:1052–5. 10.1136/hrt.2008.146589 [DOI] [PubMed] [Google Scholar]
- 12. Chang SS, Dong JZ, Ma CS, et al. Current status and time trends of oral anticoagulation use among Chinese patients with nonvalvular atrial fibrillation: the chinese atrial fibrillation registry study. Stroke 2016;47:1803–10. 10.1161/STROKEAHA.116.012988 [DOI] [PubMed] [Google Scholar]
- 13. Xiong Q, Shantsila A, Lane DA, et al. Sex differences in clinical characteristics and inpatient outcomes among 2442 hospitalized Chinese patients with nonvalvular atrial fibrillation: The Nanchang Atrial Fibrillation Project. Int J Cardiol 2015;201:195–9. 10.1016/j.ijcard.2015.08.076 [DOI] [PubMed] [Google Scholar]
- 14. Ellrodt AG, Fonarow GC, Schwamm LH, et al. Synthesizing lessons learned from get with the guidelines: the value of disease-based registries in improving quality and outcomes. Circulation 2013;128:2447–60. 10.1161/01.cir.0000435779.48007.5c [DOI] [PubMed] [Google Scholar]
- 15. National Bureau of Statistics of China. China Statistical Year book 2013. Beijing, China: China Statistics Press, 2013. [Google Scholar]
- 16. McNamara RL, Brass LM, Drozda JP, et al. American College of Cardiology American Heart Association. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Commitee to Develop Data Standards on Atrial Fibrillation). J Am Coll Cardiol 2004;44:475–95. 10.1016/j.jacc.2004.06.041 [DOI] [PubMed] [Google Scholar]
- 17. Buxton AE, Calkins H, Callans DJ, et al. ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology). J Am Coll Cardiol 2006;48:2360–96. 10.1016/j.jacc.2006.09.020 [DOI] [PubMed] [Google Scholar]
- 18. Hao Y, Liu J, Liu J, et al. Rationale and design of the Improving Care for Cardiovascular Disease in China (CCC) project: A national effort to prompt quality enhancement for acute coronary syndrome. Am Heart J 2016;179:107–15. 10.1016/j.ahj.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 19. Spertus JA, Eagle KA, Krumholz HM, et al. American College of Cardiology and American Heart Association methodology for the selection and creation of performance measures for quantifying the quality of cardiovascular care. Circulation 2005;111:1703–12. 10.1161/01.CIR.0000157096.95223.D7 [DOI] [PubMed] [Google Scholar]
- 20. Estes NA, Halperin JL, Calkins H, et al. ACC/AHA/Physician Consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and the Physician Consortium for Performance Improvement (Writing Committee to Develop Clinical Performance Measures for Atrial Fibrillation): developed in collaboration with the Heart Rhythm Society. Circulation 2008;117:1101–20. 3rd 10.1161/CIRCULATIONAHA.107.187192 [DOI] [PubMed] [Google Scholar]
- 21. Bonow RO, Douglas PS, Buxton AE, et al. ACCF/AHA methodology for the development of quality measures for cardiovascular technology: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures. Circulation 2011;124:1483–502. 10.1161/CIR.0b013e31822935fc [DOI] [PubMed] [Google Scholar]
- 22. Huang C, Zhang S, Huang D, et al. Current knowledge and management recommendations of atrial fibrillation: 2015. Chinese Journal of Cardiac Arrhythmias 2015;5:321–85. [Google Scholar]
- 23. Eapen ZJ, Fonarow GC, Dai D, et al. Comparison of composite measure methodologies for rewarding quality of care: an analysis from the American Heart Association’s Get With The Guidelines program. Circ Cardiovasc Qual Outcomes 2011;4:610–8. 10.1161/CIRCOUTCOMES.111.961391 [DOI] [PubMed] [Google Scholar]
- 24. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282:1458–65. [DOI] [PubMed] [Google Scholar]
- 25. Berthiaume JT, Tyler PA, Ng-Osorio J, et al. Aligning financial incentives with "Get With The Guidelines" to improve cardiovascular care. Am J Manag Care 2004;10:501–4. [PubMed] [Google Scholar]
