Abstract
Objectives
To determine the effect of heading a soccer ball on serum neurofilament light (NF-L) protein, plasma tau protein and symptom metrics including total number of symptoms reported and symptom severity scores on the Standardized Concussion Assessment Tool— 3rd edition (SCAT3).
Methods
Eleven male collegiate soccer players were recruited to take part in three experimental conditions including heading, sham and control conditions. Participants were required to perform 40 headers in 20 min in the heading condition, and control 40 soccer balls directed at them with their hands, chest or thigh in the sham condition. No ball contact was made during the control condition. Blood sampling and SCAT3 symptom assessments were completed prior to and 1 hour following conditions. A subset of participants returned 3 weeks following the heading condition for blood sampling.
Results
NF-L was elevated at 1 hour (p=0.004) and 1 month (p=0.04) following the heading condition, and at 1 hour (p=0.02) following the control condition. Tau levels remained unchanged following all conditions. The total number of symptoms (TS) and symptom severity (SS) scores from the SCAT3 were both elevated following the heading condition (p=0.01 and p=0.03, respectively). Both TS and SS decreased following sham (p=0.04 and p=0.04) and control conditions (p=0.04 and p=0.04).
Conclusion
An acute bout of soccer heading is associated with increased NF-L concentrations at 1 hour and 1 month following the session and can lead to symptoms commonly reported following sport-related concussion.
Keywords: protein, soccer, prospective, football, head
What are the main findings?
Heading in soccer can lead to biochemical signs of axonal damage in serum.
Repetitive subconcussive impacts in the form of soccer headers can lead to symptoms associated with sport-related concussion.
Longitudinal studies should be conducted to explore biochemical changes in conjunction with imaging abnormalities.
Introduction
Soccer, the most popular sport in the world with over 270 million active participants,1 is unique in that players are allowed to make contact with the ball using their head. The number of headers performed vary based on position and typically range from 6 to 12 per game with ball speeds of 80 km/h or greater.2 There is concern surrounding the potential negative, long-term consequences of subconcussive, repetitive head impacts sustained in contact sport, including headers in soccer; for review, see Blennow et al.3 Several retrospective cohort and case study reports describe a variety of neurodegenerative diseases in the brains of retired soccer players including chronic traumatic encephalopathy,4 5 amyotrophic lateral sclerosis6 and Alzheimer’s disease.7 Researchers have proposed a link between repetitive subconcussive head trauma and the development of neurodegenerative disease; however, with respect to heading in soccer, the link is not yet well established. To date, there is limited evidence of neuronal damage in active soccer players measured in vivo under controlled circumstances.
Decreased fractional anisotropy from diffusion tensor MRI, thought to reflect white matter structural disruption, has been shown to relate to the number of headers performed in a year;8 however, when expanded over the length of a career no differences on MRI were seen between soccer players and control (ie, track and field) athletes.9 Similarly, conflicting results have been found for blood-based biomarkers of brain trauma. In an observational study, serum S100B concentration was shown to be elevated following a competitive match in elite female soccer players compared with pre-game levels and correlated with the number of headers performed in the game;10 however, no difference was found in serum S100B concentration in participants who performed up to 20 headers when compared with control participants who did not participate in a heading intervention.11 Several studies have shown an increase in serum S100B following physical exertion in the absence of head trauma in sports including basketball,12 cycling,13 ice hockey,12 and running,13 which might explain why soccer players have higher S100B levels after a competitive soccer match,14 while under controlled conditions, S100B levels do not change.11
To date, no fluid biomarker has shown promise at detecting neuronal injury as a result of repetitive head impacts in the form of soccer headers. Tau, a microtubule-associated protein primarily found in thin, unmyelinated axons of the central nervous system,15 and neurofilament light (NF-L), highly expressed in large, myelinated axons,3 are promising biomarkers for the detection of sport-related concussion (SRC) in athletes with persistent symptoms.16 17 Further, serum NF-L is significantly elevated in patients with poor clinical outcome following severe traumatic brain injury18 and is ~10-fold higher than individuals who suffer a SRC,19 suggesting a dose-dependent response to central nervous system (CNS) trauma. Plasma tau is elevated in boxers within 6 days of a bout compared with controls,20 and serum NF-L has also shown usey in detecting neuronal trauma from repetitive, subconcussive head impacts in American soccer players over the course of a season.21 Taken together, it is clear plasma tau and serum NF-L are promising candidate biomarkers for CNS damage in a variety of settings. Therefore, we examined the effects on CNS structural integrity by examining both proteins in blood in response to an acute bout of soccer heading.
