Abstract
BACKGROUND:
The Acute Pain Service (APS) was initially introduced to optimize multimodal postoperative pain control. The aim of this study was to evaluate the association between the implementation of an APS and postoperative pain management and outcomes for patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC).
METHODS:
In this propensity-matched retrospective cohort study, we performed a before–after study without a concurrent control group. Outcomes were compared among patients undergoing CRS-HIPEC when APS was implemented versus historical controls (non-APS). The primary objective was to determine if there was a decrease in median total opioid consumption during postoperative days 0–3 among patients managed by the APS. Secondary outcomes included opioid consumption on each postoperative day (0–6), time to ambulation, time to solid intake, and hospital length of stay.
RESULTS:
After exclusion, there were a total of 122 patients, of which 51 and 71 were in the APS and non-APS cohort, respectively. Between propensity-matched groups, the median (quartiles) total opioid consumption during postoperative days 0–3 was 27.5 mg intravenous morphine equivalents (MEQs) (7.6–106.3 mg MEQs) versus 144.0 mg MEQs (68.9–238.3 mg MEQs), respectively. The median difference was 80.8 mg MEQs (95% confidence interval, 46.1–124.0; P < .0001). There were statistically significant decreases in time to ambulation and time to solid diet intake in the APS cohort.
CONCLUSIONS:
After implementing the APS, CRS-HIPEC patients had decreased opioid consumption by >50%, as well as shorter time to ambulation and time to solid intake. Implementation of an APS may improve outcomes in CRS-HIPEC patients.
KEY POINTS.
Question: Is the implementation of a dedicated Acute Pain Service, that manages all aspects of multimodal analgesia during the perioperative period, associated with reduced opioid consumption after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC)?
Findings: Compared to historical controls, the implementation of an Acute Pain Service was associated with >50% reduction in total opioid consumption during the first 4 hospital days after CRS-HIPEC.
Meaning: Services such as this should be seriously considered in institutions performing CRS-HIPEC and other major surgeries to help reduce opioid consumption and related side effects.
Poorly controlled postoperative pain often leads to increased stress response, which in turn affects various organ systems leading to reduced quality of life, longer hospital stays, increased costs, and decreased patient satisfaction.1–3 Postoperative pain significantly affects patients’ recovery, and the majority of patients reports it as the biggest source of anxiety when undergoing a surgical procedure. Despite developments in new pain management protocols and incorporation of pain as the fifth vital sign, 80% of patients continue to report suboptimal acute pain management.4 Likewise, patients with persistently poorly controlled pain throughout their admission are more likely to have 30-day readmission or subsequent emergency department visits.5
Opioid analgesics remain the mainstay in the treatment of postoperative pain; however, they are associated with adverse drug events.6 The use of multimodal analgesia has been shown to limit the amount of opioids consumed and provides more effective pain control than opioids alone. Current evidence suggests that multimodal analgesia should be the standard of care.7 Practice guidelines for perioperative pain management recommend that multimodal therapy should be used in all postsurgical patients.8 Hospitals have begun to introduce a multidisciplinary Acute Pain Service (APS) to increase awareness of the importance of perioperative opioid-sparing analgesia and to create surgery-specific analgesic protocols that reflect this multimodal approach.
APS has been linked to decreases in pain intensity levels, improved patient satisfaction scores, and fewer adverse events.9 In October 2015, our institution, in response to the high postoperative opioid consumption and poor patient satisfaction rates, endorsed the development of APS. We are unaware of any other studies that evaluate the impact of APS on opioid consumption in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC).
CRS-HIPEC is a complex surgical procedure requiring xiphoid to pubis incision for adequate tumor debulking and delivery of high-dose hyperthermic chemotherapy to the abdominal cavity. It is considered one of the most painful major abdominal procedures, and thus thoracic epidural analgesia has been a mainstay of the postoperative management at our institution. In this retrospective study, we hypothesized that the establishment of a dedicated service focused on multimodal opioid-sparing management, including placement and management of thoracic epidural analgesia, would have a positive association with a reduction of postoperative opioid requirements in patients undergoing CRS-HIPEC.
