Abstract
Background
At present, the predictive ability of the prognostic indicator of hepatocellular carcinoma (HCC) is still limited. This study aims to analyze the relationship between the preoperative high-sensitivity C-reactive protein to lymphocyte ratio (HCLR) and the clinicopathologic characteristics of HCC.
Patients and methods
A total of 229 HCC patients undergoing surgical resection were retrospectively analyzed. The majority of the patients (132/229) had tumors larger than 5 cm, and 45 out of 229 had more than one tumor focus. Receiver operating characteristic curve analysis was used to decide the cutoff value of HCLR. The overall survival (OS) and progression-free survival (PFS) rates were evaluated by adopting the Kaplan–Meier method.
Results
The cutoff value of HCLR for the best discrimination of HCC prognosis was 1.3 with a sensitivity of 75.5% and a specificity of 71.8%. The area under the receiver operating characteristic curve was 0.791 (95% CI, 0.731–0.840). Preoperative HCLR at a high level (>1.3) was positively correlated with large tumor size, TNM stage, microvascular invasion, and recurrence. The mean OS and PFS in patients with HCLR >1.3 were significantly shorter than in those with HCLR ≤1.3. Univariate and multivariate analyses revealed the HCLR was an independent predictor of OS and PFS.
Conclusion
HCLR was an important independent predictor of dismal prognosis in HCC patients and can be used as a sensitive indicator for the dynamic monitoring of postoperative patients.
Keywords: hepatocellular carcinoma, HCLR, marker, prognosis, survival
Video abstract
Introduction
Hepatocellular carcinoma (HCC) is widely known for its high morbidity, high malignancy, and high tendency of metastasis and recurrence, but its mechanism is still unclear, leading it to pose a great threat to public health. Sub-Saharan Africa and Asia are found to be high-prevalence areas of HCC, with China alone accounting for nearly 50% of HCC cases worldwide. A recent study showed that there were 4,661,000 new HCC cases and 4,221,000 deaths in China during 2015.1 In the last half century, studies in the field of epidemiology, etiology, and the basic diagnosis and treatment of HCC have made significant progress. The postoperative survival rate has risen to some degree as well. Nonetheless, the reality is that the prognosis of HCC is still very disappointing, and postoperative recurrence and metastasis rate at 5 years is still up to 60%–70%.2,3 At present, serum alpha-fetoprotein (AFP) remains the most widely used marker for HCC diagnosis, despite its relatively low sensitivity and specificity as well as common false-negative/-positive phenomena. A credible marker or index for early diagnosis and prognosis prediction is still absent in current clinical practice. A recent study suggested that serum AFP has little prognostic value in compensated cirrhosis patients with a single, small HCC.4 Thus, a novel and accurate marker that may improve the postoperative surveillance of early HCC in clinical practice is highly desirable.
Accumulating evidence shows that a number of major inflammatory mediators may exert significant impact on the formation of tumor microenvironment and contribute to the progression of HCC, some of which may be promising biomarkers for the early diagnosis and prognostic prediction of HCC,5–15 such as tumor necrosis factor-α, interleukin-6, intercellular adhesion molecule-1,5,6 neutrophil to lymphocyte ratio (NLR), which is a previous study of our group,7–9 C-reactive protein (CRP),10,11 high-sensitivity C-reactive protein (Hs-CRP),12–15 and systemic immune-inflammation index (SII).16 Although these indicators may have some predictive power, we found that Hs-CRP to lymphocyte ratio (HCLR) may serve as a more optimized novel indicator for the prognosis of HCC in our previous study.
This study was designed to investigate the optimal cutoff value of HCLR with the best discriminatory ability and evaluate the prognostic power of HCLR in HCC patients who underwent surgical resection.
Patients and methods
Patients and clinical data
From July 2004 to March 2009, 229 cases of patients who underwent hepatectomy at the Affiliated Hospital of Guilin Medical University (Guilin, People’s Republic of China) were included in this study. The majority of the patients (132/229) had tumors larger than 5 cm, and 45 out of 229 had more than one tumor focus. Five of the patients with recurrence (95/229) were extrahepatic. All patients were diagnosed by clinical, serological examination, ultrasonography (US), magnetic resonance imaging, computerized tomography of the thorax and abdomen, and bone scintigraphy. All were verified by pathologic examination. More than 90% were Child–Pugh class A patients. In addition, all the patients underwent curative resection, which was defined as a complete resection of the tumor with a resection margin of at least 1 cm and no new lesions determined by two observations not less than 4 weeks apart.17 Demographic characteristics, cirrhosis, hepatitis B surface antigen, tumor size, AFP, HCLR, complete blood count, albumin, globulin, total bilirubin, direct bilirubin, alanine aminotransferase, aspartate aminotransferase (AST), alkaline phosphatase, and γ-glutamyl transpeptidase (γ-GT) are listed in Table 1. All methods were performed according to the Affiliated Hospital of Guilin Medical University’s guidelines and regulations. This study conformed to the Declaration of Helsinki and has received approval from the research ethics committee of the Affiliated Hospital of Guilin Medical University. Written informed consent was obtained from all patients.
Table 1.
