Abstract
Object
In patients with focal nerve injury and neuropathic pain cutting the nerve to obtain permanent pain reduction can be considered. Surgery is indicated only if a diagnostic nerve block provides temporary pain relief. We evaluated the predictive value of a block on the outcome of surgery.
Methods
In total, three blocks were performed at two week intervals. Patients were blinded to injections containing lidocaine 1% and a placebo was included. Surgery was offered regardless of the effect of the blocks. Twenty-four patients received 72 blocks. Sixteen patients opted for surgery, 5 patients refrained from surgery, and in 3 the blocks provided permanent pain relief. The predictive ability of the block on the outcome of surgery was assessed by calculating the area under a Receiver Operating Characteristic curve (AUC).
Results
The AUC of the first lidocaine block was 0.35 with a 95% confidence interval from 0.077 to 0.62. At 95% confidence (two-sided), the AUC is less than 0.62, and hence the predictive ability of the block was poor. The outcome of the second lidocaine block and saline block did not change the conclusion of the first block.
Conclusions
We conclude that the use of blocks to select patients for surgery should be critically appraised.
Perspective
A pain relieving response to one open block is currently considered mandatory before patients with focal nerve injury and neuropathic pain are offered surgery. Blinded blocks including a placebo show that responses for selection should be carefully interpreted because they may not be as predictive as generally presumed.
Introduction
Chronic pain of moderate to severe intensity occurs in 19% of adult Europeans, 4% of which is caused by nerve damage.[1,2] The neuropathic pain that arises from nerve damage is associated with abnormal sensation or hypersensitivity in the area of the affected nerve, which can be adjacent to or combined with areas with reduced sensation. Patients experience paraesthesia (i.e., skin crawling sensation or tingling), spontaneous (not stimulus-induced) ongoing pain, and shooting, electric shock-like sensations.[3] Neuropathic pain due to a nerve lesion is difficult to treat. Only a minority of patients have an adequate response to pharmacotherapy.[3,4] Alternatives such as neurostimulation therapy are increasingly applied, but their role needs yet to be defined. So far, most trials on neurostimulation for pain relief did not comply with the requirements of evidence-based medicine.[5]
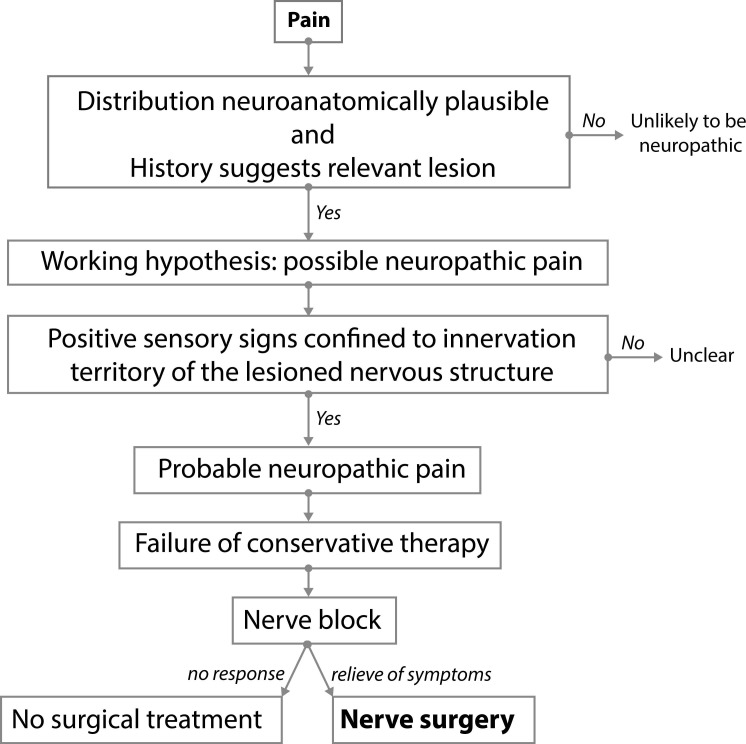
Surgical treatment of the damaged nerve is considered as an option when all other treatments have failed. The surgical technique consists of either neurolysis and releasing the nerve from the scarred area or neuroma resection with relocation and burying of the proximal stump.[6] The nerve innervating the area of pain can be identified by assessing the area of sensory abnormality, tapping over the course of the nerve and by blocks with local anaesthetics.[7] Nerve blocks are also used in the decision-making process to determine whether to perform a surgical intervention. Adequate temporary pain relief following the block is considered mandatory before surgery is undertaken.[7–55] [56–62] (Fig 1) There is no objective evidence base for blocking a nerve before nerve surgery is undertaken. Only one study assessed the predictive value of a diagnostic nerve block for the pain relieving effect of surgery. However, patients who did not respond to the block were not operated thereby introducing a selection bias.[52] The weakness of the currently used algorithm appears that the decision whether to operate or not is based on just one “open” block that might be effective due to an inherent effect on the damaged nerve or that may be contaminated by a placebo effect.
Fig 1. Current selection algorithm for nerve surgical treatment of patients with neuropathic pain due to a focal trauma to a nerve.
The decision whether to operate or not is based on one “open” nerve block. Adequate temporary pain relief following the block is considered mandatory before surgery is undertaken. The nerve is then cut proximal to the painful area to obtain permanent pain reduction.
