Abstract
BACKGROUND:
Despite its central role in early trauma coagulopathy, abnormal fibrinolysis continues to be poorly understood. Excessive fibrinolysis is a known contributor to mortality. Recent studies with thromboelastography (TEG) suggest decreased fibrinolysis (or shutdown) may be just as harmful. Considering the broad use of 2 different viscoelastic assays, which are not interchangeable, we proposed for the first time to define and characterize fibrinolysis shutdown using rotational thromboelastometry (ROTEM).
METHODS:
Retrospective cohort study of severely injured patients with admission ROTEM. Shutdown was defined by the best Youden index value of the maximum lysis. Fibrinolysis phenotypes were physiologic, hyperfibrinolysis, and shutdown. Multivariable logistic regression evaluated association between Injury Severity Score and the fibrinolysis phenotypes, and the association among shutdown phenotype with mortality, blood transfusion, and thrombotic events.
RESULTS:
Five hundred fifty patients were included. Maximum lysis <3.5% was selected to define shutdown. Predominant phenotype was physiologic (70.7%), followed by shutdown (25.6%) and hyperfibrinolysis (3.6%). Shutdown patients had higher Injury Severity Score, lower base excess, and required more transfusions than physiologic group. Shutdown was associated with acidosis (base excess: odds ratio [OR] for a 1 mEq/L increase, 0.93; 95% confidence interval [CI], 0.88–0.98; P = .0094) and the combination of clotting derangements, higher clot firmness (maximum clot formation: OR for a 2 mm increase, 1.8; 95% CI, 1.5–2.27; P < .0001), lower fibrinogen (OR for a 0.5 g/dL decrease, 1.47; 95% CI, 1.18–1.84; P = .0006), and poor clot formation dynamics (clot formation time: OR for a 5 seconds increase, 1.25; 95% CI, 1.15–1.36; P < .0001). Fibrinolysis shutdown was not independently associated with mortality (OR, 0.61; 95% CI, 0.28–1.33; P = .21), massive transfusion (OR, 2.14; 95% CI, 0.79–5.74; P = .1308), or thrombotic events (OR, 1.08; 95% CI, 0.37–3.15; P = .874). Shutdown was associated with increased 24-hour transfusion (OR, 2.24; 95% CI, 1.24–4.04; P = .007).
CONCLUSIONS:
Despite higher injury burden, evidence of shock, and greater need for blood transfusions, early fibrinolysis shutdown was not associated with mortality, suggesting that it could represent an adaptive physiologic response to life-threatening trauma.
KEY POINTS.
Question: Posttrauma fibrinolysis shutdown was recently described using thromboelastography analyzer TEG and associated to increased mortality. We sought to define fibrinolysis shutdown with rotational thromboelastometry or ROTEM because these viscoelastic tests are not always interchangeable and are used independently by different trauma centers.
Findings: Using the same methods as described for TEG, the ROTEM maximum lysis <3.5% defined fibrinolysis shutdown. Shutdown patients had higher injury severity, worse acidosis, and significant hemostatic activation with concomitant fibrinogen consumption and poor clot formation dynamics. Despite the association of shutdown and the need for early blood transfusions, shutdown was not associated with mortality.
Meaning: Fibrinolysis shutdown (decreased clot degradation) in severely injured patients at hospital admission may not be necessarily harmful but may potentially represent a physiologic response to life-threatening injuries.
Bleeding and thrombotic complications remain leading causes of preventable deaths after traumatic injury.1,2 The mechanisms driving trauma-induced coagulopathy are complex and heterogeneous, from early critical deficits of fibrinogen and other clotting factors,3,4 endotheliopathy, to excessive fibrinolysis.5–8
Abnormal fibrinolysis is a key contributor to trauma-induced coagulopathy. In 2010, the Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage 2 (CRASH-2) trial demonstrated a reduced mortality in bleeding trauma patients with the empirical administration of an antifibrinolytic drug, suggesting that fibrinolysis could be harmful to this population.9 Little is known about the mechanisms regulating the postinjury fibrinolysis response. A murine model showed a divergent fibrinolysis response after different insults. While ischemia led to an overwhelming clot degradation (hyperfibrinolysis), tissue destruction triggered the opposite, fibrinolysis suppression or shutdown.10 Viscoelastic tests like thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are among the few diagnostic tools capable of evaluating fibrinolysis during trauma resuscitation because conventional fibrinolysis assays are complex and often unavailable after hours. Thromboelastography contributed to establish the association of hyperfibrinolysis with mortality,11,12 massive transfusion,13,14 and organ dysfunction.8,15 While the link between hyperfibrinolysis and poor outcomes is well recognized, the potential harm of fibrinolysis shutdown is a recent concept. In 2014, Moore et al16 defined fibrinolysis shutdown using TEG LY30 value below 0.9% (LY30 is the percentage of clot amplitude reduction at 30 minutes after the maximum clot amplitude) in 180 severely injured patients. Among this cohort, shutdown was diagnosed in 63% of the patients, and the mortality incidence across the fibrinolysis spectrum followed a U-shaped curve with higher mortality at both extremes (shutdown and hyperfibrinolysis), and a plateau in the physiologic range.16 Subsequently, a bi-institutional cohort of 2540 patients showed similar results and the independent association of fibrinolysis shutdown with mortality.17
To date, all studies describing postinjury fibrinolysis shutdown have exclusively used TEG.16–19 Because the results of TEG and ROTEM are not interchangeable,20 and because of the lack of studies with the latter, our primary objective is to establish a cutoff value for fibrinolysis shutdown using ROTEM. Second, we propose an exploratory analysis assessing whether injury severity is associated with fibrinolysis shutdown and the relation between a shutdown phenotype with clinical outcomes in a population of severely injured patients.
