Abstract
Purpose
To assess the incremental cost associated with the management of patients with primary non-squamous non-small cell lung cancer (NSCLC) with brain metastases at the time of diagnosis.
Methods
Data were extracted from the French Hospital medical information database (Programme de Médicalisation des Systèmes d’Information (PMSI)). Patients with non-squamous NSCLC were identified through a diagnosis of lung cancer and a prescription of bevacizumab or pemetrexed. All such patients hospitalised with lung cancer for the first time in 2013 and with metastases identified at the first hospitalisation were eligible. Two cohorts were identified, one with brain metastases (group B: n=971) and one with metastases at other sites (group A: n=1529). For each patient, total in-hospital medical resource consumption associated with the initial hospitalisation in 2013 and with any follow-up stays in the following 24 months was documented. Costs were attributed from official French national tariffs and expressed in 2017 euros.
Results
The mean number of hospitalisations per patient in the 24-moth follow-up period was 17 in group A and 21 in group B. >99% of patients in both groups received chemotherapy. 58% of patients in group B and 13% in group A were managed by radiotherapy. 37% in group B and 24% in group A received palliative care. The associated cost was €2979 per patient-month for patients in group B and €2426 for patients in group A, representing a differential cost of €553 per month. Radiotherapy (+€164/month) and palliative care (+€130/month) were the principal drivers of the incremental cost.
Conclusions
The presence of brain metastases at the time of diagnosis of non-squamous NSCLC carries a significant burden, and ways of lowering this burden are needed.
Keywords: non-squamous non-small cell lung cancer, brain metastasis, cost, PMSI, radiotherapy, anaplastic lymphoma kinase.
Key questions.
What is already known about this subject?
Patients with non-squamous non-small cell lung cancer are at highrisk of developing metastases in the brain during disease management.
What does this study add?
Compared with metastases in other sites, the presence of metastases in the brain carries a significant burden to the health system.
How might this impact on clinical practice?
Prevention of brain metastases is a key objective for the management of patientswith non-small cell lung cancer.
Introduction
Lung cancer is the most common cancer worldwide and the principal cause of cancer-related death.1 In France, there were an estimated 45 000 incident cases of lung cancer and 30 500 lung cancer-related deaths in 2015.2 In most cases, metastases are already present when lung cancer is diagnosed. The presence of metastases dramatically impacts survival since 5-year survival in patients with stage IV lung cancer is <5%. However, there may be some discrepancies in treatment and outcome according to where the metastases occur. In particular, central nervous system metastases are frequent in patients with lung cancer. Data from over 20 000 patients with lung cancer in the Swedish Family Cancer Database, an exhaustive nationwide database, reported that 56% of patients with lung cancer developed metastases, the most frequent site being the nervous system, accounting for 39% of all metastases.3 Interestingly, some subgroups of patients with lung cancer may experience higher rates of brain metastases at diagnosis, such as patients with non-squamous non-small cell lung cancer (NSCLC) bearing rearrangements of the anaplastic lymphoma kinase (ALK) gene, in whom brain metastases are found in one-third of cases at the time of diagnosis.4
Central nervous system metastases are associated with significant morbidity related to neurocognitive or motor deficits and loss of autonomy.5 Quality of life in these patients is generally poor and can be further deteriorated by side effects of treatment.6 Caregiver burden is also high due to the high risk of dependence of patients with brain metastases.7 Treatment typically involves surgery, when possible, or radiotherapy. Standard chemotherapy is generally thought to be of limited and delayed efficacy for the treatment of brain metastases.5 However, in the setting of oncogene addicted NSCLC, the recent development of inhibitors of the epidermal growth factor receptor (EGFR) or ALK, which penetrate the brain, has shown promise for the prevention and treatment of brain metastases.5
Retrospective studies of North American insurance claims databases have evaluated the economic burden of brain metastases in patients with primary lung cancer and have shown that direct health costs increase around fourfold following diagnosis of brain metastases.8 9 In particular, use of stereotactic brain surgery may markedly increase the cost of treatment.10 However, since the cost of management of lung cancer rises substantially following evolution to the metastatic stage, regardless of the site of metastasis,11 it is not clear how much of the extra cost determined in these studies is inherent to management of metastases specifically arising in the central nervous system.