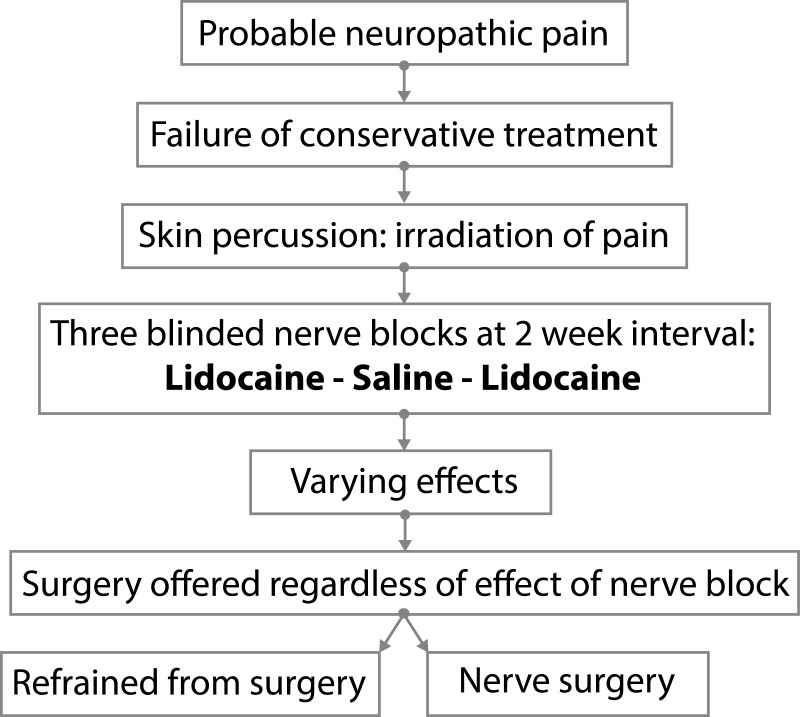
We used a different protocol in our patients. The protocol is applied exclusively to patients with probable neuropathic pain due to nerve injury[2] and who had insufficient pain reduction or severe side effects from pharmacotherapy. The involved nerve is blocked three times in 4 weeks (with a 2-week interval between injections) and consists of two single-blind injections with lidocaine and one with placebo.(Fig 2) All patients are given the opportunity of surgery irrespective of the outcome of the block injections.
Fig 2. The LUMC protocol applied exclusively to patients with persistent postsurgical or nerve injury–induced probable neuropathic pain related to sensory nerves.
All patients had positive sensory signs confined to the innervation territory of the damaged nervous structure and had insufficient pain reduction or severe side effects from pharmacotherapy. The involved nerve is blocked three times in 4 weeks (with a 2-week interval between injections) and consists of two single-blind injections with lidocaine and one with placebo. All patients are given the opportunity of surgery irrespective of the outcome of the block injections.
We looked for the level of evidence for the use of a block for selection (Fig 1) by assessing the predictive value of a block on the outcome of surgery.
Methods
Leiden nerve block protocol
A standardized diagnostic nerve block protocol was applied in the work-up of patients in whom nerve surgery to treat neuropathic pain was considered.(Fig 2) All of these patients had had extensive conservative pain treatment prior to referral, which was insufficient or induced significant side effects. For this study, only patients were included who had persistent postsurgical or nerve injury–induced neuropathic pain related to sensory nerves and who had positive sensory signs confined to the innervation territory of the damaged nervous structure. Prior to the block, the area with aberrant skin sensation (allodynia, hypaesthesia, dysaesthesia) was assessed. The involved nerve and site of maximum pain provocation was anatomically localised by percussion from distal to proximal over the course of the suspected nerve and scar. Neuroma provocation was considered present when tapping increased the pain and provoked tingling in the area with aberrant skin sensation. In total, three injections were given at two-week intervals. Patients remained blinded for the nature of the injections (active or placebo). No information was provided about the potential effects or duration of effects. The first and third injections were performed with lidocaine 1%; the second injection with placebo (water Sodium Chloride 0.9% solution, saline); each injection had a volume of 4 cc. All injections were performed by the same surgeon with a vast experience in nerve surgery. The site of injection was assessed by tapping from distal, starting in the area with the sensory deficit, to proximal, over the anatomical trajectory of the damaged nerve. The injection was positioned just proximal to the area of maximal pain provocation upon tapping. The direction of the needle and depth of the tip was repositioned several times in order to circumvent the nerve and provide an adequate infiltration as regarding to depth and size of the area of the involved nerve. After removing the needle, pressure was applied to improve the spread of the fluid. All three injections were performed at the same location. Two weeks after the injection the response to the injection was documented and arranged in four groups: 0 = no effect of the injection; 1 = pain relief for several hours; 2 = pain relief for days or permanent pain relief; 3 = increase of pain for hours or days. Two weeks after the third block, the content of all three provided injections was revealed to the patient and the effect of the injections was subsequently discussed. The patients were informed about the current treatment paradigm (Fig 1) implying that surgery is offered when the response to lidocaine was positive. Regardless of the outcome of the three blocks, all patients were offered nerve surgery. All patients provided verbal informed consent. The Leiden University Medical Center Institutional Review Board declared that ethical approval was not required for this study.
Nerve surgery
Patients were operated under general anaesthesia. The nerve trunk proximal to the area of maximal pain was dissected free and dissection continued distally into the zone of tissue and nerve damage. The responsible nerve branch ending was identified and cut just proximally to the neuroma into healthy looking nerve. Skin branches leaving the nerve trunk proximal to the neuroma and which were not involved in the lesion were left intact. The neuroma of the damaged nerve was resected. The proximal nerve stump was loosely buried as deep as possible away from potential sites of mechanical pressure and joints. The stump was enveloped by fat tissue and secured with a 5.0 vicryl suture. Finally, wound closure was performed according to standard procedures. The effect of surgery on pain was documented using five point scale: 0 = no effect, 1 = partial pain reduction, 2 = (as good as) pain free, 3 = temporary pain reduction, 4 = increase of pain as compared to the pre-operative level.