METHODS
We performed a retrospective cohort study at St Michael’s Hospital, a level I trauma center located in Toronto, Canada. The study was approved by the Institutional Review Board, and the requirement for written informed consent was waived by the Institutional Review Board. Using the institutional trauma registry, we selected consecutive injured patients admitted to our institution requiring trauma team activation from November 2014 to April 2016. We included patients with severe injuries, defined as 1 or more body area with an Abbreviated Injury Scale (AIS) >2, in addition to a ROTEM assay at hospital admission. We excluded patients with a known bleeding disorder, without evidence of significant traumatic injury, or discharged home within 48 hours of the injury. We collected patient demographics, physiological and coagulation parameters at hospital admission, tranexamic acid (TXA) administration, and clinical outcomes (mortality, any blood product transfusion within the first 24 hours of hospital admission, massive transfusion, and thrombotic events). TXA administration is driven by physician discretion, usual dose 1 g intravenous bolus, with indications including but not limited to patients at risk of significant hemorrhage and within 3 hours of injury, receiving massive transfusion, or with diagnosis of hyperfibrinolysis. We defined massive transfusion as administration of >6 red blood cell units in 4 hours or 10 units within 24 hours. Our institution has a massive transfusion protocol in place; the first blood product delivery consists of 6 units of packed red blood cells, 4 units of fresh frozen plasma, and 1 adult pool of platelets. Thrombotic events were defined as a composite outcome comprising deep venous thrombosis (established by a positive venous duplex ultrasound scanning of the extremities), pulmonary embolism (diagnosed by a positive computed tomography pulmonary angiogram), myocardial infarction (as defined by the Joint European Society of Cardiology/American College of Cardiology definition21), and ischemic stroke (defined as a new neurological deficit corresponding with head computed tomography or magnetic resonance imaging findings involving different territories from the initial injury). The patient’s outcome assessor was unaware of the fibrinolysis group to avoid information bias.
Using a citrated whole blood sample, a ROTEM assay (delta device; Tem Innovations GmbH, Munich, Germany) was performed within 1 hour of hospital arrival by trained technicians of the hospital laboratory, in accordance with the manufacturer’s instructions. Only ROTEM FIBTEM and EXTEM tests were done, and for each, the following measurements were collected (Supplemental Digital Content 1, Figure S1, http://links.lww.com/AA/C313): the time from the test start until the clot amplitude reaches 2 mm, clotting time (seconds); the time between 2 and 20 mm of clot amplitude is reached, clot formation time (CFT, seconds); the maximum clot firmness (MCF, mm); the difference between the MCF and the lowest clot amplitude after MCF is reached, maximum lysis (ML, %); and the ratio of clot amplitude and the MCF at 30 (or 60) minutes after clotting time, lysis index at 30 minutes (LI30) or 60 minutes (LI60).
ML and LI30/LI60 are 2 frequently used ROTEM parameters to measure fibrinolysis. Because there is no exact equivalent for TEG LY30 in ROTEM, we selected EXTEM ML instead of EXTEM LI30 to define fibrinolysis shutdown to avoid including patients with intermediate hyperfibrinolysis11 (patients with total clot lysis within 30 and 60 minutes of the test) during the best cutoff value calculation. Furthermore, ML is the parameter routinely used in our institution for evaluation of fibrinolysis.
Statistical Analysis
Because no other study has defined posttraumatic fibrinolysis shutdown with ROTEM, we used the same methods previously described for TEG. To select the ROTEM cutoff, the area under the receiver operating characteristic curve was calculated for mortality as the dependent variable and EXTEM ML as a predictor variable, excluding patients with hyperfibrinolysis and patients who received TXA before the assay. Then, sensitivity and specificity were obtained for every ML value below 15%; we selected the cut-point after calculating the Youden index (J) for every value (using the formula J = sensitivity + specificity – 1), and selected the cutoff based on the highest J number for discriminating among survivors and nonsurvivors.22
Last, we stratified patients into 3 fibrinolysis groups according to their fibrinolysis value: hyperfibrinolysis, physiologic, and shutdown. The shutdown range was defined as the values below the cutoff point established by the Youden Index. Hyperfibrinolysis was diagnosed with ML values >15% as a previously established cut-point.23 The physiologic group was defined as the intermediate group with ML values above shutdown range and below hyperfibrinolysis threshold. Diagnostic performance of the fibrinolysis shutdown cutoff to discriminate among survivors and nonsurvivors is presented as sensitivity and specificity (percentage) with its associated 95% confidence intervals (CIs).
Descriptive statistics are presented as the median (with first and third quartile) for continuous variables and proportions for categorical variables. To test a statistical difference among 3 groups in median values and proportions, we applied the Kruskal-Wallis test and χ2 test (or Fisher exact test, in case of a low number of patients), respectively. Bonferroni correction was used to determine significance in the setting of multiple comparisons (significance level, α = .016).
To ascertain the association between different fibrinolysis phenotypes (dependent variable) and Injury Severity Scale (ISS) (independent variable), we estimated 3 different multivariable logistic regression models for each phenotype. We selected a priori the independent variables included in the models according to those used in previous studies in the area and others with biological plausibility, to reduce the risk of overfitting.24,25 In detail, the logistic models used to assess association between ISS and for physiologic and fibrinolysis shutdown were adjusted for age, gender, AIS head, mechanism of injury, prehospital TXA administration, base excess, hemoglobin and fibrinogen level, platelets count, international normalized ratio (INR), activated prothrombin time, ROTEM parameters, MCF, and CFT. The hyperfibrinolysis model was adjusted for systolic blood pressure and base excess.
For our exploratory analysis, we assessed the possible association of fibrinolysis (ML used as a continuous independent variable) on death, transfusion in 24 hours, massive transfusion, and thrombotic events. Given that the outcomes of interest were binary, we performed 4 univariate logistic regression models for each outcome and then a multivariable analysis was performed to estimate the association of fibrinolysis shutdown accounting for major confounders. In detail, for the mortality model, we adjusted for age, ISS, AIS head, transfusion within 24 hours, and base excess. For the 24-hour transfusion model, we adjusted for age, ISS, TXA administration, INR, and base excess. In the massive transfusion model, we adjusted for ISS, systolic blood pressure, and hemoglobin level at admission. And last, for the thrombotic events model, we adjusted for age, ISS, and transfusions within 24 hours. We examined the collinearity among variables and influential observations. Results are displayed graphically and presented as the odds ratio (OR) with its 95% CIs. All P values were 2-sided, with P values <.05 considered as statistically significant.