Clinical and biochemical data of examined patients
| Parameter | Mean ± SDa |
|---|---|
| Age (years) | 50.46±10.88 |
| Gender: female/male (n) | 27/202 |
| Alcohol abuse: yes/no (n) | 91/138 |
| Cirrhosis: yes/no (n) | 217/12 |
| HBsAg: positive/negative (n) | 191/38 |
| Tumor size (range, cm) | 7.28±3.93 |
| AFP (ng/mL): median, range | 123.4 (0.23–38,030.00) |
| WBC (×109/L) | 6.47±2.13 |
| LYMPH (×109/L) | 1.74±0.66 |
| Platelets (×109/L) | 189.75±80.21 |
| Albumin (g/L) | 37.89±4.56 |
| Globulin (g/L) | 32.35±5.99 |
| TB (μmol/L): median, range | 12.8 (4.7–159.05) |
| DB (μmol/L): median, range | 5.04 (1.83–128.99) |
| ALT (U/L) | 46.14±53.42 |
| AST (U/L) | 51.52±53.76 |
| ALP (U/L) | 99.50±70.59 |
| γ-GT (U/L): median, range | 63.4 (15.1–689.0) |
| HCLR level: median, range | 2.07 (0.01–32.8) |
Note:
Data presented as mean ± SD or others.
Abbreviations: γ-GT, γ-glutamyl transpeptidase; AFP, alpha-fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DB, direct bilirubin; HBsAg, hepatitis B surface antigen; HCLR, high-sensitivity C-reactive protein to lymphocyte ratio; LYMPH, lymphocyte count; TB, total bilirubin; WBC, white blood cell.
Surveillance after hepatic resection
For the criteria for patients’ inclusion and exclusion, we referred to our previous reports,6,9 as well as the content and requirements for the periodical follow-ups. Liver function tests, AFP levels, and US were regularly monitored every 2 months. A chest radiograph was taken every 6 months during the first two postoperative years and then every 3–6 months. All the patients had routine follow-ups. If the AFP level or US test was abnormal, a computerized tomography scan or magnetic resonance imaging was done immediately. Progression-free survival (PFS) was defined from the date of surgery to the date of recurrence, metastasis, death, or last follow-up, while the overall survival (OS) was defined from the date of surgery to the date of death or last follow-up.
Receiver operating characteristic (ROC) curve
ROC, also called the sensitivity curve, is named for the same sensitivity of each point on the curve. The points are all the reactions on the same signal stimulation and are merely different results under two criteria. The false-positive rate functions as the horizontal axis of ROC and the true-positive rate as the vertical axis. The curve is concluded from the various results obtained from different criteria of the subjects under the given stimulus.
Selection of cutoff value
The cutoff value is the judgment standard, which is the boundary value between the positive and the negative values. ROC curve analysis was performed to determine the cutoff value of HCLR. The point with the highest sensitivity and specificity was selected as the optimal cutoff value. Discriminatory ability of using the optimal cutoff value was evaluated by the sensitivity, specificity, positive/negative predictive value, and positive/negative likelihood ratio. The area under ROC curve (AUC) was also calculated to assess the overall accuracy.
Statistical analysis
Analyses for all statistics were performed using SPSS 18.0 (SPSS Inc, Chicago, IL, USA) and MedCalc 11.3.0.0 (MedCalc Software, Ostend, Belgium). Data conforming to Gaussian distribution were expressed as the mean ± SD and assessed by independent t-test. Categorical data were compared by the Pearson’s chi-squared test or the Fisher’s exact test. The Kaplan–Meier method was used to calculate OS and PFS, and survival distributions were compared with the log-rank test. Univariate analysis and multivariate Cox proportional hazards regression models were performed to determine independent prognostic factors. P<0.05 was considered significant.
Results
The optimal cutoff value of HCLR
The AUC of the HCLR was 0.791 (95% CI, 0.731–0.840) for predicting the prognosis in patients with HCC. Based on the ROC curve, the optimal cutoff value of preoperative HCLR level was 1.3, with a sensitivity of 75.5% and a specificity of 71.8% (Figure 1; Table 2). The classification performance of other AUC resulted from previously published literature, such as Hs-CRP, NLR, and SII, which were also calculated and are shown in Figure 1 and Table 2, and the AUCs were 0.760 (95% CI, 0.700–0.814), 0.678 (95% CI, 0.613–0.738), and 0.676 (95% CI, 0.611–0.736), respectively. These results suggested that HCLR is more valuable than the other three indices or parameters in predicting the prognosis of HCC.
Figure 1.
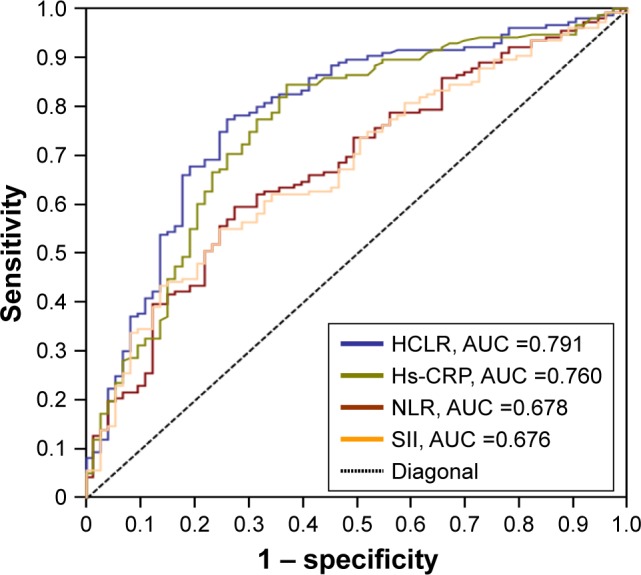
ROC curve analysis was performed to assess the prognostic value of preoperative HCLR.