The objective of this study was to assess the incremental cost of management of patients with primary NSCLC who had developed brain metastases at the time of diagnosis. To this end, costs accrued over the 2-year period following diagnosis were compared between patients with brain metastases and those with metastases at other sites in a nationwide cohort of patients hospitalised in France. We chose to focus on non-squamous NSCLC, which is the subtype of lung cancer with the higher risk of brain metastases.
Materials and methods
Study design
This retrospective study analysed data extracted from the French Hospital medical information database (PMSI). The study population consisted of all patients hospitalised for a non-squamous NSCLC in France in 2013. Data extraction and analysis followed the guidelines for exploitation of the PMSI database published by the French Health Ministry.12 The study was conducted in accordance with relevant requirements. Use of the PMSI-MCO (Medicine, Surgery, Obstetrics) database for this type of study has been approved by the French national data protection agency (Commission Nationale de l’Informatique et des Libertés (CNIL); annual authorisation number 1419102 v7—2015-111111-56-18/order M14N056 and M14L056).
PMSI database
The French PMSI-MCO is an exhaustive medico-administrative hospital discharge database which covers all overnight or day hospitalisations in the public and private sectors involving short-term stays in medical, surgical or obstetric facilities in France.13 Individual patients can be tracked across multiple hospitalisations through a unique anonymous patient identifier. Outpatient consultations at hospitals are not documented in the PMSI. In 2013, almost 30 million stays by over 13 million patients were reported in this database.
All hospital procedures are documented by hospital stay at the time of final discharge in the form of a standardised discharge summary (SDS), irrespective of the number and type of hospital departments in which the patient has been cared. For costing purposes, each stay is attributed to a diagnosis-related group (DRG) which reflects the reasons for hospitalisation. These are identified by the physician through International Classification of Diseases, 10th Revision (ICD-10) codes,14 either as principal diagnoses (PD: the condition for which the patient was hospitalised), related diagnoses (RD: any underlying condition which may have been related to the PD) or as significantly associated diagnoses (SAD: comorbidities or complications which may affect the course or cost of hospitalisation). Sociodemographic information (gender, age and residence code) and medical information on the PD/RD that led to hospital admission, the nature of treatments received and examinations carried out, underlying comorbidities and possible complications (SAD), are available for epidemiologic studies. Medication use is integrated into the DRG, and therefore cannot be individualised, except for expensive drugs, which are individually identified and recorded in a separate database (FICHCOMP), which can be linked to the PMSI-MCO database for individual stays.
Since the introduction of a DRG-based prospective payment system in 2005, the PMSI-MCO database has been used as the basis for the funding of services in all hospitals, with each hospital receiving DRG-based payments according to national tariffs. For this reason, expenditure on hospitalisations by public health insurance is expected to be well documented in the PMSI-MCO database. Information in the database is exhaustive (all public and private French hospitals are included, and no sampling is performed) and is of high quality, with limited coding errors.
Study population
All hospital stays in France with at least one ICD-10 code for lung cancer (code C34*: malignant neoplasm of bronchus and lung; online supplementary table 1) documented as a PD, RD or SAD were identified from the PMSI-MCO database between 1 January 2013 and 31 December 2013. Hospitalisations with at least one ICD-10 code for metastatic cancer (C77*, C78*, C79* codes; online supplementary table 2) documented in the SDS, but without any other primary cancers stated as a PD/RD/SAD were also retained.
esmoopen-2018-000414supp001.pdf (241.6KB, pdf)
Hospital stays for each patient admitted were extracted over a 12-month period before the initial index hospitalisation in 2013 (the retrospective period) and over a 24-month period following this index stay (the prospective period). For example, if the initial index hospitalisation was 1 April 2013, all hospital stays of this patient were extracted from 1 April 2012 until 30 March 2015. Patients who had any primary or metastatic cancer, including lung cancer, during the 12-month retrospective period were excluded in order to restrict the study to patients with incident lung cancer with synchronous metastasis.