MATERIAL AND METHODS
Study Sample and Design
Data were collected retrospectively from the data warehouse of the University of California, San Diego Healthcare Systems. All data from surgical patients that were scheduled for a CRS-HIPEC from January 1, 2014, to December 1, 2016, were extracted. The resulting dataset remained deidentified and did not contain sensitive patient-health information as defined by the University of California, San Diego, Human Research Protections Program, and therefore, was exempt from the informed consent requirement and approved by our Institutional Review Board. This is a retrospective cohort study, in which we conducted a before–after study without a concurrent control group. The primary objective was to determine if there was a decrease in median total opioid consumption during postoperative days (PODs) 0–3 among patients managed by APS. We chose this as the primary outcome because it is during the first few days after CRS-HIPEC that patients exhibit the most pain and are aggressively attempting to reach milestones. Secondary outcomes included opioid consumption during each POD (up until POD 6), antiemetic use during PODs 0–6, hospital length of stay, intensive care unit (ICU) length of stay, time to ambulation, time of bladder catheter removal, time to first solid oral intake, number of unsuccessful epidural placements (defined as failure of placing an epidural catheter after multiple attempts), and duration of indwelling epidural catheters. The manuscript adheres to the applicable Enhancing the Quality And Transparency Of health Research (EQUATOR) guidelines.
Establishment of the APS
Thoracic epidural analgesia has been a mainstay for postoperative pain control in CRS-HIPEC patients in our institution since the surgical program was established. Before October 2015, all such epidurals were placed by the operating room (OR) anesthesia team in the OR before induction of anesthesia. Once the patient arrived in the postanesthesia care unit, the regional anesthesia team managed epidural catheters until the day of removal. The primary surgical team was responsible for all other pain management (ie, opioid orders).
APS began in October 2015 by a group of 6 anesthesiologists of regional anesthesia as well as chronic pain backgrounds. The service consisted of a single attending anesthesiologist provider on a 24-hour service for 7 days at a time. There was no trainee involvement. APS implemented an opioid-sparing pain management regimen that included patient identification and communication with the surgical attending 1 day before surgery (Figure 1). APS placed all thoracic epidurals with sensory block to ice confirmation in the preoperative holding area, and managed all infusions until catheter removal. In addition, APS provided intraoperative opioid-sparing pain management recommendations to the OR anesthesia team, which included suggestions for intraoperative epidural management, preoperative gabapentinoid, nonopioid adjuvants, and antiemetic utilization. Furthermore, in contrast to the pre-APS era, APS was now responsible for prescribing and managing all of the patients’ pain regimen until 1-day postepidural catheter removal and/or the transition to oral pain medications. Postoperative management included utilization of nonopioid adjuvants (ie, lidocaine patches, acetaminophen, nonsteroidal anti-inflammatory drugs), management of thoracic epidural infusions, and opioid management. All patients received lidocaine patches and around-the-clock acetaminophen. Ketorolac was only used occasionally after approval from surgical team. APS maintained open communication with surgical team and conducted twice daily rounds on patients (not always with the surgical team). To the best of our knowledge, there have not been any hospital policy changes for inpatient pain management during the study period. Likewise, there have not been any surgical staffing changes in that time frame.
Figure 1.
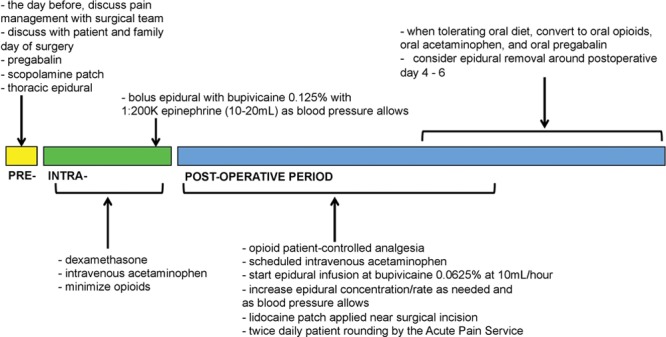
Diagram illustrating the multimodal approach by the Acute Pain Service for postoperative opioid-sparing analgesia for patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy.