Abbreviations: AUC, area under the curve; HCLR, high-sensitivity C-reactive protein to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; ROC, receiver operating characteristic; SII, systemic immune-inflammation index; Hs-CRP, high-sensitivity C-reactive protein.
Table 2.
The accuracy of different indices or parameters in predicting the prognosis of HCC patients
| Group | Cutoff value | AUC | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| HCLR | 1.3 | 0.791 | 0.755 | 0.718 | 0.844 | 0.606 |
| 95% CI | 0.731–0.840 | 0.678–0.821 | 0.609–0.807 | 0.772–0.901 | 0.501–0.706 | |
| Hs-CRP | 2.2 | 0.760 | 0.735 | 0.702 | 0.836 | 0.559 |
| 95% CI | 0.700–0.814 | 0.659–0.803 | 0.585–0.802 | 0.761–0.893 | 0.452–0.662 | |
| NLR | 2.2 | 0.678 | 0.586 | 0.736 | 0.820 | 0.448 |
| 95% CI | 0.613–0.738 | 0.502–0.667 | 0.631–0.833 | 0.735–0.887 | 0.361–0.539 | |
| SII | 416 | 0.676 | 0.556 | 0.751 | 0.818 | 0.446 |
| 95% CI | 0.611–0.736 | 0.473–0.636 | 0.640–0.845 | 0.736–0.885 | 0.359–0.537 |
Abbreviations: AUC, area under the receiver operating characteristics curve; HCC, hepatocellular carcinoma; HCLR, Hs-CRP to lymphocyte ratio; Hs-CRP, high-sensitivity C-reactive protein; NLR, neutrophil to lymphocyte ratio; NPV, negative predictive value; PPV, positive predictive value; SII, systemic immune-inflammation index.
The relationship between preoperative HCLR level and clinical characteristics
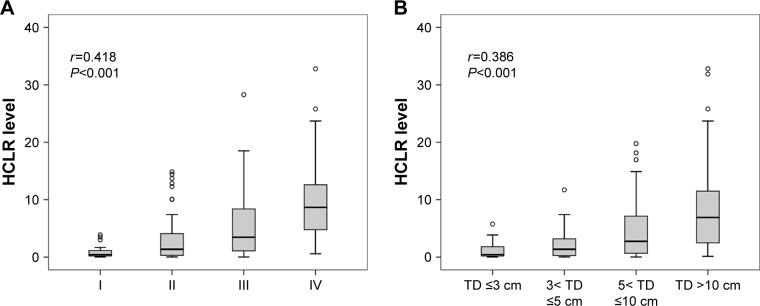
The relationships between preoperative HCLR level and clinicopathologic parameters of HCC patients were analyzed and are shown in Table 3. All cases were divided into two groups: the low HCLR level group (HCLR ≤1.3) and the high HCLR level group (HCLR >1.3). Other clinical characteristics were dichotomized as shown in Table 3. The results suggested that positive relationships existed between HCLR level and large tumor size (>5 cm) (χ2=20.85, P<0.001), TNM III–IV stage (χ2=23.69, P<0.001), microvascular invasion (χ2=14.43, P<0.001), recurrence (χ2=6.85, P=0.009), and serum AST level (χ2=6.92, P=0.009). The strong correlations of TNM stage and tumor size in relation to HCLR level can be seen in Figure 2A and B. As the TNM stage and size of tumor increased, the level of HCLR gradually increased as well (Spearman’s correlation coefficient r=0.418, P<0.001 and r=0.386, P<0.001, respectively). However, no significant relationships were found with gender, age, hepatitis B surface antigen, tumor number, drinking, and serum AFP level (all P>0.05).
Table 3.
Correlation between the clinicopathologic variables and HCLR in HCC
| Clinical character | Clinical variable | No of patients | HCLR level
|
χ2 | P-value | |
|---|---|---|---|---|---|---|
| ≤1.3, no (%) | >1.3, no (%) | |||||
| Gender | Female | 27 | 10 (37.0) | 17 (63.0) | 0.162 | 0.687 |
| Male | 202 | 83 (41.1) | 119 (58.9) | |||
| Age (years) | ≤55 | 153 | 58 (37.9) | 95 (62.1) | 1.39 | 0.237 |
| >55 | 76 | 35 (46.1) | 41 (53.9) | |||
| HBsAg | Negative | 38 | 13 (34.2) | 25 (65.8) | 0.77 | 0.379 |
| Positive | 191 | 80 (41.9) | 111 (58.1) | |||
| Tumor size (range, cm) | ≤5 | 92 | 54 (58.7) | 38 (41.3) | 20.85 | <0.001 |
| >5 | 137 | 39 (28.5) | 98 (71.5) | |||
| Tumor number | Single | 184 | 72 (39.1) | 112 (60.9) | 0.85 | 0.356 |
| Multiple | 45 | 21 (46.7) | 24 (53.3) | |||
| Drinking | Absent | 138 | 59 (42.8) | 79 (57.2) | 0.661 | 0.416 |
| Present | 91 | 34 (37.4) | 57 (62.6) | |||
| TNM stage | I–II | 118 | 66 (55.9) | 52 (44.1) | 23.69 | <0.001 |
| III–IV | 111 | 27 (24.3) | 84 (75.7) | |||
| Microvascular invasion | Absent | 181 | 85 (47.0) | 96 (53.0) | 14.43 | <0.001 |
| Present | 48 | 8 (16.7) | 40 (83.3) | |||
| Recurrence | Absent | 134 | 64 (47.8) | 70 (52.2) | 6.85 | 0.009 |
| Present | 95 | 29 (30.5) | 66 (69.5) | |||
| AFP (ng/mL) | ≤20 | 88 | 41 (46.6) | 47 (53.4) | 2.11 | 0.146 |
| >20 | 141 | 52 (36.9) | 89 (63.1) | |||
| AST (U/L) | ≤40 | 139 | 66 (47.5) | 73 (52.5) | 6.92 | 0.009 |
| >40 | 90 | 27 (30.0) | 63 (70.0) | |||
Note: Bold figures represent as statistically significant.