The software used was the R project (R Foundation for Statistical Computing, Vienna, Austria) for statistical computing.26–28 This manuscript adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
RESULTS
From a total of 1680 patients admitted to the St Michael’s Hospital Trauma Centre between November 2014 and April 2016, 1126 patients had a ROTEM on admission. We excluded 528 patients without severe injuries, 5 patients discharged home within 48 hours of the injury, and 43 patients with known bleeding disorders. Thus, 550 patients with severe injuries were analyzed (Supplemental Digital Content 1, Figure S2, http://links.lww.com/AA/C313). The median age was 43 years (interquartile range, 27–60), 72.9% were males, with a median ISS of 19 (interquartile range, 14–26). There were 54 deaths (9.8%), 29 massive transfusions (5.3%), and 17 thrombotic events (3.1%) (Table 1).
Table 1.
Clinical Characteristics According to Fibrinolysis Groups
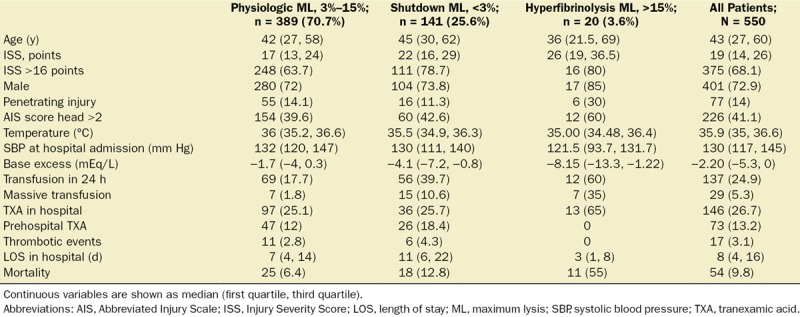
An ML value <3.5% had the best Youden index (J = 0.19) to define fibrinolysis shutdown, with sensitivity 42.5% (95% CI, 27–57) and specificity 76.5% (95% CI, 51–88). The patients were then stratified according to their fibrinolytic response.
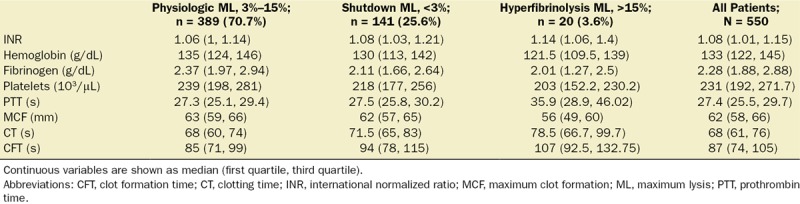
The physiologic fibrinolysis group (ML value, 4%–15%) was the most common phenotype (389 patients, 70.7%), followed by fibrinolysis shutdown (141 patients, 25.6%) and hyperfibrinolysis (20 patients, 3.6%). The baseline characteristics were significantly different across the groups (Table 1). Compared to the physiologic group, hyperfibrinolysis and shutdown patients had higher ISS (17 vs 26 vs 22; P < .001) and lower base excess (−1.7 mEq/L vs −8.1 mEq/L vs −4.1; P < .001). More patients with hyperfibrinolysis and fibrinolysis shutdown received massive transfusions (1.8% vs 10.6% vs 35%; P < .001) and any blood transfusion within the first 24 hours (17.7% vs 37.7% vs 60%; P < .001). Mortality was also significantly different between the groups with the highest mortality observed in the hyperfibrinolysis group (55%), followed by shutdown with a mortality of 12.8%, approximately 2-fold higher than the physiologic phenotype (6.4%; P = .029). Traumatic brain injury was the most common cause of death across all groups, while multiple organ failure was similarly distributed among all 3 phenotypes (Supplemental Digital Content 1–2, Figure S3, http://links.lww.com/AA/C313, Table S1, http://links.lww.com/AA/C314). We observed significant differences in all coagulation tests in all groups (INR, platelets count, hemoglobin, serum fibrinogen levels, and ROTEM parameters; Table 2).
Table 2.
Coagulation Parameters According to Fibrinolysis Groups
Association Between Fibrinolysis Phenotypes and ISS
Using a multivariable logistic regression model for each of the fibrinolysis groups, we found that higher base excess (OR for 1 mEq/L increase, 0.93; 95% CI, 0.88–0.98; P = .0094), lower fibrinogen level (OR for 0.5 g/dL decrease, 1.47; 95% CI, 1.18–1.84; P = .0006), delayed CFT (OR for 5 seconds increase, 1.25; 95% CI, 1.15–1.36; P < .0001), and higher MCF (OR for 2 mm increase, 1.8; 95% CI, 1.5–2.27; P < .0001) were independently associated with shutdown after accounting for age, gender, AIS head, mechanism of injury, prehospital TXA use, hemoglobin level, platelet count, activated prothrombin time, and INR (Table 3 and Figure 1). Moreover, ISS had no significant association with shutdown (OR for 5 points increase, 1.01; 95% CI, 0.89–1.14; P = .7) or the physiologic fibrinolysis response (OR for 5 points increase, 0.94; 95% CI, 0.83–1.06; P = .3) (Supplemental Digital Content 2, Table S2, http://links.lww.com/AA/C314). The variables independently associated with the hyperfibrinolysis phenotype were ISS (OR for 5 points increase, 1.27; 95% CI, 1.09–1.47; P = .0015) and the base excess (OR for 1 mEq/L increase, 0.88; 95% CI, 0.83–0.95; P = .0005) (Supplemental Digital Content 2, Table S3, http://links.lww.com/AA/C314).
Table 3.