Data Collection
All patients with peritoneal metastatic disease who were scheduled for a CRS-HIPEC from January 1, 2014, to December 1, 2016, were extracted. All cases who were performed laparoscopically, aborted intraoperatively, or did not receive HIPEC were excluded from the analysis. Furthermore, only patients who were offered a thoracic epidural were included. Some patients were not considered for an epidural due to relative/absolute contraindications or patient/surgeon refusal. All patients with chronic pain (identified by International Classification of Diseases, 9th Revision, diagnosis code for “chronic pain,” patients using oral oxycodone >20 mg equivalents per day, or patients using fentanyl patches) were excluded. Figure 2 illustrates the exclusion/inclusion methodology. All cases performed before October 2015 were considered non-APS, while patients after that time period were APS. For each case, total opioid use (intravenous morphine equivalents [MEQs]) was calculated during each day from PODs 0–6. Total antiemetic use (ondansetron, metoclopramide, and promethazine), defined as number of times a patient received an antiemetic, was calculated for PODs 0–6. We analyzed the differences in opioid and antiemetic use for each day to further assess specifically which days had the biggest impact from APS. The number of days until patient first ambulated and tolerated solid oral diet was recorded. Total hospital length of stay and postoperative ICU length of stay were collected. Other outcomes collected included days until epidural was removed/dislodged, occurrence of an unsuccessful epidural placement (defined as failure to place epidural catheter after multiple attempts), and days until bladder catheter removal.
Figure 2.
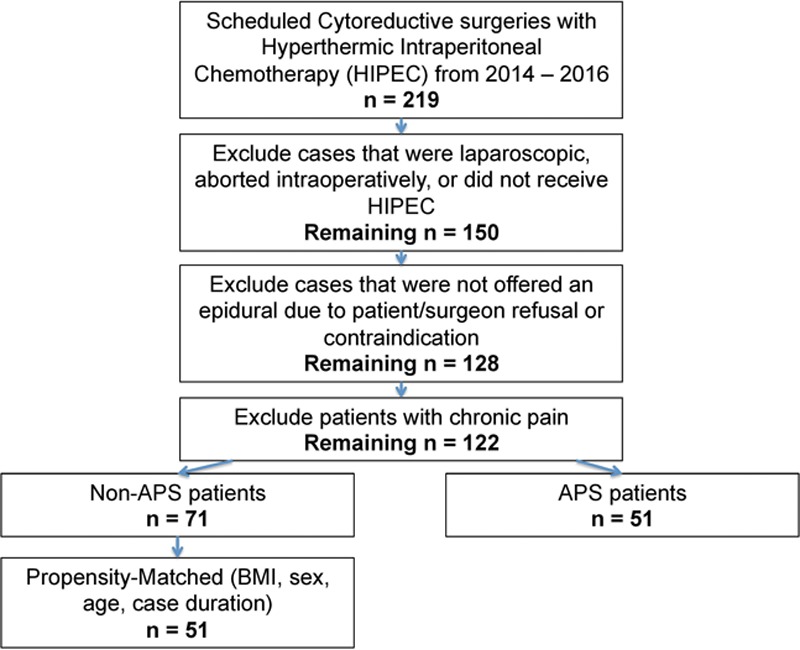
Exclusion and inclusion methodology. APS indicates Acute Pain Service; BMI, body mass index; HIPEC, hyperthermic intraperitoneal chemotherapy.
Statistical Analysis
R, a software environment for statistical computing (R version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria), was used to perform all statistical analyses. The primary outcome of interest was median total opioid consumption during PODs 0–3. There were no missing values for any of the covariates or outcomes of interest. The “MatchIt” package for R statistical software was used for propensity score matching; it improves statistical models by preprocessing data with nonparametric matching methods.10 A 1:1 propensity score matching method using nearest neighbor matching without replacement was utilized to create matched cohorts. The caliper was set at 0.2 SDs of the logit of the estimated propensity score. Thus, the nearest match between subjects from each cohort (based on propensity score) within a subset of potential patients (that are within the selected caliper range) was matched together. Propensity score for cohort (APS versus non-APS) was determined using logistic regression based on age, body mass index (BMI), sex, and case duration (as these are potential confounders for postoperative pain). To determine balance between matched groups, an absolute standardized mean difference that is <0.2 for each variable was considered adequate. These propensity-matched groups were then used for the primary analysis. Of note, all patients in our study population were assigned an American Society of Anesthesiologists physical status score of 3, underwent a surgical incision from the xiphoid to pubis, and each CRS-HIPEC was performed by one of 3 surgical oncology specialists.