Abbreviations: AFP, alpha-fetoprotein; AST, aspartate aminotransferase; HBsAg, hepatitis B surface antigen; HCLR, high-sensitivity C-reactive protein to lymphocyte ratio; HCC, hepatocellular carcinoma; TNM, tumor-node-metastases.
Figure 2.
Box plots of HCLR levels according to the TNM stage (A) and TD (B).
Abbreviations: HCLR, high-sensitivity C-reactive protein to lymphocyte ratio; TD, tumor diameter; TNM, tumor, node and metastases.
Association of HCLR and AFP with postoperative OS and PFS
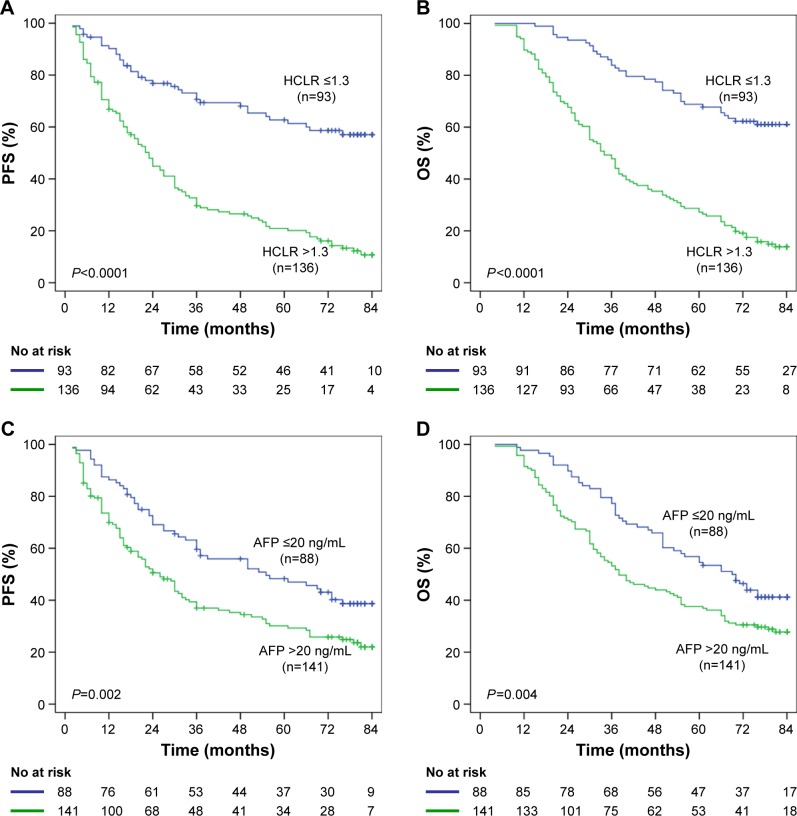
Kaplan–Meier survival analysis showed that the HCLR >1.3 group was significantly associated with poorer PFS and OS in 229 HCC patients (Figure 3A and B). Median OS was 41.58 months in the HCLR >1.3 group and 68.22 months in the HCLR ≤1.3 group (P<0.0001), while median PFS was 31.85 and 60.77 months, respectively. Compared with the AFP ≤20 ng/mL group, the AFP >20 ng/mL group was also associated with poorer PFS (P=0.002; Figure 3C) and OS (P=0.004; Figure 3D).
Figure 3.
The relationship of HCLR and AFP with PFS or OS in the overall population.
Notes: (A, B) Kaplan–Meier analysis revealed significantly shorter PFS (A) and OS (B) in HCC patients with HCLR >1.3 than in those with HCLR ≤1.3. (C, D) PFS (C) and OS (D) of HCC patients with AFP >20 ng/mL were shorter than in those with AFP ≤20 ng/mL.
Abbreviations: AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; HCLR, high-sensitivity C-reactive protein to lymphocyte ratio; OS, overall survival; PFS, progression-free survival.