Multivariable Logistic Regression Model to Evaluate Association Between ISS and a Fibrinolysis Shutdown Phenotype
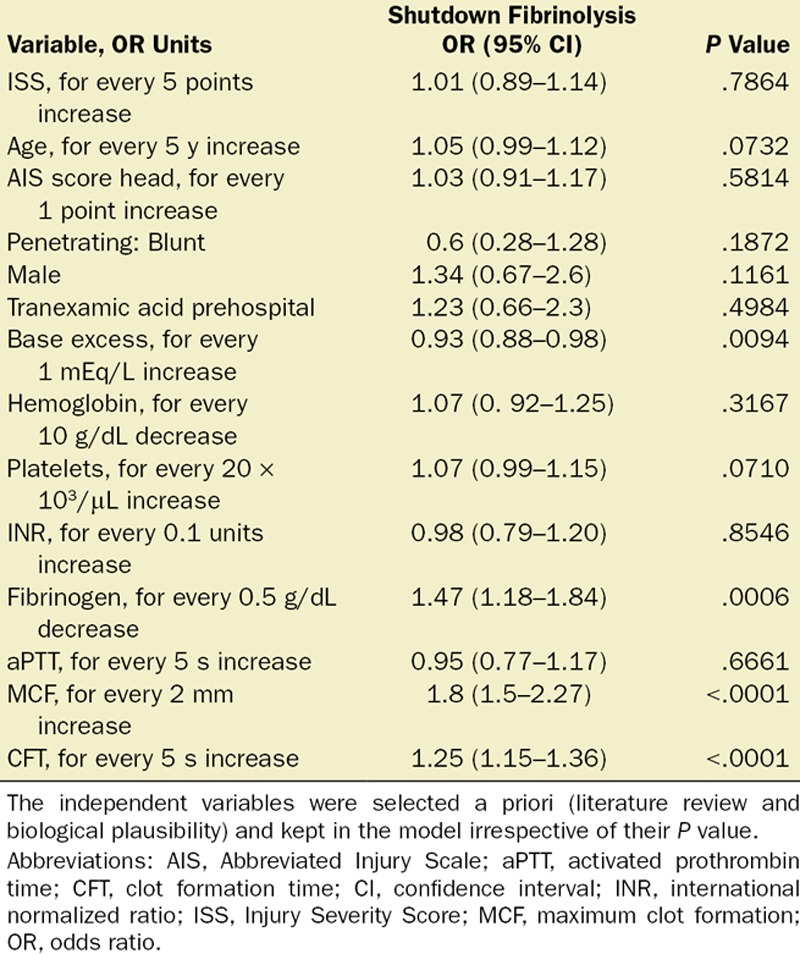
Figure 1.
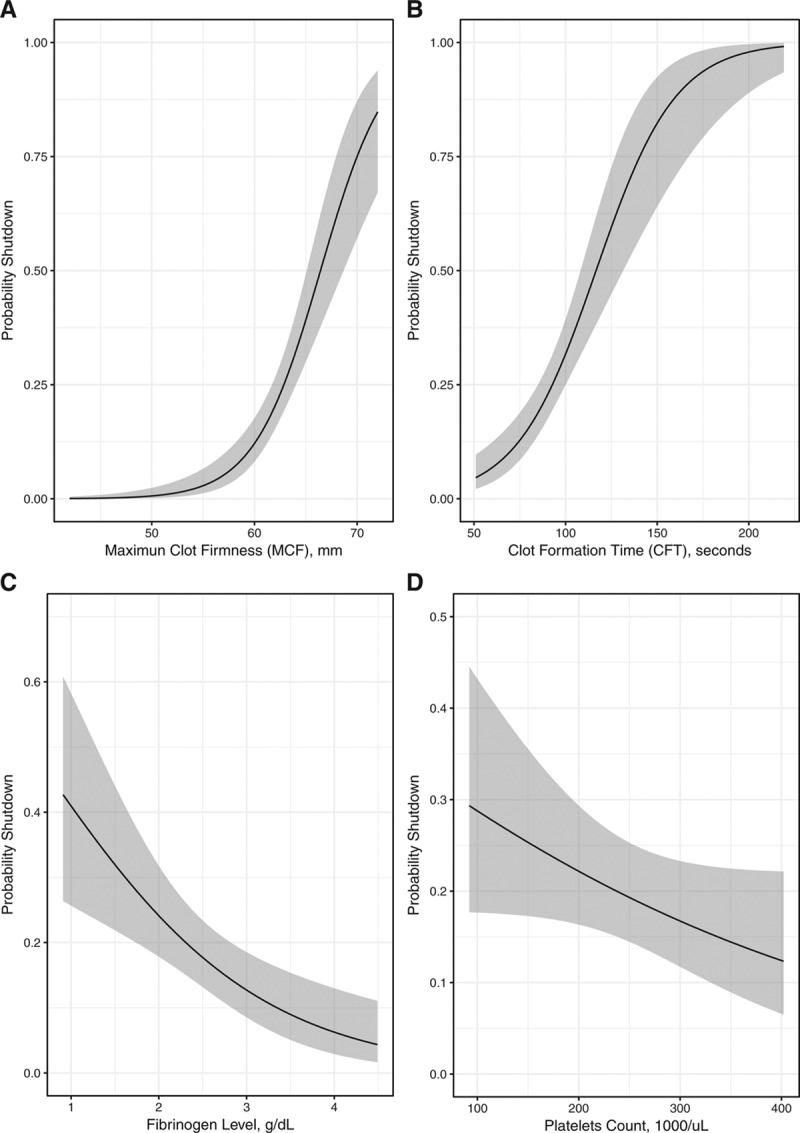
Association between fibrinolysis shutdown and coagulation parameters. The panels show the association of MCF, fibrinogen level, CFT, and platelets count with fibrinolysis shutdown. A logistic model estimated higher probabilities of fibrinolysis shutdown with greater values of MCF (A, OR 1.8 for a 2 mm increase; 95% CI, 1.5–2.27, P < .0001), a delayed CFT (B, OR 1.25 for a 5 s increase; 95% CI, 1.15–1.36, P < .0001), lower fibrinogen (C, OR 1.47 for a 0.5 g/dL decrease; 95% CI, 1.18–1.84, P = .0006), and a higher platelet count (D, OR 1.07 for a 20 × 103/μL increase; 95% CI, 0.99–1.15, P = .07). Association was adjusted for the average patients’ characteristics (age = 43 y, male, no head injury, blunt trauma, base excess = −2.2 mEq/L, hemoglobin level = 133 g/dL, Injury Severity Score = 19, no prehospital tranexamic administration). CFT indicates clot formation time; CI, confidence interval; MCF, maximum clot formation; OR, odds ratio.