Data analysis
To assess whether the response to the nerve block is predictive of the success of the surgery, we proceeded as follows. The result of the block was ordered according to a (presumed) increasing likelihood of a successful surgery: a) no effect; b) increase of pain for hours or days; c) pain relief for days; d) pain relief for several hours. The outcome of surgery was dichotomized as unsuccessful (those surgeries with no effect, or temporary pain reduction, or an increase of pain as compared to the pre-operative level) or as successful (partial pain reduction or (as good as) pain free following surgery). A decision rule for recommending surgery would involve a cut-point for the result of the block. For instance, one might recommend surgery to patients who score better than b) on the block. Such a decision rule will result in two types of errors: recommending surgery to some patients who do not benefit, and not recommending surgery to patient who do benefit. Changing the cut-point will impact the probabilities of both types of errors. Plotting the sensitivity versus 1-specificity of the decision rule for varying cut-points yields a so-called Receiver Operating Curve (ROC). The area under this ROC cure (AUC) is a commonly used measure of predictive ability. The AUC is expressed between 0 and 1. The predictive ability and accuracy of a diagnostic block is excellent if the AUC is between 0.90–1.0. The test is good with an AUC between 0.80–0.90, fair between 0.70–0.80, poor between 0.60–0.70, and failed if the AUC is between 0.50–0.60. An AUC less than 0.5 is worse than random guessing.[63;64] We used a Bootstrap test with 10000 replications as implemented in the R package pROC[65] for correlated ROC curves to assess whether the first lidocaine block differs from the second lidocaine block.
Results
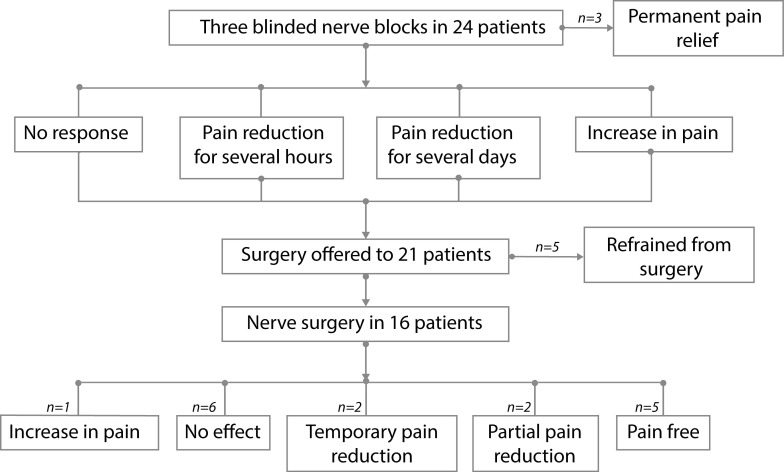
Between 2008 and 2013, a total of 24 patients (11 male, 13 female) met the inclusion criteria. (Fig 3) The mean age at first consultation was 43 years (median 39, SD 14). The mean interval between the nerve lesion and the first block was 40 months (median 15, SD 20). The sensory nerves involved were located in the lower extremity in 18 patients (75%), in the upper extremity in 4 (16%) patients and at the thoracic level in 2 (8%) (Table 1, S1 Minimal Data Set).
Fig 3. The effect on pain of three blinded nerve blocks in 24 patients with persistent postsurgical or nerve injury–induced probable neuropathic pain related to sensory nerves and results of nerve surgery.
Two weeks after each injection the response to the injection was documented. Two weeks after the third block, the content of all three provided injections was revealed to the patient and the effect of the injections was subsequently discussed. Regardless of the outcome of the three blocks, all patients were offered nerve surgery. A permanent positive effect on the pain following surgery was seen in 7/16 (44%) of the patients. No beneficial effect or an increase was reported by 9/16 (56%) of the patients. Five of the 16 patients (31%) were (as good as) pain free, and 2 (12%) had partial pain reduction. In 6/16 (38%) there was no effect of the surgery, whereas a temporary pain reduction was seen in 2 (12%) of the patients and an increase in 1 (6%).
Table 1. Effect of blocks and surgery on pain.