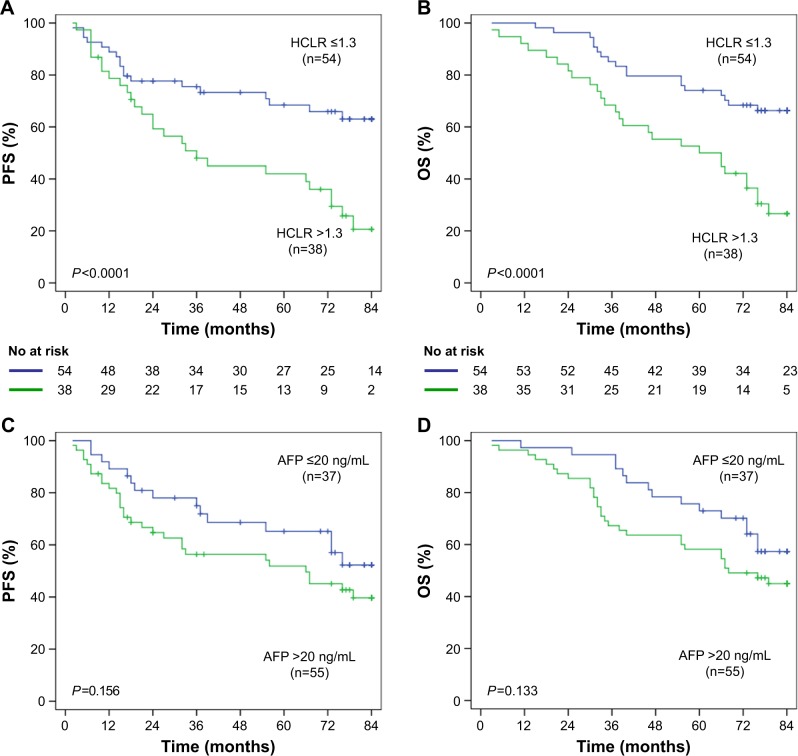
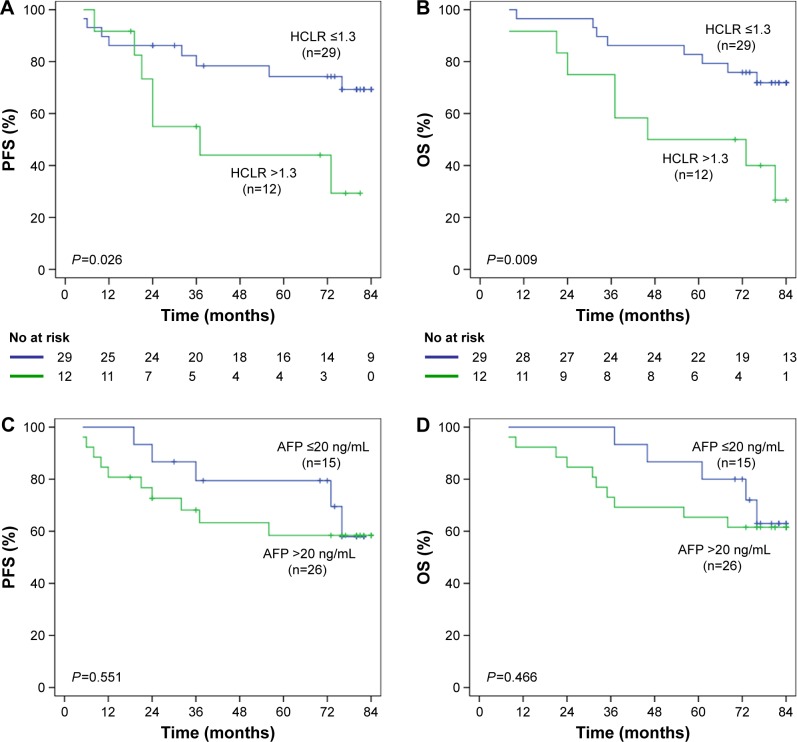
Furthermore, we analyzed the postoperative survival of HCC patients in different tumor size subgroups. In HCC patients with tumor size ≤5 cm group (n=92), mean PFS was 44.59 months in the HCLR >1.3 group and 63.53 months in the HCLR ≤1.3 group (P<0.0001, Figure 4A), while the mean OS was 54.62 months and 70.53 months (P<0.0001, Figure 4B), respectively. However, there was no significant PFS (Figure 4C) or OS (Figure 4D) difference between the subgroup of AFP ≤20 ng/mL and >20 ng/mL (all P>0.05). In HCC patients with tumor size ≤ 3 cm group (n=41), the mean PFS was 47.67 months in the HCLR >1.3 group and 68.82 months in the HCLR ≤1.3 group (P= 0.026, Figure 5A), while the mean OS was 55.81 months and 73.65 months (P=0.009, Figure 5B), respectively. Nevertheless, there was no significant PFS (Figure 5C) or OS (Figure 5D) difference between the subgroup of AFP ≤20 ng/mL and >20 ng/mL (all P>0.05) in HCC patients with tumor size ≤3 cm. These results suggest that HCLR level still has prognostic value in middle- and small-sized HCC patients.
Figure 4.
The relationship of HCLR and AFP with PFS or OS in patients with tumor size ≤5 cm.
Notes: (A, B) HCLR >1.3 significantly correlated with shorter PFS (A) and OS (B) in patients with tumor size ≤5 cm. (C, D) There was no significant difference in PFS (C) or OS (D) between AFP ≤20 ng/mL group and AFP >20 ng/mL group in patients with tumor size ≤5 cm.
Abbreviations: AFP, alpha-fetoprotein; HCLR, high-sensitivity C-reactive protein to lymphocyte ratio; OS, overall survival; PFS, progression-free survival.
Figure 5.
The relationship of HCLR and AFP with PFS or OS in patients with HCC ≤3 cm.
Notes: (A, B) Kaplan–Meier analysis revealed significantly shorter PFS (A) and OS (B) in HCC ≤3 cm patients with HCLR >1.3 than in those with HCLR ≤1.3. (C, D) There was no significant difference in PFS (C) or OS (D) in the subgroup with AFP level ≤20 or >20 ng/mL in patients with tumor size ≤3 cm.