- 26. Lewis WR, Peterson ED, Cannon CP, et al. An organized approach to improvement in guideline adherence for acute myocardial infarction: results with the Get With The Guidelines quality improvement program. Arch Intern Med 2008;168:1813–9. 10.1001/archinte.168.16.1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chinese Hospital Association. Manual of single disease quality management. Beijing, China: Scientific and technical documentation press, 2008. [Google Scholar]
- 28. Sun Y, Wang Y, Jiang J, et al. Renal Dysfunction, CHADS2 Score, and adherence to the anticoagulant treatment in nonvalvular atrial fibrillati. Clin Appl Thromb Hemost 2017;23:248–54. 10.1177/1076029615611250 [DOI] [PubMed] [Google Scholar]
- 29. Sun Y, Hu D, Li K, et al. Predictors of stroke risk in native Chinese with nonrheumatic atrial fibrillation: retrospective investigation of hospitalized patients. Clin Cardiol 2009;32:76–81. 10.1002/clc.20232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lip GY, Rushton-Smith SK, Goldhaber SZ, et al. GARFIELD-AF Investigators. Does sex affect anticoagulant use for stroke prevention in nonvalvular atrial fibrillation? The prospective global anticoagulant registry in the FIELD-Atrial Fibrillation. Circ Cardiovasc Qual Outcomes 2015;8:S12–S20. 10.1161/CIRCOUTCOMES.114.001556 [DOI] [PubMed] [Google Scholar]
- 31. Huisman MV, Rothman KJ, Paquette M, et al. Antithrombotic Treatment Patterns in Patients with Newly Diagnosed Nonvalvular Atrial Fibrillation: The GLORIA-AF Registry, Phase II. Am J Med 2015;128:e1301:1306–13. 10.1016/j.amjmed.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 32. Lip GY, Laroche C, Ioachim PM, et al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry). Eur Heart J 2014;35:3365–76. 10.1093/eurheartj/ehu374 [DOI] [PubMed] [Google Scholar]
- 33. Lewis WR, Piccini JP, Turakhia MP, et al. Get With The Guidelines AFIB: novel quality improvement registry for hospitalized patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2014;7:770–7. 10.1161/CIRCOUTCOMES.114.001263 [DOI] [PubMed] [Google Scholar]
- 34. Kakkar AK, Mueller I, Bassand JP, et al. International longitudinal registry of patients with atrial fibrillation at risk of stroke: Global Anticoagulant Registry in the FIELD (GARFIELD). Am Heart J 2012;163:e11:13–19. 10.1016/j.ahj.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 35. Piccini JP, Fraulo ES, Ansell JE, et al. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT-AF. Am Heart J 2011;162:e601:606–12. 10.1016/j.ahj.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 36. Lamberts M, Gislason GH, Olesen JB, et al. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol 2013;62:981–9. 10.1016/j.jacc.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 37. Piccini JP, Hernandez AF, Zhao X, et al. Quality of care for atrial fibrillation among patients hospitalized for heart failure. J Am Coll Cardiol 2009;54:1280–9. 10.1016/j.jacc.2009.04.091 [DOI] [PubMed] [Google Scholar]
- 38. Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation 2013;128:721–8. 10.1161/CIRCULATIONAHA.113.002927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wieloch M, Själander A, Frykman V, et al. Anticoagulation control in Sweden: reports of time in therapeutic range, major bleeding, and thrombo-embolic complications from the national quality registry AuriculA. Eur Heart J 2011;32:2282–9. 10.1093/eurheartj/ehr134 [DOI] [PubMed] [Google Scholar]
- 40. Vinereanu D, Lopes RD, Bahit MC, et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet 2017;390:1737–46. 10.1016/S0140-6736(17)32165-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-020968supp001.pdf (1.1MB, pdf)