| Patient | Nerve | Age | Interval Block 1-lesion | Block 1 Lidocaïne |
Block 2 Saline |
Block 3 Lidocaïne |
Result surgery |
|---|---|---|---|---|---|---|---|
| 1 | superficial radial | 62 | 4 | 1 | 0 | 1 | 0 |
| 2 | cutaneous lateral femoral | 15 | 69 | 3 | 2 | 3 | 0 |
| 3 | saphenous | 70 | 20 | 0 | 0 | 0 | 0 |
| 4 | infrapatellar | 35 | 25 | 1 | 2 | 3 | 0 |
| 5 | infrapatellar | 28 | 62 | 2 | 0 | 0 | 0 |
| 6 | distal tibial | 38 | 15 | 0 | 3 | 0 | 0 |
| 7 | superficial radial | 40 | 57 | 2 | 3 | 2 | 1 |
| 8 | sural | 63 | 38 | 0 | 3 | 0 | 1 |
| 9 | intercostobrachial | 37 | 45 | 0 | 3 | 0 | 2 |
| 10 | palmar digital II | 44 | 20 | 1 | 3 | 1 | 2 |
| 11 | medial plantar | 49 | 6 | 0 | 0 | 3 | 2 |
| 12 | cutaneous lateral femoral | 36 | 59 | 2 | 2 | 0 | 2 |
| 13 | cutaneous superficial peroneal | 61 | 19 | 2 | 0 | 0 | 2 |
| 14 | cutaneous superficial peroneal | 34 | 23 | 1 | 3 | 1 | 3 |
| 15 | cutaneous superficial peroneal | 50 | 23 | 2 | 0 | 2 | 3 |
| 16 | infrapattelar | 32 | 32 | 1 | 3 | 1 | 4 |
| 17 | intercostal 4 | 54 | 9 | 2 | 2 | 2 | * |
| 18 | dorsal branch ulnar | 38 | 9 | 2 | 2 | 2 | * |
| 19 | calcaneal and medial plantar | 20 | 16 | 2 | 0 | 2 | * |
| 20 | cutaneous posterior femoral | 36 | 33 | 0 | 2 | 3 | ** |
| 21 | infrapatellar | 42 | 290 | 1 | 3 | 1 | ** |
| 22 | cutaneous deep peroneal | 50 | 12 | 0 | 0 | 0 | ** |
| 23 | cutaneous superficial peroneal | 37 | 6 | 2 | 0 | 0 | ** |
| 24 | sural | 60 | 15 | 1 | 2 | 3 | ** |
Age at nerve lesion in years. Interval block 1 and lesion in months. Effects of the lidocaine or saline injection: 0) No effect; 1) Pain relief for several hours; 2) Pain relief for days or permanent pain relief; 3) Increase of pain for hours or days. Result of surgery: 0) no effect, 1) partial pain reduction, 2) (as good as) pain free, 3) temporary pain reduction, 4) increase of pain as compared to the pre-operative level.
* Permanent pain relieving effect as response to the block
** Refrained from surgery
The pain started after a surgical intervention in 18/24 of the patients (75%), of which the pain developed immediately following surgery in 13/16 (80%), and in 3/16 (20%) of the patients after weeks to months. In 6/24 (25%) of patients, the pain started following a trauma; in 3 immediately following trauma, and in the other 3 patients weeks to months later.
Effect of nerve blocks
The different effects of the blocks on pain are shown in Table 1 and summarized in Table 2.
Table 2. Summary of the different effects of the three blocks on pain.
| Blocks | Lidocaine 1 (n = 24) |
Lidocaine 2# (n = 24) |
Lidocaine 1+2 (n = 48) | Saline (n = 24) |
Total (n = 72) |
|---|---|---|---|---|---|
| No effect | 7 (29) | 9 (38) | 16 (33) | 9 (38) | 25 (35) |
| Relief for several hours | 7 (29) | 5 (21 | 12 (25) | 0 | 12 (17) |
| Relief for days or permanent | 9 (38) | 5 (21) | 14 (29) | 7 (29) | 21 (29) |
| Any relief | 16 (66) | 10 (42) | 26 (54)* | 7 (29) | 33 (46) |
| Increase for hours or days | 1 (4) | 5 (21) | 6 (13) | 8 (33) | 14 (19) |
Percentages are between brackets.
# The effect of the second lidocaine block was similar to the first in 16/24 (66%).
* Pain reduction following both lidocaine blocks was found in 10/24 (42%) of the patients.
In 26 of the 48 lidocaine blocks (54%) a pain reducing effect was observed. The effect of the second lidocaine block was similar to the first in 16/24 (66%). Pain reduction following both lidocaine blocks was found in 10/24 (42%) of the patients.
A pain relieving effect of the first lidocaine block was obtained in 16/24 (66%) of the patients, which lasted for several hours in 7/24 (29%) and for many days in 9/24 (38%). There was no effect of the first lidocaine block in 7/24 (29%) of the patients. One patient of the 24 (4%) observed more pain following the first lidocaine block and 5/24 (21%) after the second lidocaine block. In total, 16/24 (66%) of the patients had strong pain reduction in at least one of the two lidocaine blocks regardless of the duration of the effect.
A pain relieving effect of placebo lasting for days was noted in 7/24 (29%) of the blocks. No effect on pain following saline injection was noted in 9/24 (38%) of patients. A temporary increase of pain following saline injection was seen in 8/24 (33%).
No permanent adverse effects of the injections were seen. In 3 of the 24 patients, the injections induced a continuous pain relieving effect to such an extent that these patients did not need surgery nor did they require further pain treatment. Five of the remaining 21 patients who were offered surgery refrained. They requested a 100% guarantee to get significant pain relief following surgery which could not be given, or nerve surgery was judged to be too radical.
Nerve surgery
Sixteen of the 21 patients opted for surgery. The mean interval between the nerve lesion and the operation for pain was 38 months (Median 27, SD 20). The mean interval between the first block and the operation was 6 months (Median 5, SD 3). The mean follow-up after the operation was 15 months (Median 11, SD 13).
At surgery, the nerve was cut and buried in fat.(Fig 4) A permanent positive effect on the pain following surgery was seen in 7/16 (44%) of the patients.(Table 1) No beneficial effect or an increase was reported by 9/16 (56%) of the patients. Five of the 16 patients (31%) were (as good as) pain free, and 2 (12%) had partial pain reduction. In 6/16 (38%) there was no effect of the surgery, whereas a temporary pain reduction was seen in 2 (12%) of the patients and an increase in 1 (6%).