Non-squamous NSCLC cannot be identified explicitly in the PMSI database as there is no equivalent ICD-10 code. For this reason, specific lung cancer therapy was used as a proxy marker. Patients who had received bevacizumab or pemetrexed at least once at the index hospitalisation or during the 24-month prospective period were considered to have non-squamous NSCLC. The justification for this is that these two agents were the only two agents licensed exclusively for the treatment of metastatic non-squamous NSCLC and documented in the FICHCOMP database. At the time of enrolment, the FICHCOMP databases where bevacizumab and pemetrexed could be tracked were only available for public hospitals. Patients with metastatic non-squamous NSCLC treated with either of these drugs in private hospitals were therefore de facto excluded from the study.
In the next step, two subgroups of patients were identified, namely (A) a reference group of patients with synchronous metastasis at the index hospitalisation in sites other than the brain, and (B) patients with synchronous brain metastases at the index hospitalisation. The database was searched for documentation of brain metastases documented in the SDS for the index hospitalisation SDS as a PD, RD or SAD (ICD-10 code C97.3). Patients with documented brain metastases were assigned to group B. Due to potential undercoding of metastases, all hospital stays for patients in group A were reviewed both retrospectively and prospectively to identify any signs or symptoms of brain metastases or specific procedures for their treatment through the relevant DRG codes. These codes related to epilepsy, hemiplegia, cerebral surgical excision, stereotactic radiotherapy or standard radiotherapy (≤10 sessions). In order to restrict the reference group to patients without brain metastases at any time during the observation period, patients with codes for brain metastases, related signs or treatments for any stay during the retrospective or prospective periods were also excluded from group A.
For each patient in each group, all stays during the prospective period were extracted and reviewed by medical experts in order to identify those stays related to the management of the lung cancer or its metastases. All stays with C34* code as PD/RD were automatically retained, as well as stays where the PD/RD code was considered to be related to lung cancer or its metastases, including ‘anaemia in chronic diseases classified elsewhere’ (D63*), ‘other aplastic anaemias’ (D61*) or ‘pleural effusion in conditions classified elsewhere’ (J61*). Stays with other codes for cancer (such as oesophagus, liver or brain) documented as the PD or RD were eligible if a C34* code was documented as an SAD or if no other primary cancer was documented.
Data collection
The sociodemographic characteristics of patients were collected (age at inclusion, gender). For each hospital stay, the distribution per type of management was analysed based on the DRG codes: radiotherapy (stereotactic or standard), chemotherapy (including bevacizumab, pemetrexed and any other chemotherapy administered in hospital), palliative care, medical management, surgical procedures, interventional procedures and others.
Total in-hospital medical resource consumption, including standard medication, associated with the initial index hospitalisation in 2013 and with any follow-up stays occurring in the 24 months following the index hospitalisation was documented. Medication prescribed or delivered during outpatient consultations at the hospital was not considered.
Costing
Costing was restricted to direct costs and determined from the perspective of the French social security system (National Health Insurance, NHI). Costs were attributed from French national tariffs for medical acts applicable in France from 2013 to 2015, and were expressed in 2017 euros. A standard national tariff was applied to each hospitalisation based on the DRG code attributed in the PMSI database. These standard tariffs include medical and related procedures, nursing care, treatments (except specific expensive drugs), food and accommodation and investment costs for hospitalised patients. Additional costs per day of hospitalisation in an intensive care unit were added to the DRG tariffs when appropriate. For private hospitals, where physicians are reimbursed on a fee-for-service basis, physician fees were identified from the Echelle Nationale des Coûts à méthodologie Commune (the French observatory of real-world spending on healthcare) and added to the DRG tariffs. Expensive drugs and implants were costed using the retail price for the public FICHCOMP database and the official tariff for the private FICHCOMP database (at the time of costing, the private FICHCOMP had just been made available).
The costs per patient-month of all hospitalisations related to lung cancer or its metastases were determined for each cohort, with and without chemotherapy. The cost difference between the two cohorts was assessed. An analysis of the mean cost per patient for each month following inclusion was then performed for each cohort.
Statistical analysis
Analysis of the data was purely descriptive and no a priori hypotheses were tested. Statistical Analysis System software, V.9.2 for Windows (SAS Institute) was used for all analyses.