Because we are comparing 2 cohorts from 2 distinct time periods, we assessed change in the primary outcome over time in the pre-APS and the APS cohort. The purpose was to (1) assess whether there was a linear trend over time in the primary outcome before APS was implemented; (2) compare that trend with what was observed after APS was implemented; and (3) assess whether there is evidence of a change in the primary outcome in the short period after APS was implemented compared to just before it was implemented. To do this, we performed segmented regression to estimate and compare slopes and assess for a change in the mean of the total outcome just before APS implementation.11 The following linear regression model was performed:
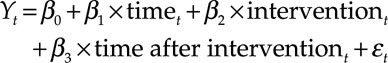 |
Where Yt is the mean total opioid consumption (POD 0–3) in time t; “time” is a continuous variable indicating time in days since start of study period; “intervention” is an indicator for time t occurring before implementation of APS or after; and “time after intervention” is a continuous variable counting the number of days after APS was implemented at time t. In this segmented regression model, β0 estimates the mean opioid consumption at time zero; β1 estimates the change in mean opioid consumption before APS was implemented; β2 estimates the level change in opioid consumption immediately after the intervention; and β3 estimates the change in the trend in mean opioid consumption after APS was implemented.11
If the results suggest that there was no improvement in the trend in the primary outcome before APS was implemented and that there was positive evidence of change in the primary outcome immediately after APS was implemented, this would suggest that APS indeed was an important component of any changes in opioid consumption that may have occurred. Therefore, the next step would be to demonstrate improved outcomes in the APS group versus historical controls. Between matched cohorts, Wilcoxon rank sum test was used to calculate the difference in the primary outcome (total opioid consumption during PODs 0–3), as the data followed a skewed distribution. The 25% and 75% quartiles were reported with each median value. The median difference and 95% confidence interval (CI) were calculated using the Hodges–Lehman estimator. A Welch 2-sample t test and a Pearson χ2 test were utilized to measure differences between continuous and categorical variables, respectively, for the secondary outcomes. A P value of <.05 was considered statistically significant. For each POD, a Wilcoxon rank sum test was used to calculate differences in opioid/antiemetic use. Because of multiple comparisons (7 PODs), a P value of <.007 was considered statistically significant when comparing opioid consumption for each day.
Based on historical reports at our institution, the mean (standard deviation) opioid consumption during PODs 0–3 after CRS-HIPEC was 100 mg MEQs (100 mg). With a sample size of 102 (51 in each matched group) and α = .05, we had a power of 0.95 to detect a clinically significant difference of 50% decrease in opioid consumption. We used a t test calculation to estimate power, as distribution of opioid consumption was not known before data collection. After data collection, opioid consumption demonstrated a nonparametric distribution, and therefore, it was determined to use Wilcoxon rank sum test as the primary statistic.
RESULTS
There were a total of 219 patients scheduled for CRS-HIPEC at this single institution from 2014 to 2016. After exclusion, there were a total of 122 patients, of which 51 and 71 were in the APS and non-APS cohort, respectively. In an unadjusted analysis, total opioid consumption during PODs 0–3 was lower for APS, with median (quartiles) 27.5 mg (7.6–106.3 mg) MEQs versus 131.7 mg (65.7–212.9 mg) MEQs for non-APS, with median difference of 67.8 mg MEQs (95% CI, 37.7–106.7; P < .0001).
Propensity-matched cohorts based on age, BMI, sex, and case duration were developed; there were no clinically important differences in these variables between cohorts based on the absolute standardized mean difference (Table 1). Figure 3 illustrates the trend in total opioid consumption (PODs 0–3) based on date/time of surgery (ordered chronologically) in the historical control (non-APS) or APS cohorts. Segmented regression analysis demonstrated that (1) just before the beginning of the study period, the mean opioid consumption (PODs 0–3) was 153.9 mg (P < .0001); (2) before initiation of APS, there was no significant day-to-day change in mean opioid consumption for PODs 0–3 (β coefficient = .05; P = .62); (3) immediately after the initiation of APS, the estimated mean opioid consumption (PODs 0–3) dropped abruptly by 138.7 mg (P = .007); and (4) there was no significant change in the day-to-day trend in the mean total opioid consumption (PODs 0–3) after implementation of APS (β coefficient = 0.11; P = .53).