Abbreviations: AFP, alpha-fetoprotein; HCLR, high-sensitivity C-reactive protein to lymphocyte ratio; OS, overall survival; PFS, progression-free survival; HCC, hepatocellular carcinoma.
Predictors of OS and PFS in the univariate and multivariate analyses
The results of univariate analysis are shown in Table 4. With an unadjusted hazard ratio (HR) of 3.21 (95% CI, 2.24–4.60), HCLR >1.3 was an important prognostic factor of poor OS. In addition, tumor size >5 cm, TNM III–IV stage, microvascular invasion, serum AFP >20 ng/mL, and serum AST >40 U/L were also predictors of poor OS. After adjusting other statistically significant predictors, the seven factors mentioned above were assessed using the stepwise multivariate Cox proportional hazards model, and microvascular invasion, serum AST >40 U/L, and HCLR >1.3 remained to be significant independent predictors of shorter OS (HR, 95% CI, and P-value are listed in Table 4). Similarly, in the univariate analysis, tumor size >5 cm, III–IV of TNM stage, microvascular invasion, serum AFP >20 ng/mL, serum AST >40 U/L, and HCLR >1.3 were poor predictors of PFS. However, only serum AST >40 U/L and HCLR >1.3 were independent predictors of shorter PFS (HR, 95% CI, and P-value are listed in Table 4).
Table 4.
Univariate and multivariate Cox regression analyses of the HCLR with clinicopathologic characteristics
| Variable | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Overall survival | ||||||
| Gender (male vs female) | 1.02 | 0.62–1.66 | 0.944 | |||
| Age, years (>55 vs ≤55) | 0.83 | 0.59–1.17 | 0.290 | |||
| HBsAg (positive vs negative) | 0.82 | 0.55–1.23 | 0.343 | |||
| Tumor size, cm (>5 vs ≤5) | 2.27 | 1.61–3.20 | <0.001 | 1.42 | 1.02–1.96 | 0.063 |
| Tumor number (multiple vs single) | 1.01 | 0.67–1.52 | 0.948 | |||
| Drinking (present vs absent) | 1.11 | 0.81–1.53 | 0.497 | |||
| TNM stage (III–IV vs I–II) | 2.55 | 1.85–3.52 | <0.001 | 1.26 | 0.84–1.90 | 0.264 |
| Microvascular invasion (present vs absent) | 3.05 | 2.12–4.37 | <0.001 | 2.13 | 1.38–3.29 | 0.001 |
| Recurrence (present vs absent) | 0.98 | 0.97–1.01 | 0.062 | |||
| AFP, ng/mL (>20 vs ≤20) | 1.53 | 1.09–2.13 | 0.012 | 1.21 | 0.86–1.71 | 0.257 |
| AST, U/L (>40 vs ≤40) | 1.91 | 1.38–2.62 | <0.001 | 1.67 | 1.19–2.34 | 0.003 |
| HCLR level (>1.3 vs ≤1.3) | 3.21 | 2.24–4.60 | <0.001 | 2.64 | 1.81–3.86 | <0.001 |
| Progression-free survival | ||||||
| Gender (male vs female) | 1.01 | 0.61–1.63 | 0.983 | |||
| Age, years (>55 vs ≤55) | 0.83 | 0.59–1.17 | 0.288 | |||
| HBsAg (positive vs negative) | 0.86 | 0.57–1.29 | 0.473 | |||
| Tumor size, cm (>5 vs ≤5) | 2.04 | 1.45–2.88 | <0.001 | 1.39 | 0.95–2.03 | 0.086 |
| Tumor number (multiple vs single) | 1.04 | 0.69–1.56 | 0.845 | |||
| Drinking (present vs absent) | 1.16 | 0.85–1.60 | 0.340 | |||
| TNM stage (III–IV vs I–II) | 2.22 | 1.61–3.06 | <0.001 | 1.17 | 0.77–1.77 | 0.458 |
| Microvascular invasion (present vs absent) | 2.30 | 1.61–3.28 | <0.001 | 1.39 | 0.91–2.12 | 0.127 |
| AFP, ng/mL (>20 vs ≤20) | 1.61 | 1.15–2.24 | 0.005 | 1.28 | 0.90–1.82 | 0.170 |
| AST, U/L (>40 vs ≤40) | 1.94 | 1.41–2.68 | <0.001 | 1.49 | 1.04–2.13 | 0.028 |
| HCLR level (>1.3 vs ≤1.3) | 2.97 | 2.07–4.26 | <0.001 | 2.36 | 1.61–3.45 | <0.001 |
Note: Bold figures represent as statistically significant.
Abbreviations: AFP, alpha-fetoprotein; AST, aspartate aminotransferase; HBsAg, hepatitis B surface antigen; HCLR, high-sensitivity C-reactive protein to lymphocyte ratio; HR, hazard ratio; TNM, tumor, node and metastases.