Association Between Fibrinolysis Shutdown and Outcomes
The univariable logistic regression models that assessed the association of ML values on each outcome (mortality, 24-hour transfusion, massive transfusion, and thrombotic events) are displayed in Figure 2. The association between ML values and the outcomes of interest results in a J-shaped curve, except for thrombotic events. Thus, the predicted probabilities are higher at the extremes of the fibrinolysis spectrum, notably more elevated in the hyperfibrinolysis than in the shutdown range.
Figure 2.
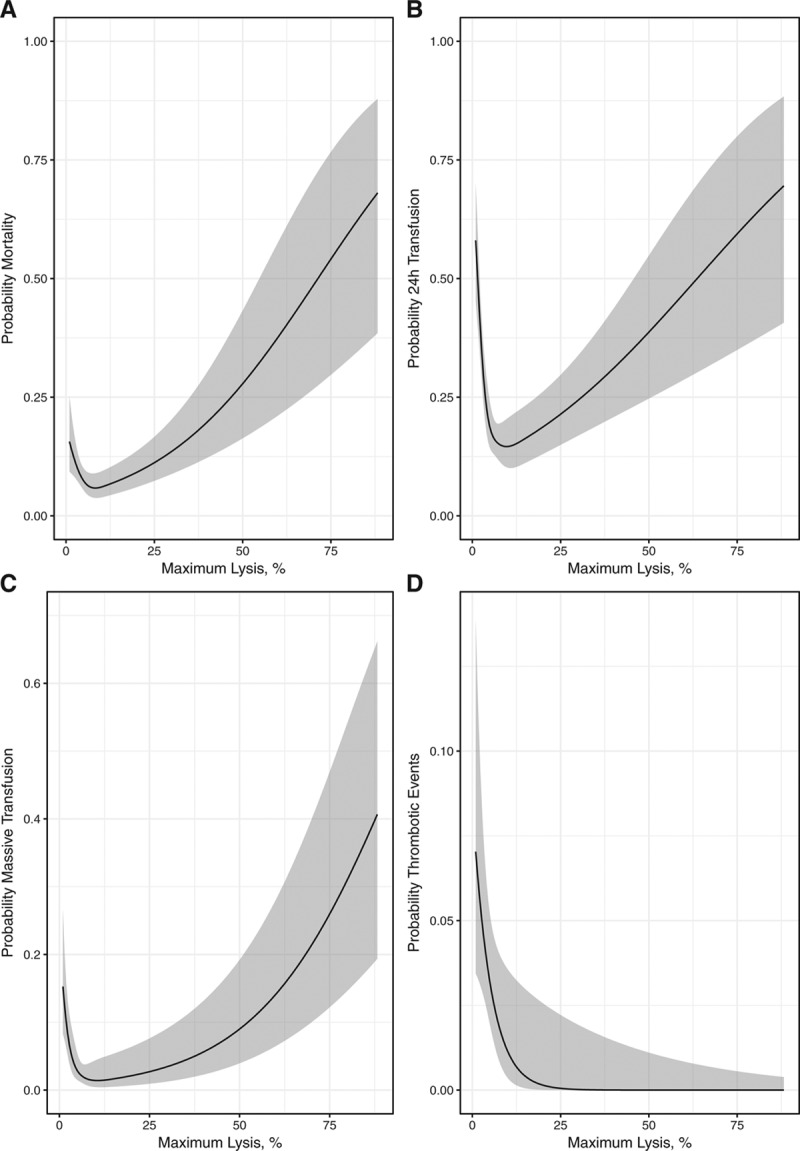
Association between the fibrinolysis spectrum and trauma outcomes. The panels show the association of the fibrinolysis values on death (A), 24-h transfusion (B), massive transfusion (C), and thrombotic events (D). Logistic models estimated high probabilities of the outcomes for extremes values of the fibrinolysis spectrum (fibrinolysis shutdown and hyperfibrinolysis), depicting a J-shaped curve, excepting for thrombotic events.
Furthermore, the multivariable logistic regression model showed that fibrinolysis shutdown was associated with a higher likelihood of transfusion within the first 24 hours (OR, 1.92; 95% CI, 1.07–3.43; P = .026) after adjusting for age, ISS, TXA use, systolic blood pressure, hemoglobin level, INR, and base excess. Moreover, a greater ISS (OR for 5 points increase, 1.18; 95% CI, 1.03–1.35; P = .015), TXA administration (OR, 6.59; 95% CI, 3.8–11.3; P < .0001), lower systolic blood pressure (OR for 10 mm Hg decrease, 1.26; 95% CI, 1.13–1.41; P < .0001), a lower hemoglobin level (OR for 10 g/dL decrease, 1.27; 95% CI, 1.08–1.5; P = .0037), and a higher INR (OR for 0.1 units increase, 1.27; 95% CI, 1.02–1.59; P = .029) also significantly increased the likelihood of 24-hour transfusion (Table 4).
Table 4.