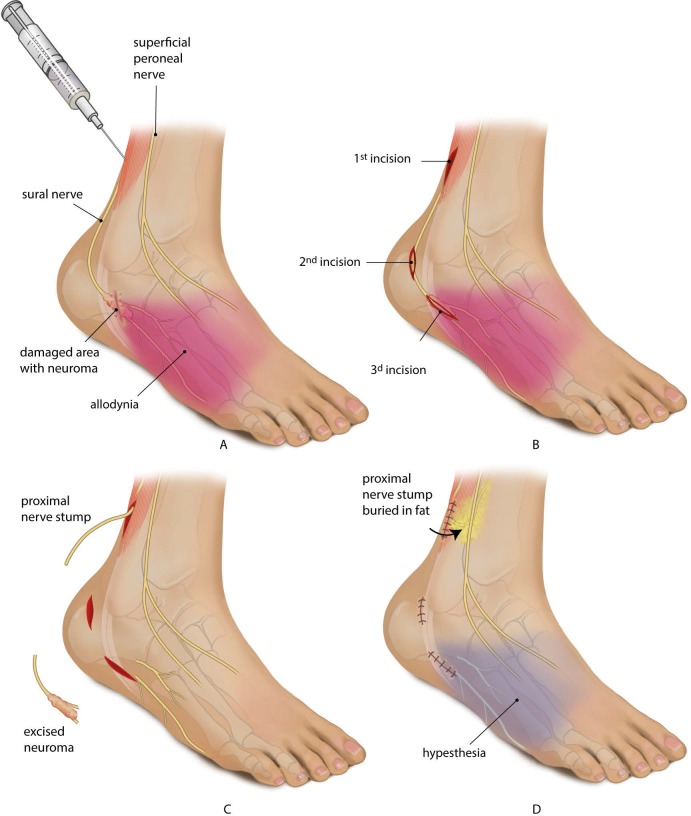
Fig 4. Case illustration of a 63 year patient (number 8, Table 1) in whom blocking a nerve had no pain relieving effect, but nerve surgery had.
Following the current selection algorithm, this patient would not have been operated. The patient had complaints befitting ankle arthrosis. An isolated subtalar arthrodesis was performed by placing compression screws. Immediate postoperative, the patient had severe neuropathic pain with allodynia in the sural nerve area limiting the walking distance to around 150 meters. Conservative treatment failed. A: At inspection 38 months after the onset of the pain, a scar of the screw placement was seen around 4 centimetres below the lateral malleolus. Percussion in the scarred damaged area provoked irradiating painful sensations in the sural nerve area. The sural nerve was blocked three times in 4 weeks (with a 2-week interval between injections) consisting of two single-blind injections with a volume of 4 cc. lidocaine 1% and one with placebo. There was no effect of the lidocaine injections and following saline injection the pain increased temporarily. B: At surgery, the sural nerve was identified in undamaged area (1st incision) and dissected free subcutaneously. A second incision was made and the sural nerve was followed distally towards the scar. Subsequently, a third incision was made over the scar and a damaged sural nerve was identified. C: The damaged sural nerve was cut and the abnormal looking nerve tissue was resected. Pathological examination of the abnormal tissue showed traumatic neuroma. The resection plane of the proximal stump showed normal myelinated fibers and fascicles. D: The proximal stump of the sural nerve was loosely buried in fat proximal to the ankle joint. Postoperatively, his pain decreased significantly. The area with allodynia disappeared and became hypesthetic. The patient could walk again for at least one hour.
The predictive ability of the nerve block on the outcome of surgery
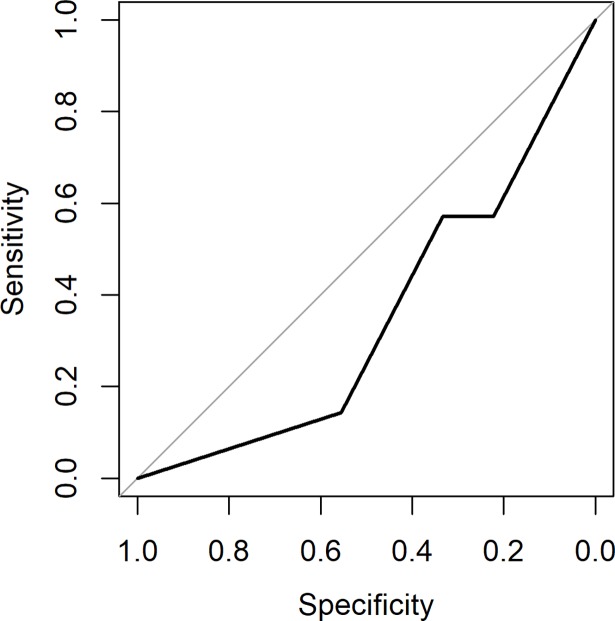
The AUC of the first lidocaine block was 0.35 with a 95% confidence interval (CI) from 0.077 to 0.62.(Fig 5) We conclude at 95% confidence (two-sided) that the AUC is less than 0.62, and hence that the predictive ability of the lidocaine nerve block is poor.
Fig 5. The area under the Receiver Operating Curve (AUC) of the first lidocaine block.
The AUC was 0.35 with a 95% confidence interval (CI) from 0.077 to 0.62. The AUC is less than 0.62 at 95% confidence (two-sided), and hence the predictive ability of the lidocaine nerve block is poor.