Results
Identification of patients with non-squamous NSCLC
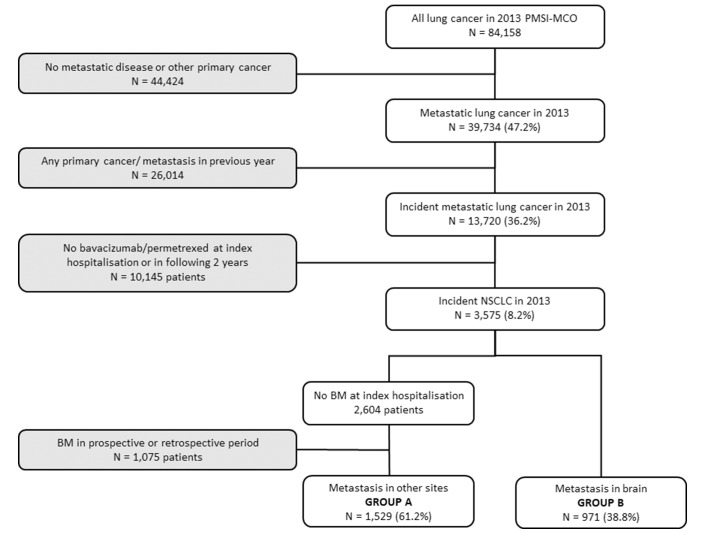
In 2013, a total of 84 158 patients with a C34* ICD-10 code for lung cancer were identified in the PMSI-MCO database, of whom 39 734 (47.2%) had metastatic disease that could not be associated with another primary cancer at the index hospitalisation. After exclusion of patients who had already been hospitalised with lung cancer or any other primary or metastatic cancer during the previous year, 13 720 patients were identified as incident cases. Of these, 3575 patients had received bevacizumab or pemetrexed at the index hospitalisation or during the retrospective or prospective periods and were considered to have non-squamous NSCLC. These consisted of 971 patients with brain metastases at the index hospitalisation and 2604 patients without (figure 1). In the latter group, 1075 were excluded due to the presence of brain metastases (or signs thereof) documented in the retrospective or prospective periods. The final study population consisted of 2500 patients, divided into group A with no documented brain metastases (1529 patients; 61%) and group B with synchronous brain metastases (971 patients; 38.8%). The flow diagram for the selection of patients is illustrated in figure 1.
Figure 1.
Flow diagram for the selection of the study population. BM, brain metastasis; NSCLC, non-small cell lung cancer.
Characteristics of patients with non-squamous NSCLC
In group A (patients without brain metastases), the mean age at inclusion was 62.6±9.8 years old (men: 62.6±9.6 years old; women: 62.4±10.3 years old), and 69.0% were men. Patients in group B (with synchronous brain metastases) were on average younger, with a mean age at inclusion of 58.6±9.1 years old (men: 59.5±9.1 years old; women: 57.2±8.9 years old), 59.3% of them being men.
Identification of stays related to lung cancer or its metastases
During the 2-year prospective period, the 2500 included patients made 47 230 hospital stays. After medical review, 1989 stays were excluded as being unrelated to lung cancer. The analysis was performed on all the remaining hospital stays related to the management of lung cancer or its metastases. The 1529 patients in group A (without brain metastases) made 25 244 related stays (on average, 17 stays per patient) and the 971 patients in group B (with brain metastases) made 19 997 related stays (on average, 21 stays per patient).
Type of management
The type of management of the enrolled patients is presented in table 1. Most patients in both groups received chemotherapy. Over one in two patients in group B (57.7%) were managed by radiotherapy (vs 12.9% for patients in group A). Patients in group B were more likely to be admitted into palliative care than patients in group A (37.2% vs 23.5%) and to have at least one stay in medical management (94.3% vs 86.6%).
Table 1.