Multivariable Logistic Regression Models to Evaluate Association Between Fibrinolysis Shutdown and Mortality, and Fibrinolysis Shutdown and Transfusion Within 24 Hours
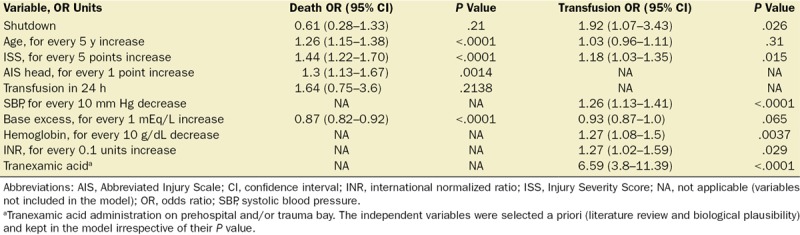
However, a multivariable logistic regression analysis demonstrated that fibrinolysis shutdown was not associated with mortality (OR, 0.61; 95% CI, 0.28–1.33; P = .21). Older age (OR for 5 years increase, 1.26; 95% CI, 1.15–1.38; P < .0001), a higher ISS (OR for 5 points increase, 1.44; 95% CI, 1.22–1.70; P < .0001), an increased severity of head injury or a higher AIS head (OR for 1 point increase, 1.3; 95% CI, 1.13–1.67; P = .0014), and a lower base excess (OR for 1 mEq/L increase, 0.87; 95% CI, 0.82–0.92; P < .0001) were independently associated with death (Table 4). Fibrinolysis shutdown was not associated with increased odds of receiving a massive transfusion (OR, 2.14; 95% CI, 0.79–5.74; P = .1308) after accounting for other covariates (Supplemental Digital Content 2, Table S4, http://links.lww.com/AA/C314). However, a higher ISS (OR for 5 points increase, 1.23; 95% CI, 1.04–1.45; P = .0149), a decreased systolic blood pressure (OR for 10 mm Hg decrease, 1.68; 95% CI, 1.38–2.04; P < .0001), and a lower hemoglobin level (OR for 10 g/dL decrease, 1.29; 95% CI, 1.02–1.64; P = .0332) had an independent association with massive transfusion. Finally, fibrinolysis shutdown had no significant association with thrombotic events (OR, 1.08; 95% CI, 0.37–3.15; P = .874) after adjusting for ISS, age, and 24-hour blood products administration. Older age (OR for 5 years increase, 1.16; 95% CI, 1.02–1.31; P = .0159) and 24-hour transfusion (OR, 3.17; 95% CI, 1.11–9.06; P = .031) were independently associated with thrombotic events (Supplemental Digital Content 2, Table S5, http://links.lww.com/AA/C314).
DISCUSSION
This is the first study to characterize fibrinolysis shutdown using ROTEM and its association with clinical outcomes. Following the same methodology used with TEG, a ROTEM ML value <3.5% was selected to define fibrinolysis shutdown. For this cut-point, sensitivity is low (42.5%), and specificity is moderate (76.7%). In earlier studies with TEG, fibrinolysis shutdown was the most common phenotype (present in 45%–64% of patients),16–19 while using ROTEM, the physiologic was the predominant phenotype (70.7%). The proportion of patients with hyperfibrinolysis was also noticeably lower (3.6%) than in previous studies. The discrepancy in the distribution of the phenotypes appears to reflect the limitations of the Youden index to select a cutoff point. The Youden index is an appropriate method when sensitivity and specificity are seen as equally important since the cutoff selection does not consider the clinical implications of favoring one over the other.29 However, to characterize a new clinical entity in a selected group from the general trauma population (severe injury), a higher specificity is preferred over sensitivity to reduce the number of false-positive results. Because the present study cutoff has a higher specificity than sensitivity, it reduces the number of patients erroneously classified as fibrinolysis shutdown, and the total number of patients in this group. The sensitivity and specificity of TEG LY30 <0.9 has not been reported, but we expect higher sensitivity than specificity compared to the present study, explaining the larger number of patients in the shutdown group, arguably many inaccurately classified as such. Furthermore, the discordance of the distribution of the groups could also reflect a difference in the sensitivity to fibrinolytic activity between ROTEM and TEG because the devices’ results are not always interchangeable.20 Last, it is plausible that patients’ characteristics and local epidemiology of traumatic injuries contributed to the differences found.
Reduced fibrinogen levels, longer CFT, higher MCF, and lower base excess were independent predictors for the shutdown phenotype. This combination of coagulation abnormalities suggests that amid a significant hemostatic activation (higher MCF), coexists a fibrinogen consumption and poor clot formation dynamics (delayed CFT), signs of reduced fibrin polymerization, impaired thrombin generation, and platelets abnormalities. Consistently, the shutdown group had a 2-fold increase in 24-hour blood transfusion requirements compared to the physiologic group, and this association remained significant after adjusting for confounders (OR, 1.92; 95% CI, 1.07–3.43; P = .026). However, fibrinolysis shutdown was not independently associated with massive transfusion or mortality. Other studies reported a greater incidence of 24-hour blood transfusion18 and massive transfusion in the shutdown compared to the physiologic group,16 but previously no study has done adjusted analysis.
Contrasting with previous reports, shutdown patients had a 2-fold increase in mortality compared to the physiologic group but this group was not independently associated with mortality (OR, 0.61; 95% CI, 0.28–1.33; P = .21). Nevertheless, the association of initial shutdown phenotype and death is inconsistent among different studies. In the largest multicenter cohort to date, Moore et al17 found no difference in the survival probability between the physiologic and shutdown groups after adjusting for age, mechanism of injury, head injury, and systolic blood pressure. An analysis of 181 trauma patients found no difference in mortality among patients with shutdown and physiologic phenotype at hospital admission.19 A pediatric cohort of 130 trauma patients reported an increased mortality among the shutdown group; however, this group had significantly lower Glasgow Coma Scale scores, thus severe head injury was a potential confounder for the increased mortality.18 Hence, our key findings are robust clot amplitude despite lower fibrinogen, and poorer clot formation dynamics and lack of association with mortality despite bleeding (increased 24-hour blood transfusion); early posttraumatic fibrinolysis shutdown could be interpreted as a physiological response to life-threatening insults rather than a harmful pathological entity.