Discussion
HCC is a multifactor and multistep malignant tumor that mostly develops slowly from liver cirrhosis, atypical hyperplasia (precancerous), and other chronic inflammation.18,19 The biological behaviors of tumor cells, such as proliferation, invasion, and necrosis, can damage the adjacent tissues and cells. These cells can release inflammatory mediators and lead to inflammation, which can accelerate tumor proliferation and invasion, resulting in an unfavorable prognosis. For instance, Hs-CRP is a sensitive acute phase reactive protein generated by the liver and is closely related with infection, inflammation, and injury in the body.20,21 It has a vital significance in assessing the prognosis of some malignant tumors such as gastric carcinoma,22 metastatic colorectal cancer,23 nasopharyngeal carcinoma,24 breast cancer,25 HCC,12–15 and so on. In addition, lymphocytes measure the performance of the host immune response against cancer, and a relative lymphopenia may observably weaken the host’s antitumor immunity.
Determining tumor markers in peripheral blood based on the “liquid biopsies” method for early diagnosis and prognostic prediction is followed in recent cancer studies.26 As prognostic markers for HCC patients, Hs-CRP and lymphocyte count are highly popular among investigators. It is effortless for patients to carry out real-time and dynamic monitoring ascribed to the easy detection of Hs-CRP and lymphocyte count in peripheral blood. In this study, we demonstrated that the optimal cutoff value of HCLR level was 1.3 with relatively high sensitivity and specificity. The AUC of HCLR was found to be higher than those of Hs-CRP, NLR, and SII. These findings suggest that HCLR can be an eligible marker for clinical practice and predicting the prognosis for HCC patients. The study found that HCLR >1.3 was a risk factor for poor survival after hepatectomy. Meanwhile, elevated HCLR was positively related with large tumor size (>5 cm), clinical TNM stage (III–IV), recurrence, micro-vascular invasion, and AST level (>40 U/L). These results suggest that elevated HCLR may be associated with HCC biological aggressiveness, with persistence of microscopic tumor foci after surgery and with poor prognosis.
The univariate analysis showed that tumor size >5 cm, TNM III–IV stage, microvascular invasion, AFP >20 ng/mL, AST >40 U/L, and HCLR >1.3 were predictors of OS and PFS. The multivariate Cox regression analysis revealed that AST >40 U/L and HCLR >1.3 were independent predictors of OS and PFS, while microvascular invasion was an independent predictor for OS only. Some factors have shown their predictive ability to predict the prognosis of liver cancer in univariate analysis, but not in multivariate Cox regression analysis, and many previous researches have considered these variables as a predictor of prognosis of liver cancer. For example, high AFP level, tumor size >5 cm, and TNM III–IV stage were considered as risk factors for recurrence and survival time after hepatectomy in some studies.27–29
Recently, accumulating evidence has shown that serum AFP level has limited predictive ability in patients with small HCCs.4,30 Consistent with previous reports, our results also indicated that serum AFP level had no prognostic role in HCC patients with tumor size ≤5 and ≤3 cm. Encouragingly, this study demonstrated that the HCLR level still had a significant prognostic value in the subgroups of middle and small tumor size. Therefore, HCLR may be a very good surveillance tool to predict the prognosis for early HCC patients.
Conclusion
Our research suggests that an elevated HCLR level (HCLR >1.3) is an independent predictor of OS and PFS. Preoperative elevated HCLR can indirectly reflect an inflammatory situation of the whole body or cancer tissue in HCC patients and accelerate tumor cells invasion and metastasis. Therefore, further research on the cause and mechanism of hepatic inflammation may contribute greatly to the early diagnosis, treatment, and prevention of recurrence of HCC. It should be noted that HCLR is a typical inflammatory factor in routine clinical detection and is mostly used as an observation target for anti-inflammatory therapy. HCLR is rarely used for follow-up monitoring of cancer patients who had undergone surgical resection. The fact that the optimal cutoff value has not been figured out might account for this situation. Since this study is a retrospective analysis and the sample source is limited in one department, further study that involves specimens from multiple departments is of great significance. To better confirm the prognostic value of HCLR in clinical practice, a prospective study will be appreciated.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81773148) and the Program of Guangxi Zhuang Autonomous Region Health and Family Planning Commission (No Z2016258).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med. 2008;359(19):2045–2047. doi: 10.1056/NEJMe0807581. [DOI] [PubMed] [Google Scholar]
- 3.Poon RT. Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology. 2011;54(3):757–759. doi: 10.1002/hep.24569. [DOI] [PubMed] [Google Scholar]
- 4.Giannini EG, Marenco S, Borgonovo G, et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology. 2012;56(4):1371–1379. doi: 10.1002/hep.25814. [DOI] [PubMed] [Google Scholar]
- 5.Guo W, Liu S, Cheng Y, et al. ICAM-1-related noncoding RNA in cancer stem cells maintains ICAM-1 expression in hepatocellular carcinoma. Clin Cancer Res. 2016;22(8):2041–2050. doi: 10.1158/1078-0432.CCR-14-3106. [DOI] [PubMed] [Google Scholar]