Management of patients with metastatic non-squamous NSCLC in 2013
| Group A (no BM) | Group B (with BM) | |||||||
| Patients | Stays | Patients | Stays | |||||
| n | % | n | % | n | % | n | % | |
| Radiotherapy | 197 | 12.9 | 4535 | 18.0 | 560 | 57.7 | 7008 | 35.0 |
| Chemotherapy | 1522 | 99.5 | 15 892 | 63.0 | 970 | 99.9 | 9541 | 47.7 |
| Palliative care | 359 | 23.5 | 445 | 1.8 | 361 | 37.2 | 483 | 2.4 |
| Surgical procedures | 583 | 38.1 | 701 | 2.8 | 316 | 32.5 | 399 | 2.0 |
| Medical management | 1324 | 86.6 | 3953 | 15.7 | 916 | 94.3 | 2982 | 14.9 |
| Interventional procedures | 151 | 9.9 | 180 | 0.7 | 71 | 7.3 | 81 | 0.4 |
| Others | 194 | 12.7 | 331 | 1.3 | 108 | 11.1 | 176 | 0.9 |
BM, brain metastasis; NSCLC, non-small cell lung cancer.
Of the 560 patients in group B receiving radiotherapy, 11.1% (62 patients) had stereotactic radiotherapy alone during the 24-month follow-up, 75.0% (n=420) received whole brain radiotherapy alone and 13.9% (n=78) both stereotactic and whole brain radiotherapy. For the 197 patients in group A, all but five patients received standard radiotherapy alone (n=192; 97.5%).
Patient follow-up
The duration of follow-up was 2 years for patients who were still alive by the end of the study period, and, for patients who died before the end of follow-up, the difference between the death date and the entry date. For patients in group A, the mean duration of follow-up was 435±272 days (median 426 (162– 730) days); for patients in group B, it was 415±250 days (median 376 (189–730) days).
Overall, 942 patients in group A and 695 patients in group B died during hospitalisation, corresponding to a 2-year hospital fatality rate of 61.6% (95% CI 59.2% to 64.0%) in group A and of 71.6% (95% CI 68.7% to 74.4%) in group B. The number of deaths was analysed for each month of follow-up and a very similar pattern was observed in both groups, with half the deaths having occurred in the first 6–9 months (online supplementary table 3). The median survival duration after the index hospitalisation was 252±179 days (median 204 (108–363) days) in group A and 290±179 days (median 250 (147–416) days) in group B.
Hospital economic burden
From the NHI perspective, the cost was €2979 per patient-month for patients in group B and €2426 for patients in group A, representing a differential cost of €553 per month (€6636 per year). After removing chemotherapy from the total cost, the differential cost was €526 per month (€6312). Table 2 presents the per capita costs in the two groups, as well as the differential cost according to the type of care received by the patient.
Table 2.
Differential cost associated with the presence of brain metastases in the management of patients with metastatic non-squamous NSCLC in 2013–2014 (NHI perspective)
| Cost per patient-month | Differential annual per capita cost | |||
| Group A (no BM) n=1529 |
Group B (BM) n=971 |
Differential cost attributable to BM | ||
| Total | €2426 | €2979 | +€553 | +€6636 |
| Chemotherapy | €1301 | €1329 | +€27 | +€324 |
| Total without chemotherapy | €1125 | €1651 | +€526 | +€6312 |
| Radiotherapy | €52 | €216 | +€164 | +€1560 |
| Palliative care | €158 | €288 | +€130 | +€1570 |
| Medical management | €652 | €861 | +€210 | +€2520 |
| Surgery procedures | €244 | €270 | +€26 | +€314 |
| Interventional procedures | €10 | €6 | −€3 | −€36 |
| Others | €10 | €9 | −€1 | −€12 |
BM, brain metastasis; NHI, National Health Insurance; NSCLC, non-small cell lung cancer.
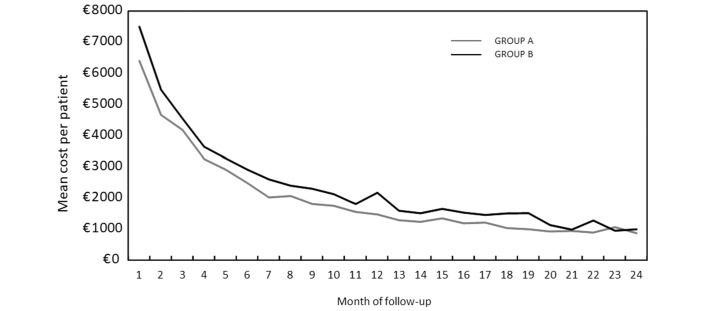
Mean cost per patient for each month of follow-up
The mean cost per patient for each month of follow-up was determined from the NHI perspective (figure 2). For each month following inclusion, all costs were added, and then divided by the number of patients who had not died. In group A, the mean cost was €6401 per patient in month 1 and €862 in month 24, whereas in group B was €7491 per patient in month 1 and €981 in month 24.