Materials and methods
Study population and blood sample processing
This prospective controlled cohort study enrolled 11 male soccer players, mean (SD) age 23.7 (3.9) years, with at least 5 years of experience playing at a highly competitive level, and included three separate test conditions, completed in a pseudorandom order across participants, spaced at least 1 week apart. The three conditions were (1) heading, (2) sham and (3) control. For the heading condition, participants stood 25 m from a JUGS machine (JUGS International, Tualatin, Oregon, USA) and performed 40 approved headers in 20 min, with 30 s separating each trial. The soccer used was a FIFA regulation size 5 ball inflated to 13.0 psi and was propelled from the JUGS machine. Launch velocity for the study was determined by measuring launch velocity of corner kicks in a small pilot study through the use of an Adidas Smart Ball (Adidas America, Portland, Oregon, USA) and mimics the velocity from corner kicks in collegiate and professional soccer matches. The launch velocity for the heading condition was 77.4 (2.7) km/h (mean (SD)). Participants were asked to return at 22 days following the heading condition for a subsequent blood draw. The sham condition was identical to the heading condition except instead of using their head, participants made contact with the soccer balls directed at them with either the hands, chest or thigh. Launch velocity for the sham condition was 80.6 (3.1) km/h (mean (SD)). No soccer balls were launched during the control condition; participants were taken to the testing area, waited 20 min and then returned to the laboratory for post-condition data collection.
Blood sampling and quantification procedures
Blood samples were collected by standard venipuncture through a vein in the antecubital fossa into one K2EDTA tube for plasma and one tube for serum immediately before and 1 hour following each testing condition, and again at 3 weeks following the heading condition. Plasma samples were kept on ice until centrifugation, which occurred within 60 min of collection. Serum samples collected in the laboratory at the University of British Columbia were kept at room temperature for at least 30 min to allow for coagulation.
Quantification of plasma tau and serum NF-L occurred on the single molecule array (Simoa) HD-1 analyser (Quanterix, Lexington, Massachusetts, USA), as previously described.22 The Simoa HD-1 analyser platform isolates individual capture beads in arrays of femtolitre-sized reaction wells, resulting in assay sensitivity 1000 times greater than conventional ELISA techniques.23 24 Lower limit of quantification values were 1.22 pg/mL and 0.58 pg/mL for tau and NF-L, respectively, using standard, on-board 4-fold sample dilutions. Samples were run in duplicate over two separate runs on the same lot of reagents with samples from the same athlete run on the same plate by board-certified technicians blinded to the experimental conditions. The coefficient of variation values for high-quality and low-quality control samples run in conjunction with study samples were <12.6%.
Symptom assessment
The Standardized Assessment of Concussion—3rd edition (SCAT3) was used to record the total number of symptoms and symptom severity scores before and after all conditions. The SCAT3 includes 22 symptoms of somatic, cognitive and neurobehavioural nature; each symptom is ranked on a Likert scale from 0 (no symptom) to 6 (severe). Total number of symptoms was determined by the total number of symptoms reported between 1 and 6 (range 0–22). Symptom severity score is assessed by adding the severity for each symptom (range 0–132).
Statistical analysis methods
Statistical analyses were conducted with SPSS V.23 (IBM) and figures were developed using GraphPad Prism V.7.0 (GraphPad Software, San Diego, California, USA). Separate paired tests were used to compare protein concentrations and SCAT3 metrics before and after each testing condition. Separate t-tests were used for comparisons between time points for normally distributed data, and the non-parametric Wilcoxon signed-rank test was used for comparisons of data that were not normally distributed. A value of p <0.05 was considered significant.
Results
Of the 11 total participants, all 11 completed the heading condition, 9 completed the sham condition and 9 completed the control condition. Of the 11 participants who completed the heading condition, 7 returned at 22 days (13–70) (median (range)) following the heading condition to provide an additional blood sample. Neurofilament light was quantifiable in all participants prior to and immediately following the heading condition and in 8 of 9 participants that completed the sham and control conditions; tau was quantifiable in 9 of the 11 participants prior to and immediately following the heading condition, 5 of the 9 participants who completed the sham condition, 8 of 9 who completed the control condition and 6 of 7 at the extended time point following the heading condition. SCAT3 metrics were recorded prior to and immediately after all conditions in all participants. All data are presented in table 1.