Table 1.
Demographics of Patients in APS and Non-APS Cohorts (Unmatched and Propensity-Matched Groups)
Figure 3.
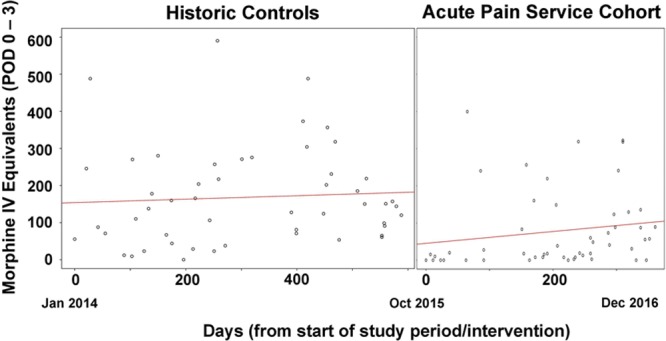
Trends in total opioid consumption during PODs 0–3 during the historical control and Acute Pain Service time periods. Each dot represents a patient ordered chronologically based on day since start of study period. IV indicates intravenous; POD, postoperative day.
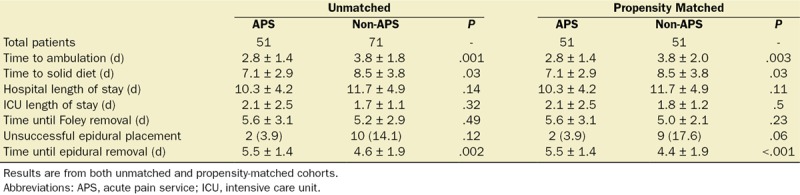
Between propensity-matched groups, the median (quartiles) total opioid consumption during PODs 0–3 was 27.5 mg MEQs (7.6–106.3 mg MEQs) versus 144.0 mg MEQs (68.9–238.3 mg MEQs), respectively. The median difference was 80.8 mg MEQs (95% CI, 46.1–124.0; P < .0001). Immediately after initiation of APS (4 months after implementation), there was a median difference of 90.86 mg MEQs (95% CI, 53.1–142.4 mg MEQs; P < .0001), in favor of the APS cohort. Figure 4 demonstrates a statistically significant decrease in median opioid consumption for each POD from 0 to 4 in the APS cohort. There was no difference between cohorts in terms of antiemetic use during all PODs studied. Table 2 lists differences in various outcomes. Specifically, time to ambulation in the APS versus non-APS group was 2.8 vs 3.8 days, respectively (P = .003); time to solid diet tolerance was 7.1 vs 8.5 days, respectively (P = .03); and duration of indwelling epidural catheter was 5.5 vs 4.6 days, respectively (P < .001).
Figure 4.
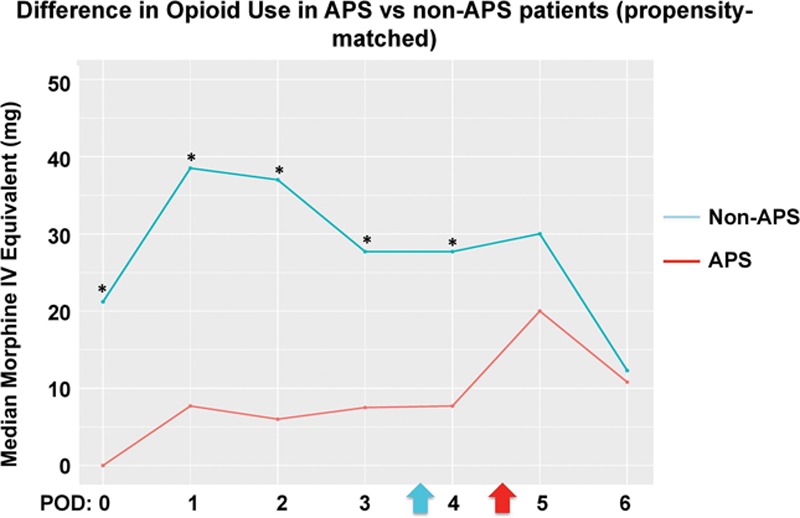
Difference in median total opioid use (mg) on each POD in patients managed by the APS versus historical controls (propensity matched). Blue arrow represents mean day at which epidural was removed/dislodged in historical controls. Red arrow represents mean day at which epidural was removed/dislodged in the APS group. *P < .007 (adjusted for multiple comparisons). APS indicates Acute Pain Service; IV, intravenous; POD, postoperative day.