Figure 2.
Evolution of mean cost per patient for each month of follow-up in patients with non-squamous non-small cell lung cancer (NSCLC) in 2013–2014 (National Health Insurance (NHI) perspective).
Discussion
This study compared hospitalisation costs of two groups of patients with incident metastatic lung cancer, one with synchronous brain metastases at the time of diagnosis, and one with synchronous metastases at other sites, in order to assess the incremental cost attributable to the brain localisation of metastases. Our results estimate this incremental cost at €553 per month (€6636 per year). The principal components associated with this increased total cost were medical management, radiotherapy and palliative care. Assuming that there are around 40 000 incident cases of lung cancer in France each year,15 that around 48% of these are non-squamous NSCLC, that around 53% of these cancers are already metastatic at the time of diagnosis16 and that around 39% of patients with metastatic non-squamous NSCLC develop brain metastases,3 then the total incremental monthly hospitalisation cost to the French health service attributable to brain metastases would be expected to be around €2.2 million.
This study has several limitations, many of which are inherent to database analyses of this type. In this study, we did not attempt to capture all patients with metastatic non-squamous NSCLC in the PMSI database, the aim being to identify two groups of patients with and without brain metastases who were matched in terms of time of diagnosis and metastatic status, who were treated with the same agents (bevacizumab and pemetrexed) and who were sufficiently numerous to generate costs with sufficient precision. Since bevacizumab and pemetrexed are the only treatments for non-squamous NSCLC identified in the FICHCOMP database, we were not able to identify patients receiving other therapies for this cancer type such as paclitaxel. Similarly, patients taking oral chemotherapy at home for non-squamous NSCLC bearing EGFR mutations (gefitinib or erlotinib) could not be retrieved. Moreover, it was not possible to identify patients treated in the private sector, since the FICHCOMP database did not cover this sector at the time of the analysis. Given the reported prevalence of different lung cancer subtypes in France, around 10 000 incident cases of metastatic non-squamous NSCLC would be expected to arise each year, and on this basis, around a third of these were captured in the present study. Finally, it should be emphasised that the PMSI database contains no information on histological type or on staging, so it was not possible to identify patients with metastatic non-squamous NSCLC directly. For the same reason, it was not possible to identify subgroups of patients with EGFR mutations or ALK rearrangements. Moreover, treatment outcome cannot be followed in the PMSI database as no clinical information other than diagnosis is documented.
In addition, hospitalisation costs determined in this study may underestimate total direct medical costs since costs generated in community medicine are not taken into account, including costs related to patient nursing care and at home hospitalisation. In particular, costs of oral chemotherapy for non-squamous NSCLC bearing EGFR or ALK mutations are not included in the cost analysis. These medications are more costly than whole brain radiotherapy delivered in hospital. At the time of the analysis (2013), only two such oral treatments were available (gefitinib and erlotinib) for patients with EGFR+NSCLC. However, since then, afatinib and osimertinib have become available for EGFR+NSCLC and crizotinib, ceritinib and alectinib for the treatment of ALK+NSCLC. Although only a minority of patients with non-squamous NSCLC have carcinomas that carry EGFR or ALK mutations, some of these new oral treatments may be of particular benefit in patients who develop brain metastases.17–19 In addition to medication, costs related to medical imaging performed in community clinics for surveillance of brain metastases will not be captured in the present analysis.