Table 1.
Biochemical variables and SCAT3 symptom metrics across all three experimental conditions
| Variable | Heading | P values | Control | P values | Sham | P values | |||
| Pre | Post | Pre | Post | Pre | Post | ||||
| NF-L, pg/mL | 14.5 (17.8) | 16.1 (17.7) | 0.004* | 26.9 (41.9) | 28.5 (44.4) | 0.19† | 10.2 (22.7) | 10.7 (23.2) | 0.02† |
| Tau, pg/mL | 3.2 (1.7) | 3.0 (1.3) | 0.16† | 4.0 (5.2) | 2.5 (3.3) | 0.14† | 3.5 (4.0) | 3.8 (2.6) | 0.58† |
| Total no of symptoms | 1.6 (1.9) | 7.0 (6.9) | 0.01* | 1 (5) | 0 (3) | 0.04† | 2.3 (2.2) | 0.9 (1.7) | 0.04* |
| Symptom severity | 0 (4) | 6 (14) | 0.03* | 1 (7) | 0 (3) | 0.04† | 2 (6) | 0 (2) | 0.04† |
*t-test; associated data are mean (SD).
†Wilcoxon signed-rank test; associated data are median (IQR).
NF-L, neurofilament light; SCAT3, Standardized Assessment of Concussion—3rd edition.
Heading condition
Neurofilament light levels were significantly higher at 1 hour following the heading condition (figure 1A), rising an average of 26%, t(10)=3.665, p=0.004. In addition, SCAT3 symptom metrics, including total number of symptoms, t(10)=2.979, p=0.01, and symptom severity score, t(10)=2.349, p=0.03, were significantly higher at 1 hour following the heading condition compared with pre-heading levels. Spearman’s correlation coefficients for the relation between neurofilament light levels and total number of symptoms and symptom severity were not significant. At 22 days following the heading condition, NF-L levels were, on average, elevated 311% above baseline values, significantly higher compared with pre-heading measures, t(6)=2.602, p=0.04 (figure 2). Tau levels remained unchanged at 1 hour, z=1.400, p=0.16, and 22 days, t(5)=1.548, p=0.18, following an acute bout of soccer heading compared with pre-heading values.
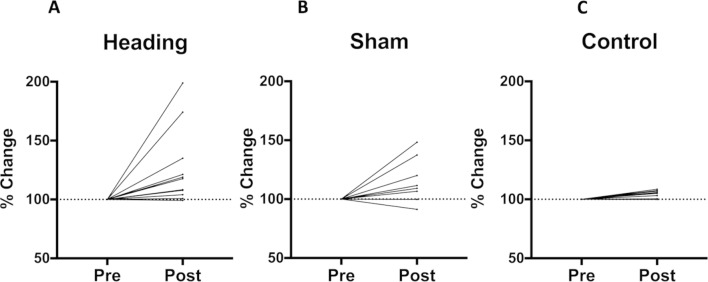
Figure 1.
Percent change in serum neurofilament light (NF-L) protein following experimental conditions. Serum NF-L was significantly elevated following the heading (p=0.004) and sham (p=0.02) conditions compared with pre-condition values. No change was seen in serum NF-L following the control condition (p>0.05).
Figure 2.