Table 2.
Differences in Outcomes Between APS Versus Non-APS Cohorts
We performed a subgroup analysis in which we only analyzed patients who had an epidural for at least 3 days. After propensity matching, the median (quartiles) of the total opioid consumption during PODs 0–3 in the APS versus non-APS patients was 20.0 mg MEQs (7.5–88.4 mg MEQs) versus 87.8 mg MEQs (53.5–185.2 mg MEQs), respectively (P < .0001), with a median difference of 53.5 mg MEQs (95% CI, 26.2–80.9 mg MEQs). There were similar improvements in time to ambulation, time to solid diet intake, and epidural duration.
DISCUSSION
In patients undergoing CRS-HIPEC, we were able to demonstrate >50% decrease in median opioid consumption during PODs 0–3, decrease in time to ambulation, and a decrease in time to solid oral intake. Even among only patients who had an epidural for at least 3 days, there was still an improvement in opioid consumption. We highlighted the value of a dedicated APS in achieving system-wide practice change and reaching quality improvement.
A recent meta-analysis had shown that epidural analgesia provided superior postoperative analgesia, decreased perioperative pulmonary-cardiac morbidity, and facilitated earlier return of gastrointestinal tract function in comparison to systemic analgesia.12 At our institution, thoracic epidural analgesia has always been the standard for patients undergoing CRS-HIPEC. We attribute the significant decrease in opioid consumption, earlier time to ambulation and solid food intake to practice changes implemented by APS. For example, every thoracic epidural placement occurred in the preoperative holding area, under the supervision of or direct placement by the APS attending anesthesiologist. All patients had a confirmed sensory block before leaving the preoperative holding area. Early epidural placement in the preoperative holding area ensured that patients had a functional epidural before surgery. In the non-APS group, epidural placement occurred in the OR, which did not allow for proper testing or troubleshooting of an epidural catheter. Likewise, APS intraoperative recommendations included bolusing epidural catheters with bupivacaine 20–30 minutes before emergence from anesthesia. Non-APS patients’ intraoperative and epidural pain management was at the discretion of the OR anesthesia team. For patients managed by APS, the on-service attending evaluated the patient in the recovery room and managed the epidural infusion and all opioid and nonopioid adjuvant medications. Non-APS patient epidurals were managed by the regional anesthesia team, and the surgical team was separately responsible for all other pain management needs. Comprehensive pain management by a single consistent dedicated team is, therefore, key to the minimization of opioids.
In addition to decreased opioid consumption, our study also demonstrated earlier time to ambulation, which may be attributed to improved pain control and fewer opioid-related side effects. This milestone plays a major role in recovery for CRS-HIPEC surgery as it promotes improved respiratory function and earlier return of bowel function. Likewise, there was a significant decrease in time to solid intake, which is an appropriate finding in the setting of earlier ambulation and reduced opioid consumption. Osseis et al13 demonstrated that a comprehensive physiotherapy program to promote early ambulation alongside thoracic epidural analgesia significantly benefits patient’s postoperative recovery and reduces the length of stay in the ICU. We did not demonstrate statistically significant differences in hospital length of stay between groups. Hospital length of stay and cost of care are multifactorial, so it is difficult to identify pain management alone as a contributor.9 In the CRS-HIPEC patient population, this is a particularly challenging outcome to study given their type of cancer, prognosis, massive fluid shifts, significant inflammation, and management of gastric tube clamping.