The excess cost associated with brain metastases compared with other metastatic sites is likely to be due in part to the use of stereotactic radiotherapy which is expensive, but also to the management of complications of brain metastases and to complications of treatment, which increase medical management costs. For example, the use of bevacizumab has been associated with an increased risk of cerebral haemorrhage, although recent data from large cohorts suggest that the incremental risk, if it exists, is minimal.20 21 In addition, >80% of patients with brain metastases present symptoms, frequently intracranial hypertension or focal neurologic deficits, which will require management.22
The cost findings of this study are difficult to compare directly with previous findings, due to differences in health resources covered and in patient groups compared. A previous study of costs documented in an insurance claims database in the USA compared costs before and after diagnosis of brain metastases in patients with ALK+NSCLC treated with crizotinib9 and reported that monthly per capita costs rose from $5983 before diagnosis to $22 645 after. Unlike our study, these costs include community medicine costs and notably delivery of crizotinib. Nevertheless, even when considering inpatient costs only, an increase was observed in monthly per capita cost from $1174 before diagnosis to $6692 afterwards. This finding is not in itself surprising since the passage from non-metastatic to metastatic disease is known to be associated with a substantial rise in resource utilisation and associated cost. A recent study by Fernandes et al 23 is perhaps more comparable with our own, in that patients with lung cancer with synchronous brain metastases at diagnosis was compared with patients with metastases in other sites. The study was again performed in a US insurance claims database and specifically evaluated patients with EGFR+NSCLC. Postdiagnosis per capita monthly costs were $21 202 for patients with synchronous brain metastases and $11 190 for patients without. Both absolute costs and the incremental cost of brain metastases were considerably higher than in our study, although it should be noted that, unlike our study, community costs were also considered, including expensive oral chemotherapy in all patients enrolled. Most recently, a third US claims database study has been published, in which a similar approach was used evaluating patients prescribed ALK inhibitors.24 In this study, costs were compared between patients who developed brain metastases and those who did not. Postdiagnosis per capita monthly costs were $29 497 for patients with brain metastases and $22 791 for patients without. Of this cost, $11 057 and $6831 represented hospitalisation costs, the incremental hospitalisation cost being $4226. Again these costs in the US setting are considerably higher than those estimated in France.
The introduction of immunotherapies and new targeted therapies (EGFR-tyrosine kinase inhibitors and ALK inhibitors) for the treatment of lung cancer may permit significant numbers of patients with NSCLC to survive much longer than with previously available treatments. In this context, it will be important to minimise the burden of brain metastases which are an important driver of impaired quality of life and functional status, as well as of cost. These considerations emphasise the need to identify effective strategies to prevent the development of brain metastases in patients with lung cancer and to develop effective and well-tolerated new treatment options that can successfully eradicate brain metastases.
In conclusion, the presence of brain metastases at the time of diagnosis of non-squamous NSCLC carries a significant burden, and ways of lowering this burden are needed, particularly in an era where durable remission of lung cancer has become a reality.
Acknowledgments
The writing and preparation of this paper was funded by Roche. Initial data analyses were undertaken by CT, who is an employee of HEVA (Lyon, France) and received funding from Roche. Writing support was provided by CT and LdL of HEVA (Lyon, France), as well as by Adam Doble of Foxymed (Paris, France), and was funded by Roche. All authors had complete access to the data that support this publication.
Footnotes
Funding: The study was funded in full by Roche.
Competing interests: NG has received personal fees from Roche, AstraZeneca, Pfizer, BMS, MSD and Novartis, in addition to being an investigator in trials involving these companies; relationships include consultancy service and membership of scientific advisory boards. DC and BT are employees of Roche. LdL, CT and AV have received grants via their company from Roche. ABC has received personal fees from AstraZeneca, Roche, Pfizer, Novartis, MSD, BMS and Takeda, grants from Novartis and Merck, and disclose collaborations with AstraZeneca, Roche, Boehringer-Ingelheim, BMS, MSD, Merck, Novartis and Pfizer.
Patient consent: Not required.