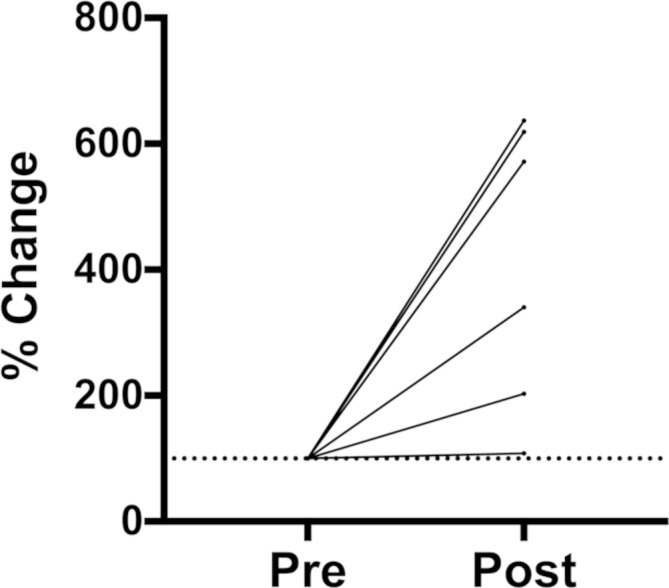
Percent change in serum neurofilament light (NF-L) 3 weeks following an acute bout of soccer heading. Serum NF-L remained elevated 3 weeks following the heading condition (p=0.04).
Sham condition
Both NF-L (figure 1B) and tau levels remained unchanged following the sham condition. Interestingly, the total number of symptoms, z=2.060, p=0.04, and symptom severity score, z=2.060, p=0.04, reported on the SCAT3 were lower following the sham condition compared with pre-condition values.
Control condition
At 1 hour following the control intervention, NF-L was significantly higher, z=2.383, p=0.02, compared with pre-intervention values (figure 1C); however, post-condition values were only 4% higher than those measured pre-condition, on average. Tau levels were unchanged from pre-condition to post-condition. Similar to the control condition, both the total number of symptoms, t(8)=2.490, p=0.04, and symptom severity scores, z=2.023, p=0.04, were lower following the control condition compared with pre-intervention values.
Discussion
The ability to accurately head the ball in soccer is a key component to the game. We believe this is the first study to measure serum NF-L, plasma tau and SCAT3 metrics immediately prior to and again at 1 hour and 22 days following an acute bout of soccer heading involving 40 headers performed at speeds mimicking those seen in professional play. Interestingly, NF-L was increased at 1 hour and 22 days following the heading condition, while tau remained unchanged. Further, an increase in NF-L was seen at 1 hour following the control condition. The total number of symptoms reported and the associated symptom severity scores both increased following an acute bout of soccer heading; however, following both the sham and control conditions, the two metrics decreased. As serum NF-L is considered a biomarker of axonal injury in the brain,25 this study suggests that repetitive, subconcussive impacts in the form of soccer headers can cause axonal damage and increase the number and severity of symptoms commonly reported post-concussion.
Although we report increased concentrations of NF-L at 1 hour and 22 days following an acute bout of soccer heading, tau remained unchanged. This could be due to the fact that increased plasma tau seems to be associated with more severe impacts. Higher levels of plasma tau are seen in those experiencing a longer return-to-play due to the presence of post-concussion symptoms and in individuals experiencing a loss of consciousness following SRC.16 26 NF-L levels were higher 1 hour and 22 days following the heading condition; this may indicate that NF-L is more sensitive to low-level impacts. Indeed, NF-L has been shown to increase over the course of an American football season,21 indicating that serum NF-L may be useful in detecting axonal trauma following repetitive subconcussive impacts.
The SCAT3 is a standardised clinical tool used to assess an athlete’s condition following a suspected SRC. Following the heading condition, both the total number of symptoms and symptom severity scores were increased compared with pre-heading values, indicating that repetitive headers in soccer travelling at 80 km/h can bring about symptoms commonly seen in those with post-concussion syndrome. This contrasts previous study findings,27 although the number of headers performed (ie, 5) was much lower than the current study (ie, 40). Intriguingly, both the total number of symptoms and symptom severity scores decreased following the sham and control conditions compared with pre-condition values. With respect to the sham condition, in which athletes guided the ball down using their chest, hands or legs, the decrease could be due to the participation, although brief, in physical activity required of the condition. Collegiate athletes have reported improved self-esteem and lower levels of depression compared with non-athletes,28 and so this may explain the decrease in SCAT3 symptom metrics.
This study is not without limitations. As this was part of a larger study, the sample size is small. This was a protracted study with three separate testing conditions lasting approximately 2.5–3 hours per condition. As such, some participants did not complete all five conditions. Although the players were not in season during the duration of the study, no restrictions could be enforced to limit exposure to head impacts in pick up soccer games or practice conditions. Symptom metrics on the SCAT3 were not recorded at the 22 days time point following the heading condition; however, as we could not limit the exposure to head impacts, and the list of symptoms included on the SCAT3 are not specific to those who experience head trauma, we believe there were too many confounding variables to accurately deduce an association with SCAT3 symptom metrics and an acute bout of soccer heading 22 days prior.