With appropriate resources, the protocol and results achieved by APS can potentially be replicated at other institutions. It has become progressively clear that the problem of undertreated postoperative pain is not secondary to absence of effective analgesics or nonpharmacologic techniques, but rather the lack of an organized dedicated team of personnel to optimize these existing multimodal therapies in addition to the lack of specific care bundles.14 This multidisciplinary-driven approach implementing well-designed protocols/guidelines has been used in successful enhanced recovery after surgery interventions.15 In addition, this patient-centered, physician-led, team-based approach is the cornerstone of the Perioperative Surgical Home, which strives to improve perioperative outcomes and reduce cost.16
A big limitation to the establishment and maintenance of a dedicated team, such as APS, is financial constraints. Resources such as designated personnel for 24-hour service, written protocols and policies for pain management, as well as ongoing patient and nursing education continue to be limited in APS programs worldwide.17 We believe that a more appropriate way of addressing this constraint is to view APS as a cost-saving rather than a money-generating service. Opioid-related adverse events after surgery are associated with increased hospital costs and length of stay.3 Comprehensive cost/benefit analysis of a 24-hour, dedicated APS is beyond the scope of this study and represents an important area of future research.
This study has several limitations, one of which is related to the retrospective design. The non-APS patients received care during a different time period, and therefore, it was difficult to control for unforeseen biases in clinical practice during these 2 distinct time points. Data were collected via manual medical chart abstraction, which relies on accurate documentation from nursing staff, physical therapy, and faculty. Ideally, such a study would be performed as a prospective randomized controlled trial; however, randomizing patients to the management of an APS or non-APS would prove unrealistic. Because the benefits of the APS, on this retrospective analysis, are compelling, it would be unethical to deprive future CRS-HIPEC patients of it for the purpose of prospective research. To test the hypothesis that a dedicated team such as APS was instrumental in affecting opioid consumption, one would have to compare driving those care bundle components by a nondedicated team. Due to the retrospective design, we were unable to accurately collect reported patient scores as these values are not recorded uniformly among nursing providers. Nonetheless, we chose opioid consumption as our primary outcome as this variable is more objective. We chose well-known confounders for opioid consumption (age, sex, BMI, and case duration); however, there are other possible confounders not included in our analysis for opioid consumption. We did, however, exclude all patients with chronic pain or preoperative opioid use and include all patients who had similar comorbidity burden based on the American Society of Anesthesiologists physical status score.
Furthermore, this is a single-center study focusing on a highly complex and specialized surgical procedure as CRS-HIPEC; we will need future studies to determine if similar results can be duplicated in the settings of less complex procedures and different patient populations. Likewise, future studies are needed to evaluate if this decrease use of opioids in the acute phase translates into long-term decreased opioid consumption and reduction in side effects of high-dose opioids.
CONCLUSIONS
Opioid-sparing techniques, including thoracic epidurals for major abdominal surgery such as CRS-HIPEC, are not novel. In this study we show that the implementation of a dedicated service that utilizes and organizes these already established multimodal opioid-sparing analgesic techniques is associated with a decrease in opioid consumption by more than 50% until POD 3, as well as improved time to ambulation and time to solid intake in patients who had CRS-HIPEC surgery. Services such as this should be seriously considered in institutions performing CRS-HIPEC and other major surgeries, to help reduce opioid consumption and related side effects.
DISCLOSURES
Name: Engy T. Said, MD.
Contribution: This author helped design and conduct the study, collect and analyze the data, and prepare the manuscript.
Conflicts of Interest: E. T. Said is an attending anesthesiologist on the Acute Pain Service.
Name: Jacklynn F. Sztain, MD.
Contribution: This author helped design and conduct the study, collect and analyze the data, and prepare the manuscript.
Conflicts of Interest: J. F. Sztain is an attending anesthesiologist on the Acute Pain Service.
Name: Wendy B. Abramson, MD.
Contribution: This author helped design the study and prepare the manuscript.
Conflicts of Interest: W. B. Abramson is an attending anesthesiologist on the Acute Pain Service.
Name: Minhthy N. Meineke, MD.
Contribution: This author helped design the study and prepare the manuscript.
Conflicts of Interest: M. N. Meineke is an attending anesthesiologist on the Acute Pain Service.
Name: Timothy J. Furnish, MD.
Contribution: This author helped design the study and prepare the manuscript.