A significant limitation of all the studies published in the area is the lack of a fibrinolysis biomarkers definition for fibrinolysis shutdown, failing to provide a precise quantification, and mechanistic insight of the posttraumatic hypofibrinolysis phenomenon. Because viscoelastic tests globally assess the hemostatic system, their fibrinolysis measure is not an isolated quantification of the fibrinolytic activation but the balance between clot formation and degradation. Thus, a high fibrinolytic activity could coexist within the fibrinolysis shutdown range if a robust clot formation is maintained.
Nonetheless, we acknowledge that the complexity of the fibrinolysis diagnostic assays and the lack of gold standard methods30 justify the use of viscoelastic tests as a first approach to the problem if interpreting with caution its results. The diagnostic performance characteristics (sensitivity and specificity) of viscoelastic tests for hypofibrinolysis are unknown since all studies defined shutdown using a surrogate outcome (ie, mortality). For hyperfibrinolysis, ROTEM is an insensitive assay as shown in an evaluation of 303 trauma patients comparing it with plasmin–antiplasmin complex and d-dimer levels; patients with moderate-to-severe fibrinolysis had no evidence of abnormal fibrinolysis with ROTEM.7 Additionally, the association of fibrinolysis shutdown and mortality is confounded by platelets abnormalities since reduced platelet number or dysfunction reduces the clot retraction in thromboelastography independently of the fibrinolysis activity31; both conditions associated with increased mortality in trauma.32
There are areas of uncertainty in the diagnosis of fibrinolysis shutdown phenomenon. First, the differences between ROTEM and TEG in diagnosing fibrinolysis are unknown, and thus, whether they are equivalent. Second, we found that prehospital TXA administration was not associated with the shutdown phenotype on hospital admission although some authors had expressed the concern that TXA could induce hypofibrinolysis.33 A significant limitation in observational studies in TXA use is the analysis without adjustment for TXA indication (eg, propensity score), leading to biased conclusions. Because transfusion is a confounder for its association with both TXA use and shutdown fibrinolysis, it could be erroneously assumed that TXA is associated with shutdown phenotype, overlooking that shutdown patients are more likely to have a transfusion and thus more likely to meet the criteria for TXA administration. We were underpowered to analyze the TXA association between shutdown. Future studies could elucidate whether TXA is universally beneficial and safe, including in patients with fibrinolysis shutdown.
Third, for a comprehensive characterization of the fibrinolysis shutdown phenomenon, future research needs to integrate other components of the hemostatic system not assessed by thromboelastography. To elucidate whether the shutdown response is driven by the endothelium activation after injury, the glycocalyx damage, increased sympathoadrenal activation,5 increased plasminogen activator inhibitor-1 levels, the plasminogen activators (tissue plasminogen activator and urokinase plasminogen activator),8 or the patients’ characteristics like older age.34
This study has important limitations. A 23.5% of false-positive results raise questions about the applicability of using this cutoff to define fibrinolysis shutdown as a clinical entity. Consequently, any recommendation of management for this population is not warranted until our understanding of the fibrinolysis shutdown phenomenon is enlightened by other biomarkers. Our results may not be generalizable to patients with lesser trauma severity since only severely injured patients were analyzed. Patients with more severe trauma have higher mortality and shutdown may have a greater impact in this group. Because we only considered a single ROTEM measurement at hospital admission, it is uncertain how the evolution of the fibrinolysis phenotype might affect patients’ outcome. On the other hand, because ROTEM was done early in the resuscitation, it provides invaluable information regarding the initial fibrinolysis response to injury with few iatrogenic interventions. Last, our sample size is limited, increasing the type II error. Nevertheless, our exploratory analysis aimed to be hypothesis generating and constitute a foundation for a multicenter collaboration to validate the ROTEM cutoff for fibrinolysis shutdown and its association with clinical outcomes.
CONCLUSIONS
Patients with fibrinolysis shutdown diagnosed using ROTEM had higher injury severity, greater signs of hypoperfusion, required more transfusions within 24 hours, and despite that fibrinolysis shutdown was not associated with increased mortality. A plausible interpretation of these findings is that decreased clot degradation (fibrinolysis shutdown) in severely injured patients at hospital admission is not necessarily a harmful response but an appropriate hemostatic activation. Presently, it is unclear whether early fibrinolysis shutdown is a pathologic phenomenon or a physiologic response to life-threatening injuries.
ACKNOWLEDGMENTS
We thank Amanda McFarlan, RN, Trauma and Acute Care Surgery Department, for her efforts in maintaining a high-quality trauma database; Michelle Sholzberg, MD, and Hina Chaudhry from the Coagulation Laboratory for assuring excellence in ROTEM measurements; and Simon Abrahamson, MD, Andrew Baker, MD, Andrea Rigamonti, MD, Federico Perez, and the Department of Critical Care at St Michael’s Hospital for their support to complete this work.
DISCLOSURES
Name: J. Carolina Gomez-Builes, MD.
Contribution: This author helped design the work, analyze the data, and write the manuscript.
Conflicts of Interest: None.
Name: Sergio A. Acuna, MD, PhD.
Contribution: This author helped analyze the data and write the manuscript.
Conflicts of Interest: None.
Name: Bartolomeu Nascimento, MD, MSc.
Contribution: This author helped analyze the data and write the manuscript.
Conflicts of Interest: B. Nascimento received speaker’s honoraria from Commonwealth Serum Laboratories Behring.
Name: Fabiana Madotto, PhD.
Contribution: This author helped analyze the data and write the manuscript.
Conflicts of Interest: None.
Name: Sandro B. Rizoli, MD, PhD, FRCP.
Contribution: This author helped design the work, analyze the data, and write the manuscript.
Conflicts of Interest: S. B. Rizoli received an honorarium as member of the scientific advisory board for Commonwealth Serum Laboratories Behring.
This manuscript was handled by: Roman M. Sniecinski, MD.
Supplementary Material
Footnotes
Published ahead of print April 19, 2018.
Funding: None.