Ethics approval: Since this was a retrospective study of an anonymised database, ethics committee approval was not required.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2. cancer Inationaldu. Les cancers en France. édition 2016: Institut national du cancer 2017. [Google Scholar]
- 3. Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014;86:78–84. 10.1016/j.lungcan.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 4. Solomon B. First-line treatment options for ALK-rearranged lung cancer. Lancet 2017;389:884–6. 10.1016/S0140-6736(17)30124-1 [DOI] [PubMed] [Google Scholar]
- 5. Owen S, Souhami L. The management of brain metastases in non-small cell lung cancer. Front Oncol 2014;4:248 10.3389/fonc.2014.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters S, Bexelius C, Munk V, et al. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev 2016;45:139–62. 10.1016/j.ctrv.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 7. Saria MG, Nyamathi A, Phillips LR, et al. The hidden morbidity of cancer: Burden in caregivers of patients with brain metastases. Nurs Clin North Am 2017;52:159–78. 10.1016/j.cnur.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guérin A, Sasane M, Dea K, et al. The economic burden of brain metastasis among lung cancer patients in the United States. J Med Econ 2016;19:526–36. 10.3111/13696998.2016.1138962 [DOI] [PubMed] [Google Scholar]
- 9. Guérin A, Sasane M, Zhang J, et al. Brain metastases in patients with ALK+ non-small cell lung cancer: clinical symptoms, treatment patterns and economic burden. J Med Econ 2015;18:312–22. 10.3111/13696998.2014.1003644 [DOI] [PubMed] [Google Scholar]
- 10. Halasz LM, Weeks JC, Neville BA, et al. Use of stereotactic radiosurgery for brain metastases from non-small cell lung cancer in the United States. Int J Radiat Oncol Biol Phys 2013;85:e109–16. 10.1016/j.ijrobp.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGuire A, Martin M, Lenz C, et al. Treatment cost of non-small cell lung cancer in three European countries: comparisons across France, Germany, and England using administrative databases. J Med Econ 2015;18:525–32. 10.3111/13696998.2015.1032974 [DOI] [PubMed] [Google Scholar]
- 12. Technical Agency for Hospital Information. Guide méthodologique de production des informations relatives l’activité médicale et sa facturation en médecine, chirurgie, obstétrique et odontologie. Bull Off 2014;2014/6 bis, 2014. [Google Scholar]
- 13. Bezin J, Duong M, Lassalle R, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: Powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf 2017;26:954–62. 10.1002/pds.4233 [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization. International Classification of Diseases 10th Revision. Geneva: WHO, 2010. [Google Scholar]
- 15. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–403. 10.1016/j.ejca.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 16. Chouaïd C, Debieuvre D, Durand-Zaleski I, et al. Survival inequalities in patients with lung cancer in France: A nationwide cohort study (the TERRITOIRE Study). PLoS One 2017;12:e0182798 10.1371/journal.pone.0182798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119–28. 10.1016/S1470-2045(14)70362-6 [DOI] [PubMed] [Google Scholar]
- 18. Zhuang H, yuan zhiyong, Wang J, et al. Phase II study of whole brain radiotherapy with or without erlotinib in patients with multiple brain metastases from lung adenocarcinoma. Drug Des Devel Ther 2013;7:1179–86. 10.2147/DDDT.S53011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clinical Cancer Research 2012;18:4406–14. 10.1158/1078-0432.CCR-12-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Besse B, Lasserre SF, Compton P, et al. Bevacizumab safety in patients with central nervous system metastases. Clinical Cancer Research 2010;16:269–78. 10.1158/1078-0432.CCR-09-2439 [DOI] [PubMed] [Google Scholar]
- 21. Besse B, Le Moulec S, Mazieres J, et al. Bevacizumab in patients with nonsquamous non-small cell lung cancer and asymptomatic, untreated brain metastases (BRAIN): a nonrandomized, Phase II Study. Clinical Cancer Research 2015;21:1896–903. 10.1158/1078-0432.CCR-14-2082 [DOI] [PubMed] [Google Scholar]
- 22. Schmieder K, Keilholz U, Combs S. The Interdisciplinary Management of Brain Metastases. Dtsch Arztebl Int 2016;113:415–21. 10.3238/arztebl.2016.0415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fernandes AW, Wu B, Turner RM. Brain metastases in non-small cell lung cancer patients on epidermal growth factor receptor tyrosine kinase inhibitors: symptom and economic burden. J Med Econ 2017;20:1136–47. 10.1080/13696998.2017.1361960 [DOI] [PubMed] [Google Scholar]
- 24. Burudpakdee C, Wong W, Seetasith A, et al. Economic impact of preventing brain metastases with alectinib in ALK-positive non-small cell lung cancer. Lung Cancer 2018;119:103–11. 10.1016/j.lungcan.2018.03.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2018-000414supp001.pdf (241.6KB, pdf)