Conflicts of Interest: T. J. Furnish is an attending anesthesiologist on the Acute Pain Service.
Name: Ulrich H. Schmidt, MD, PhD, MBA.
Contribution: This author helped design the study, analyze the data, and prepare the manuscript.
Conflicts of Interest: None.
Name: Gerard R. Manecke, MD.
Contribution: This author helped design the study, analyze the data, and prepare the manuscript.
Conflicts of Interest: None.
Name: Rodney A. Gabriel, MD, MAS.
Contribution: This author helped design and conduct the study, collect and analyze the data, and prepare the manuscript.
Conflicts of Interest: R. A. Gabriel is an attending anesthesiologist on the Acute Pain Service.
This manuscript was handled by: Honorio T. Benzon, MD.
Footnotes
Published ahead of print March 27, 2018.
Funding: None.
Conflicts of Interest: See Disclosures at the end of the article.
This work has been presented, in part, as an oral presentation at the Enhanced Recovery After Surgery US Conference, November 10, 2017, Dallas, TX.
E. T. Said and J. F. Sztain contributed equally to this manuscript.
Reprints will not be available from the authors.
REFERENCES
- 1.Gandhi K, Heitz JW, Viscusi ER. Challenges in acute pain management. Anesthesiol Clin. 2011;29:291–309.. [DOI] [PubMed] [Google Scholar]
- 2.Lucas CE, Vlahos AL, Ledgerwood AM. Kindness kills: the negative impact of pain as the fifth vital sign. J Am Coll Surg. 2007;205:101–107.. [DOI] [PubMed] [Google Scholar]
- 3.Oderda GM, Said Q, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41:400–406.. [DOI] [PubMed] [Google Scholar]
- 4.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–540.. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez-Boussard T, Graham LA, Desai K, et al. The fifth vital sign: postoperative pain predicts 30-day readmissions and subsequent emergency department visits. Ann Surg. 2017;266:516–524.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vila H, Jr, Smith RA, Augustyniak MJ, et al. The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: is patient safety compromised by treatment based solely on numerical pain ratings? Anesth Analg. 2005;101:474–480.. [DOI] [PubMed] [Google Scholar]
- 7.White PF, Kehlet H. Improving postoperative pain management: what are the unresolved issues? Anesthesiology. 2010;112:220–225.. [DOI] [PubMed] [Google Scholar]
- 8.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273.. [DOI] [PubMed] [Google Scholar]
- 9.Werner MU, Søholm L, Rotbøll-Nielsen P, Kehlet H. Does an acute pain service improve postoperative outcome? Anesth Analg. 2002;95:1361–1372.. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z. Propensity score method: a non-parametric technique to reduce model dependence. Ann Transl Med. 2017;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309.. [DOI] [PubMed] [Google Scholar]
- 12.Pöpping DM, Elia N, Van Aken HK, et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2014;259:1056–1067.. [DOI] [PubMed] [Google Scholar]
- 13.Osseis M, Weyrech J, Gayat E, et al. Epidural analgesia combined with a comprehensive physiotherapy program after cytoreductive surgery and HIPEC is associated with enhanced post-operative recovery and reduces intensive care unit stay: a retrospective study of 124 patients. Eur J Surg Oncol. 2016;42:1938–1943.. [DOI] [PubMed] [Google Scholar]
- 14.Rawal N. Current issues in postoperative pain management. Eur J Anaesthesiol. 2016;33:160–171.. [DOI] [PubMed] [Google Scholar]
- 15.Grant MC, Sommer PM, He C, et al. Preserved analgesia with reduction in opioids through the use of an acute pain protocol in enhanced recovery after surgery for open hepatectomy. Reg Anesth Pain Med. 2017;42:451–457.. [DOI] [PubMed] [Google Scholar]
- 16.Zaccagnino MP, Bader AM, Sang CN, Correll DJ. The perioperative surgical home: a new role for the acute pain service. Anesth Analg. 2017;125:1394–1402.. [DOI] [PubMed] [Google Scholar]
- 17.Stamer UM, Mpasios N, Stüber F, Maier C. A survey of acute pain services in Germany and a discussion of international survey data. Reg Anesth Pain Med. 2002;27:125–131.. [DOI] [PubMed] [Google Scholar]