In conclusion, this study suggests that exposure to repetitive, subconcussive yet high-velocity head impacts in the form of soccer headers may result in axonal damage that can be detected using ultrasensitive immunoassay methods. Players can head the ball up to 12 times per game,2 29 and with approximately 40 games played in a professional season a player may potentially head the ball between 400 and 500 times per season, not including those performed in practice. The results from the current study suggest that heading in soccer should not be overlooked as a potential mechanism for axonal damage. Indeed, more research is needed in order to help elucidate the possibility of such a relationship.
Acknowledgments
The authors would like to thank the University of British Columbia–Okanagan men’s soccer team. The authors would also like to thank the Faculty of Health and Social Development and the members of the Sports Concussion Research Laboratory at the University of British Columbia. The authors would also like to thank the Swedish Research Council, the European Research Council, the Knut and Alice Wallenberg Foundation, the Torsten Söderberg Foundation and Swedish State Support for Clinical Research (ALFGBG).
Footnotes
Contributors: CW, JDS, KeB, JB, JD, ADW and PvD all contributed to the conception of the study and data collection. CW, HZ, KaB and PvD contributed to data analysis and interpretation, drafting and revision of the manuscript, and final approval of the version submitted for publication was approved by all authors.
Funding: This research was funded by grants to PvD from the Canadian Institutes for Health Research (CIHR), Canada Foundation for Innovation (CFI) and Mitacs. CW was supported by the Mitacs Accelerate Programme.
Competing interests: HZ and KaB are co-founders of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg. HZ has served at advisory boards for Eli Lilly and Roche Diagnostics and has received travel support from Teva. KaB has served as a consultant or at advisory boards for Alzheon, BioArctic, Biogen, Eli Lilly, Fujirebio Europe, IBL International, Merck, Novartis, Pfizer and Roche Diagnostics.
Patient consent: Obtained.
Ethics approval: All procedures in this study were approved by the Clinical Research Ethics Board at the University of British Columbia and were conducted according to the declaration of Helsinki guidelines.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data sharing statement: The data will be made available upon request.
References
- 1. Count FB. 270 million people active in soccer : FIFA Communications Division, 2006. [Google Scholar]
- 2. Spiotta AM, Bartsch AJ, Benzel EC. Heading in soccer: dangerous play? Neurosurgery 2012;70:1–11. discussion11. doi 10.1227/NEU.0b013e31823021b2 [DOI] [PubMed] [Google Scholar]
- 3. Blennow K, Brody DL, Kochanek PM, et al. . Traumatic brain injuries. Nat Rev Dis Primers 2016;2:16084 10.1038/nrdp.2016.84 [DOI] [PubMed] [Google Scholar]
- 4. Hales C, Neill S, Gearing M, et al. . Late-stage CTE pathology in a retired soccer player with dementia. Neurology 2014;83:2307–9. 10.1212/WNL.0000000000001081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grinberg LT, Anghinah R, Nascimento CF, et al. . Chronic traumatic encephalopathy presenting as Alzheimer’s disease in a retired soccer player. J Alzheimers Dis 2016;54:169–74. 10.3233/JAD-160312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiò A, Benzi G, Dossena M, et al. . Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain 2005;128(Pt 3):472–6. 10.1093/brain/awh373 [DOI] [PubMed] [Google Scholar]
- 7. Ling H, Morris HR, Neal JW, et al. . Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol 2017;133:337–52. 10.1007/s00401-017-1680-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lipton ML, Kim N, Zimmerman ME, et al. . Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology 2013;268:850–7. 10.1148/radiol.13130545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jordan SE, Green GA, Galanty HL, et al. . Acute and chronic brain injury in United States National Team soccer players. Am J Sports Med 1996;24:205–10. 10.1177/036354659602400216 [DOI] [PubMed] [Google Scholar]