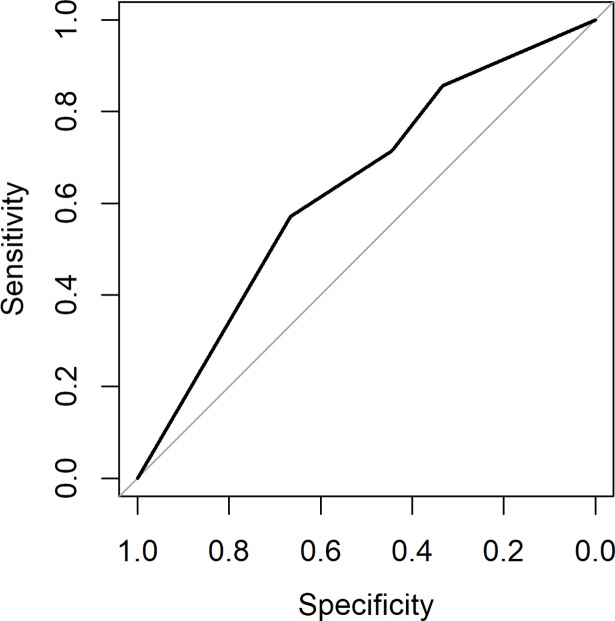
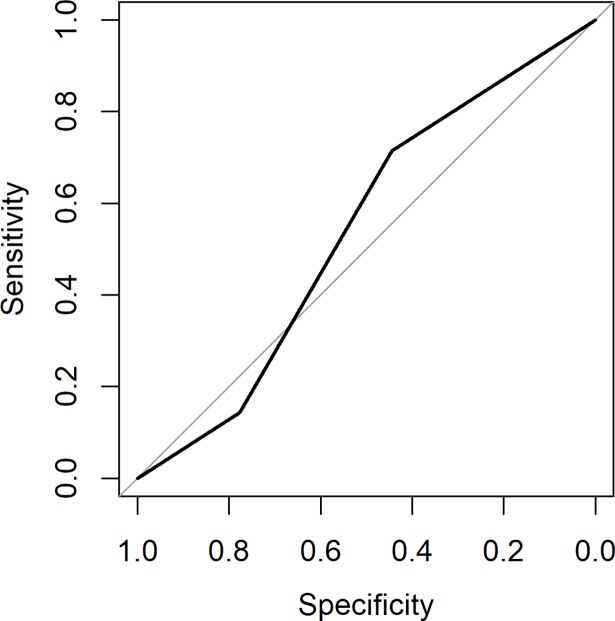
The AUC of the second lidocaine block was 0.63 (95% CI 0.36 to 0.91, Fig 6) and of the saline block 0.53 (95% CI: 0.26–0.82, Fig 7). There was no difference between the first and second lidocaine block (p = 0.24). The outcome of the second lidocaine block and saline block does not change the conclusion of the first block.
Fig 6. The AUC of the second lidocaine block was 0.63 (95% CI 0.36 to 0.9).
Fig 7. The AUC of the saline block was 0.53 (95% CI: 0.26–0.82).
Discussion
Satisfactory control of neuropathic pain following nerve injury can be extremely difficult to obtain. Nerve surgery is occasionally considered when conservative measures fail.[6] Diagnostic blocks are used to select patients who might benefit from nerve surgery.[18,52] (Fig 1) The surgery entails neuroma resection and burying of the proximal stump in an area of healthy tissue in such a way that mechanical strain or pressure forces on the stump cannot occur. (Fig 4)
Usually one lidocaine block is given in an un-blinded setting when selecting patients for surgery.[18] (Fig 1) Our block protocol for patients with persistent postsurgical or nerve injury induced probable neuropathic pain[2] consisted of three injections including a placebo. In addition, the patient was blinded. Nerve surgery to reduce pain was offered to all patients, regardless of the outcome of the three blocks. The predictive ability of the lidocaine block in this study was poor. Based on these results we conclude that block responses for selection for surgery should be carefully interpreted as they may not be as predictive as generally presumed. Actually, the use of blocks to select patients for surgery should be critically appraised.
Three technical factors potentially affect the outcome of our analysis: the blocking procedure, the surgery and the blinding. First, we may actually not have injected lidocaine in the close surroundings of the involved nerve. This would then erroneously lead to false negative blocks. Our blocks were performed following current routine. We injected 4 cc. of lidocaine to reduce the chance of lidocaine not reaching the nerve. Usually, a volume of 1–2 cc. is used.[19,20,29,30,39,50,66] We think that this factor as such, cannot explain the low predictive value. Recently, it has become possible to directly visualize and identify small nerves with high-resolution ultrasound.[67,68] Whether application of this technique might contribute to optimizing the blocking procedure, and thereby patient selection for surgery, has yet to be established.
Second, there is no consensus as to which envelopment of the proximal stump is optimal. (Fig 4) The proximal stump can be buried in bone[10,14–16,33,43,48,51], muscle,[9,10,12,14–16,18,21–29,33,35,41,46–48,51,52,60,66] vein[12,32,36–38] or nerve.[13,57,59] Local factors in the area of the damaged nerve play a role in neuropathic pain[69] and provocation might not be entirely a mechanical problem.[70] A relative excess of nerve growth factor (NGF) in the surrounding tissue may also play a role.[71] Differences in NGF content between muscle and fat are likely to exist.[72,73] Like others we routinely buried the proximal stump in fat.[55,74] Envelopment of the nerve stump in muscle[75] might give a different effect on pain reduction which will inherently change the predictive value of a block. Given the low values found in our study, it seems unlikely that the effect of envelopment will be of a sufficient magnitude to increase the value of blocking.