- 6.Zhu PP, Yuan SG, Liao Y, Qin LL, Liao WJ. High level of intercellular adhesion molecule-1 affects prognosis of patients with hepatocellular carcinoma. World J Gastroenterol. 2015;21(23):7254–7263. doi: 10.3748/wjg.v21.i23.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu SJ, Ji F, Han M, et al. Prognostic value of combined preoperative fibrinogen and neutrophil–lymphocyte ratio in patients with hepatocellular carcinoma after liver transplantation. Oncotarget. 2017;8(3):4301–4312. doi: 10.18632/oncotarget.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halazun KJ, Najjar M, Abdelmessih RM, et al. Recurrence after liver transplantation for hepatocellular carcinoma: a new MORAL to the story. Ann Surg. 2017;265(3):557–564. doi: 10.1097/SLA.0000000000001966. [DOI] [PubMed] [Google Scholar]
- 9.Liao W, Zhang J, Zhu Q, et al. Preoperative neutrophil-to-lymphocyte ratio as a new prognostic marker in hepatocellular carcinoma after curative resection. Transl Oncol. 2014;7(2):248–255. doi: 10.1016/j.tranon.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sieghart W, Pinter M, Hucke F, et al. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2224–2234. doi: 10.1002/hep.26057. [DOI] [PubMed] [Google Scholar]
- 11.Liu XY, Ma LN, Yan TT, et al. Combined detection of liver stiffness and C-reactive protein in patients with hepatitis B virus-related liver cirrhosis, with and without hepatocellular carcinoma. Mol Clin Oncol. 2016;4(4):587–590. doi: 10.3892/mco.2016.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujiwara N, Tateishi R, Nakagawa H, et al. Slight elevation of high-sensitivity C-reactive protein to predict recurrence and survival in patients with early stage hepatitis C-related hepatocellular carcinoma. Hepatol Res. 2015;45(6):645–655. doi: 10.1111/hepr.12398. [DOI] [PubMed] [Google Scholar]
- 13.Nagaoka S, Yoshida T, Akiyoshi J, et al. Serum C-reactive protein levels predict survival in hepatocellular carcinoma. Liver Int. 2007;27(8):1091–1097. doi: 10.1111/j.1478-3231.2007.01550.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu YB, Ying J, Kuang SJ, et al. Elevated preoperative serum Hs-CRP level as a prognostic factor in patients who underwent resection for hepatocellular carcinoma. Medicine. 2015;94(49):e2209. doi: 10.1097/MD.0000000000002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Peng Y, Mao B, Qian K. Thioredoxin reductase: a novel, independent prognostic marker in patients with hepatocellular carcinoma. Oncotarget. 2015;6(19):17792–17804. doi: 10.18632/oncotarget.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Ding T, He Q, et al. An in situ molecular signature to predict early recurrence in hepatitis B virus-related hepatocellular carcinoma. J Hepatol. 2012;57(2):313–321. doi: 10.1016/j.jhep.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 18.He G, Dhar D, Nakagawa H, et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell. 2013;155(2):384–396. doi: 10.1016/j.cell.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reebye V, Sætrom P, Mintz PJ, et al. Novel RNA oligonucleotide improves liver function and inhibits liver carcinogenesis in vivo. Hepatology. 2014;59(1):216–227. doi: 10.1002/hep.26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. Pre-treatment C-reactive protein as a prognostic factor for recurrence after surgical resection of hepatocellular carcinoma. Anticancer Res. 2013;33(3):1181–1188. [PubMed] [Google Scholar]
- 21.Imai N, Kinoshita A, Onoda H, et al. Persistent elevated C-reactive protein after treatment is an independent marker of a poor prognosis in patients with hepatocellular carcinoma. Clin Transl Oncol. 2013;15(7):575–581. doi: 10.1007/s12094-012-0976-y. [DOI] [PubMed] [Google Scholar]
- 22.Woo Y, Hyung WJ, Obama K, et al. Elevated high-sensitivity C-reactive protein, a marker of advanced stage gastric cancer and postgastrectomy disease recurrence. J Surg Oncol. 2012;105(4):405–409. doi: 10.1002/jso.22129. [DOI] [PubMed] [Google Scholar]
- 23.Casadei Gardini A, Carloni S, Scarpi E, et al. Prognostic role of serum concentrations of high-sensitivity C-reactive protein in patients with metastatic colorectal cancer: results from the ITACa trial. Oncotarget. 2016;7(9):10193–10202. doi: 10.18632/oncotarget.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang LQ, Hu DP, Chen QY, et al. Elevated high-sensitivity C-reactive protein levels predict decreased survival for nasopharyngeal carcinoma patients in the intensity-modulated radiotherapy era. PLoS One. 2015;10(4):e0122965. doi: 10.1371/journal.pone.0122965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frydenberg H, Thune I, Lofterød T, et al. Pre-diagnostic high-sensitive C-reactive protein and breast cancer risk, recurrence, and survival. Breast Cancer Res Treat. 2016;155(2):345–354. doi: 10.1007/s10549-015-3671-1. [DOI] [PubMed] [Google Scholar]
- 26.Strønen E, Toebes M, Kelderman S, et al. Targeting of cancer neoan-tigens with donor-derived T cell receptor repertoires. Science. 2016;352(6291):1337–1341. doi: 10.1126/science.aaf2288. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Tan Y, Jiang L, et al. Liver resection associated with better outcomes for single large hepatocellular carcinoma located in the same section. Medicine. 2017;96(10):e6246. doi: 10.1097/MD.0000000000006246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu PH, Hsu CY, Hsia CY, et al. Prognosis of hepatocellular carcinoma: assessment of eleven staging systems. J Hepatol. 2016;64(3):601–608. doi: 10.1016/j.jhep.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Chang YJ, Chung KP, Chang YJ, Chen LJ. Long-term survival of patients undergoing liver resection for very large hepatocellular carcinomas. Br J Surg. 2016;103(11):1513–1520. doi: 10.1002/bjs.10196. [DOI] [PubMed] [Google Scholar]
- 30.Farinati F, Marino D, de Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101(3):524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]