- 10. Stålnacke BM, Ohlsson A, Tegner Y, et al. . Serum concentrations of two biochemical markers of brain tissue damage S-100B and neurone specific enolase are increased in elite female soccer players after a competitive game. Br J Sports Med 2006;40:313–6. 10.1136/bjsm.2005.021584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zetterberg H, Jonsson M, Rasulzada A, et al. . No neurochemical evidence for brain injury caused by heading in soccer. Br J Sports Med 2007;41:574–7. 10.1136/bjsm.2007.037143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stålnacke BM, Tegner Y, Sojka P. Playing ice hockey and basketball increases serum levels of S-100B in elite players: a pilot study. Clin J Sport Med 2003;13:292–302. 10.1097/00042752-200309000-00004 [DOI] [PubMed] [Google Scholar]
- 13. Otto M, Holthusen S, Bahn E, et al. . Boxing and running lead to a rise in serum levels of S-100B protein. Int J Sports Med 2000;21:551–5. 10.1055/s-2000-8480 [DOI] [PubMed] [Google Scholar]
- 14. Stålnacke BM, Tegner Y, Sojka P. Playing soccer increases serum concentrations of the biochemical markers of brain damage S-100B and neuron-specific enolase in elite players: a pilot study. Brain Inj 2004;18:899–909. 10.1080/02699050410001671865 [DOI] [PubMed] [Google Scholar]
- 15. Zetterberg H, Blennow K. Fluid markers of traumatic brain injury. Mol Cell Neurosci 2015;66(Pt B):99–102. 10.1016/j.mcn.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 16. Shahim P, Tegner Y, Wilson DH, et al. . Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol 2014;71:684–9. 10.1001/jamaneurol.2014.367 [DOI] [PubMed] [Google Scholar]
- 17. Shahim P, Tegner Y, Marklund N, et al. . Neurofilament light and tau as blood biomarkers for sports-related concussion. Neurology 2018;90:e1780–e1788. 10.1212/WNL.0000000000005518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shahim P, Gren M, Liman V, et al. . Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep 2016;6:36791 10.1038/srep36791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shahim P, Zetterberg H, Tegner Y, et al. . Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017;88:1788–94. 10.1212/WNL.0000000000003912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neselius S, Zetterberg H, Blennow K, et al. . Olympic boxing is associated with elevated levels of the neuronal protein tau in plasma. Brain Inj 2013;27:425–33. 10.3109/02699052.2012.750752 [DOI] [PubMed] [Google Scholar]
- 21. Oliver JM, Jones MT, Kirk KM, et al. . Serum neurofilament light in American football athletes over the course of a season. J Neurotrauma 2016;33:1784–9. 10.1089/neu.2015.4295 [DOI] [PubMed] [Google Scholar]
- 22. Gisslén M, Price RW, Andreasson U, et al. . Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 2016;3:135–40. 10.1016/j.ebiom.2015.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rissin DM, Walt DR. Digital concentration readout of single enzyme molecules using femtoliter arrays and Poisson statistics. Nano Lett 2006;6:520–3. 10.1021/nl060227d [DOI] [PubMed] [Google Scholar]
- 24. Rissin DM, Fournier DR, Piech T, et al. . Simultaneous detection of single molecules and singulated ensembles of molecules enables immunoassays with broad dynamic range. Anal Chem 2011;83:2279–85. 10.1021/ac103161b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol 2016;12:563–74. 10.1038/nrneurol.2016.127 [DOI] [PubMed] [Google Scholar]
- 26. Gill J, Merchant-Borna K, Jeromin A, et al. . Acute plasma tau relates to prolonged return to play after concussion. Neurology 2017;88:595–602. 10.1212/WNL.0000000000003587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dorminy M, Hoogeveen A, Tierney RT, et al. . Effect of soccer heading ball speed on S100B, sideline concussion assessments and head impact kinematics. Brain Inj 2015;29:1158–64. 10.3109/02699052.2015.1035324 [DOI] [PubMed] [Google Scholar]
- 28. Armstrong S, Oomen-Early J, Connectedness S. Social connectedness, self-esteem, and depression symptomatology among collegiate athletes versus nonathletes. J Am Coll Health 2009;57:521–6. 10.3200/JACH.57.5.521-526 [DOI] [PubMed] [Google Scholar]
- 29. Mehnert MJ, Agesen T, Malanga GA. "Heading" and neck injuries in soccer: a review of biomechanics and potential long-term effects. Pain Physician 2005;8:391–7. [PubMed] [Google Scholar]