Third, in routine practice (Fig 1) patients are frequently told that a local anaesthetic will be given whereas we blinded the patient for the content of the block. We cannot assess the power of the effect of positive expectations because our patients were blinded and we did not include blocks where we revealed the potential working mechanism. It is possible that responses differ if blocks are given in an un-blinded fashion. Whether revealing the content will sufficiently raise the predictive ability of the block to make it valuable is questionable. The effect of saline could be placebo,[76] but also real and based on e.g. diluting local substances involved in the inflammatory response of neuropathic pain. The effect of saline differed in varying degrees from that of lidocaine. Multiple reasons may account for this effect, either separately or in combination. It requires further studies to elucidate this phenomenon. In itself, however, it does not conflict with our findings.
The low accuracy of blocks to predict the effect of surgery probably reflects the multifactorial complex nature and origin of neuropathic pain. Following the nerve injury, the axonopathy and demyelination trigger membrane remodelling in injured afferents and in uninjured neighbours supplying the affected region. Subsequently, cellular excitability increases in part due to sodium (Na+)- channel dysfunction. This leads to ectopic generation of action potentials constituting a primary neuropathic pain signal.[77] Lidocaine inhibits Na+ channels preventing the propagation of action potentials.[78] Theoretically, a Na+ channel-blocking agent should therefore help to relieve neuropathic pain when the impulse source is at or distal to the site of application. In our study, two-third of the patients experienced a strong pain reduction in at least one of the two lidocaine blocks. Pain reduction following both lidocaine blocks was only found in less than half of the patients.
Generally, the duration of the pain relieving effect of infiltration with 1% lidocaine is two to six hours.[79] In our study, the effect varied between several hours and days. Some patients even experienced permanent pain reduction and further treatment was not required. An explanation for these varying effects is difficult to provide. Permanent effects have been observed by others as well.[80]
The varying effect of lidocaine in terms of the duration and intensity indicate that not only Na+- channel dysfunction is at play. Multiple different sites for pain along the neural axis are involved.[69] Central sensitization may have developed since many patients were referred late potentially reducing the effect of peripheral treatment.
Lidocaine is used in the vast majority of the blocks.[7,9,10,12,19–23,29,30,36–39,42,45,46,48–50,52,56,57,60,61,66] It is not known whether a block with a different local anaesthetic, like Bupivacaine[13] with a longer duration of action may increase the accuracy of prediction.
Pain reduction can be achieved with nerve surgery, but to what extent and in which percentage of the patients cannot be summarized from the literature because of the different ways of reporting. In this series, 44% of the patients had good pain relief. Traumatic neuromas show spontaneous discharge activity and ectopic sensitivity to mechanical stimuli.[81] It might be the reduction of these phenomena that underlies the beneficial effect of resection. Strictly speaking, however, part of the effect of surgery might even be placebo.[82]
Immediate surgical repair of the damaged nerve would be optimal, but usually requires a graft. This would then imply sacrificing an intact cutaneous nerve, most often the sural nerve, in order to restore the damaged one. This trade-off is generally considered not favourable enough to justify grafting. Whether biodegradable nerve tubes[83] or cadaveric nerve grafts[84] can serve as an alternative for autologous grafts for this indication has not yet been systematically studied.
The strength of our study is that we only included patients who met the criteria for persistent postsurgical or nerve injury induced probable neuropathic pain.[2] Additionally, the patient was blinded, the blocks were repeated three times and the time course of the effect was noted. Finally, surgery was also offered when the lidocaine blocks were negative. We thereby bypassed a selection bias, which is present when surgery is only carried out in patients whom had a positive block.
We recognize several weaknesses in this study. Ideally, a prospective randomized double blinded set-up would be best. Furthermore, a relative small group of patients was surgically treated. The predictive ability of the block, however, was so low that the possibility that it would be improved by the inclusion of a larger number of patients is small. In our study, 16 different anatomical nerves were damaged, all to a different extent. The penetration of lidocaine might have been influenced by the relative contribution of supportive tissue, the fiber composition, number of fibers and fascicles and fascicular pattern which varies between nerves and individuals.[85] Also, the interval between trauma, block and surgery may have consequences for the response due to central sensitisation. Finally, we did not use questionnaires (such as the McGill one), or non-language based scales (such as the Visual Analogue Score) to document the effect of the block because all have considerable limitations.[86] The VAS score does only provide information about the amount of pain, but not on the duration of the relief. We documented the effect on pain in those categories that are clinically encountered taking both the direction as well as the duration into account.
Offering surgery to patients with focal nerve injury and neuropathic pain only if a pain relieving response to one open block is obtained is probably not a good method. Open blocks may not be as predictive as generally presumed. The selection algorithm needs to be critically re-evaluated.