Conflicts of Interest: See Disclosures at the end of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
Listen to this Article of the Month podcast and more from OpenAnesthesia.org® by visiting http://journals.lww.com/anesthesia-analgesia/pages/default.aspx.
Reprints will not be available from the authors.
REFERENCES
- 1.Stanworth SJ, Davenport R, Curry N, et al. Mortality from trauma haemorrhage and opportunities for improvement in transfusion practice. Br J Surg. 2016;103:357–365.. [DOI] [PubMed] [Google Scholar]
- 2.Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg. 2010;34:158–163.. [DOI] [PubMed] [Google Scholar]
- 3.Chin TL, Moore EE, Moore HB, et al. A principal component analysis of postinjury viscoelastic assays: clotting factor depletion versus fibrinolysis. Surgery. 2014;156:570–577.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74:1223–1229.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrowski SR, Henriksen HH, Stensballe J, et al. Sympathoadrenal activation and endotheliopathy are drivers of hypocoagulability and hyperfibrinolysis in trauma: a prospective observational study of 404 severely injured patients. J Trauma Acute Care Surg. 2017;82:293–301.. [DOI] [PubMed] [Google Scholar]
- 6.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254:194–200.. [DOI] [PubMed] [Google Scholar]
- 7.Raza I, Davenport R, Rourke C, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11:307–314.. [DOI] [PubMed] [Google Scholar]
- 8.Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41:514–521.. [DOI] [PubMed] [Google Scholar]
- 9.Shakur H, Roberts I, Bautista R, et al. ; CRASH-2 Trial Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32.. [DOI] [PubMed] [Google Scholar]
- 10.Moore HB, Moore EE, Lawson PJ, et al. Fibrinolysis shutdown phenotype masks changes in rodent coagulation in tissue injury versus hemorrhagic shock. Surgery. 2015;158:386–392.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schöchl H, Frietsch T, Pavelka M, Jámbor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2009;67:125–131.. [DOI] [PubMed] [Google Scholar]
- 12.Theusinger OM, Wanner GA, Emmert MY, et al. Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM) is associated with higher mortality in patients with severe trauma. Anesth Analg. 2011;113:1003–1012.. [DOI] [PubMed] [Google Scholar]
- 13.Ives C, Inaba K, Branco BC, et al. Hyperfibrinolysis elicited via thromboelastography predicts mortality in trauma. J Am Coll Surg. 2012;215:496–502.. [DOI] [PubMed] [Google Scholar]
- 14.Kashuk JL, Moore EE, Sawyer M, et al. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg. 2010;252:434–442.. [DOI] [PubMed] [Google Scholar]
- 15.Kutcher ME, Cripps MW, McCreery RC, et al. Criteria for empiric treatment of hyperfibrinolysis after trauma. J Trauma Acute Care Surg. 2012;73:87–93.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77:811–817.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore HB, Moore EE, Liras IN, et al. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg. 2016;222:347–355.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leeper CM, Neal MD, McKenna C, Sperry JL, Gaines BA. Abnormalities in fibrinolysis at the time of admission are associated with deep vein thrombosis, mortality, and disability in a pediatric trauma population. J Trauma Acute Care Surg. 2017;82:27–34.. [DOI] [PubMed] [Google Scholar]
- 19.Meizoso JP, Karcutskie CA, Ray JJ, Namias N, Schulman CI, Proctor KG. Persistent fibrinolysis shutdown is associated with increased mortality in severely injured trauma patients. J Am Coll Surg. 2017;224:575–582.. [DOI] [PubMed] [Google Scholar]
- 20.Rizoli S, Min A, Sanchez AP, et al. In trauma, conventional ROTEM and TEG results are not interchangeable but are similar in clinical applicability. Mil Med. 2016;181:117–126.. [DOI] [PubMed] [Google Scholar]
- 21.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined–a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969.. [DOI] [PubMed] [Google Scholar]
- 22.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35.. [DOI] [PubMed] [Google Scholar]
- 23.Lang T, Bauters A, Braun SL, et al. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis. 2005;16:301–310.. [DOI] [PubMed] [Google Scholar]
- 24.Harrell F. Bickel P, Diggle P, Fienberg SE, et al. Multivariable regression strategies. In: Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2015:2nd ed New York, NY: Springer, 64–67.. [Google Scholar]
- 25.Thorpe KE. How to construct regression models for observational studies (and how NOT to do it!). Can J Anaesth. 2017;64:461–470.. [DOI] [PubMed] [Google Scholar]
- 26.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrell FE., Jr. rms: Regression Modeling Strategies. R package version 5.1-0. 2017. Available at: https://CRAN.R-project.org/package=rms. Accessed February 1, 2018.
- 28.R Core Team. R: A Language and Environment for Statistical Computing. Available at: https://www.r-project.org. Accessed February 1, 2018.
- 29.Bewick V, Cheek L, Ball J. Statistics review 13: receiver operating characteristic curves. Crit Care. 2004;8:508–512.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilich A, Bokarev I, Key NS. Global assays of fibrinolysis. Int J Lab Hematol. 2017;35:879–877.. [DOI] [PubMed] [Google Scholar]
- 31.Katori N, Tanaka KA, Szlam F, Levy JH. The effects of platelet count on clot retraction and tissue plasminogen activator-induced fibrinolysis on thrombelastography. Anesth Analg. 2005;100:1781–1785.. [DOI] [PubMed] [Google Scholar]
- 32.Kutcher ME, Redick BJ, McCreery RC, et al. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73:13–19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore EE, Moore HB, Gonzalez E, et al. Postinjury fibrinolysis shutdown: rationale for selective tranexamic acid. J Trauma Acute Care Surg. 2015;78:S65–S69.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson PI, Sørensen AM, Perner A, et al. Elderly trauma patients have high circulating noradrenaline levels but attenuated release of adrenaline, platelets, and leukocytes in response to increasing injury severity. Crit Care Med. 2012;40:1844–1850.. [DOI] [PubMed] [Google Scholar]