Supporting information
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006. May;10(4):287–333. 10.1016/j.ejpain.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 2.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008. April 29;70(18):1630–5. 10.1212/01.wnl.0000282763.29778.59 [DOI] [PubMed] [Google Scholar]
- 3.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010. August;9(8):807–19. 10.1016/S1474-4422(10)70143-5 [DOI] [PubMed] [Google Scholar]
- 4.Attal N, Bouhassira D. Pharmacotherapy of neuropathic pain: which drugs, which treatment algorithms? Pain 2015. April;156 Suppl 1:S104–S114. [DOI] [PubMed] [Google Scholar]
- 5.Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 2010. September;17(9):1113–e88. 10.1111/j.1468-1331.2010.02999.x [DOI] [PubMed] [Google Scholar]
- 6.Lipinski LJ, Spinner RJ. Neurolysis, neurectomy, and nerve repair/reconstruction for chronic pain. Neurosurg Clin N Am 2014. October;25(4):777–87. 10.1016/j.nec.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 7.Elliot D, Sierakowski A. The surgical management of painful nerves of the upper limb: a unit perspective. J Hand Surg Eur Vol 2011. November;36(9):760–70. 10.1177/1753193411423140 [DOI] [PubMed] [Google Scholar]
- 8.Algermissen B, Philipp CM, Muller U, Urban P, Berlien H-P. Interstitial thermotherapy (ITT) using Nd:YAG laser as a new option for the treatment of neuroma. Medical Laser Application 2001;16(2):129–34. [Google Scholar]
- 9.Atherton DD, Leong JC, Anand P, Elliot D. Relocation of painful end neuromas and scarred nerves from the zone II territory of the hand. J Hand Surg Eur Vol 2007. February;32(1):38–44. 10.1016/j.jhsb.2006.10.013 [DOI] [PubMed] [Google Scholar]
- 10.Atherton DD, Fabre J, Anand P, Elliot D. Relocation of painful neuromas in Zone III of the hand and forearm. J Hand Surg Eur Vol 2008. April;33(2):155–62. 10.1177/1753193408087107 [DOI] [PubMed] [Google Scholar]
- 11.Bagheri SC, Meyer RA, Khan HA, Wallace J, Steed MB. Microsurgical repair of the peripheral trigeminal nerve after mandibular sagittal split ramus osteotomy. J Oral Maxillofac Surg 2010. November;68(11):2770–82. 10.1016/j.joms.2010.05.065 [DOI] [PubMed] [Google Scholar]
- 12.Balcin H, Erba P, Wettstein R, Schaefer DJ, Pierer G, Kalbermatten DF. A comparative study of two methods of surgical treatment for painful neuroma. J Bone Joint Surg Br 2009. June;91(6):803–8. 10.1302/0301-620X.91B6.22145 [DOI] [PubMed] [Google Scholar]
- 13.Boroumand MR, Schulz D, Uhl E, Krishnan KG. Tibioperoneal Short Circuiting for Stump Neuroma Pain in Amputees: Revival of an Old Technique. World Neurosurg 2015. September;84(3):681–7. 10.1016/j.wneu.2015.04.038 [DOI] [PubMed] [Google Scholar]
- 14.Burchiel KJ, Johans TJ, Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg 1993. May;78(5):714–9. 10.3171/jns.1993.78.5.0714 [DOI] [PubMed] [Google Scholar]
- 15.Burchiel KJ, Johans TJ, Ochoa J. Painful nerve injuries: bridging the gap between basic neuroscience and neurosurgical treatment. Acta Neurochir Suppl (Wien) 1993;58:131–5. [DOI] [PubMed] [Google Scholar]
- 16.Chiodo CP, Miller SD. Surgical treatment of superficial peroneal neuroma. Foot Ankle Int 2004. October;25(10):689–94. 10.1177/107110070402501001 [DOI] [PubMed] [Google Scholar]
- 17.Davies E, Pounder D, Mansour S, Jeffery IT. Cryosurgery for chronic injuries of the cutaneous nerve in the upper limb. Analysis of a new open technique. J Bone Joint Surg Br 2000. April;82(3):413–5. [DOI] [PubMed] [Google Scholar]
- 18.Dellon AL, Mackinnon SE. Treatment of the painful neuroma by neuroma resection and muscle implantation. Plast Reconstr Surg 1986. March;77(3):427–38. [DOI] [PubMed] [Google Scholar]
- 19.Dellon AL, Mont MA, Krackow KA, Hungerford DS. Partial denervation for persistent neuroma pain after total knee arthroplasty. Clin Orthop Relat Res 1995. July;(316):145–50. [PubMed] [Google Scholar]
- 20.Dellon AL, Mont MA, Mullick T, Hungerford DS. Partial denervation for persistent neuroma pain around the knee. Clin Orthop Relat Res 1996. August;(329):216–22. [DOI] [PubMed] [Google Scholar]
- 21.Dellon AL, Aszmann OC. Treatment of superficial and deep peroneal neuromas by resection and translocation of the nerves into the anterolateral compartment. Foot Ankle Int 1998. May;19(5):300–3. 10.1177/107110079801900506 [DOI] [PubMed] [Google Scholar]
- 22.Dellon AL, Kim J, Ducic I. Painful neuroma of the posterior cutaneous nerve of the forearm after surgery for lateral humeral epicondylitis. J Hand Surg Am 2004. May;29(3):387–90. 10.1016/j.jhsa.2004.01.014 [DOI] [PubMed] [Google Scholar]
- 23.Dellon AL, Andonian E, Rosson GD. CRPS of the upper or lower extremity: surgical treatment outcomes. J Brachial Plex Peripher Nerve Inj 2009;4:1 10.1186/1749-7221-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dellon AL. Intercostal neuroma pain after laparoscopic cholecystectomy: diagnosis and treatment. Plast Reconstr Surg 2014. March;133(3):718–21. 10.1097/PRS.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 25.Dellon L, Andonian E, Rosson GD. Lower extremity complex regional pain syndrome: long-term outcome after surgical treatment of peripheral pain generators. J Foot Ankle Surg 2010. January;49(1):33–6. 10.1053/j.jfas.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 26.Ducic I, Mesbahi AN, Attinger CE, Graw K. The role of peripheral nerve surgery in the treatment of chronic pain associated with amputation stumps. Plast Reconstr Surg 2008. March;121(3):908–14. 10.1097/01.prs.0000299281.57480.77 [DOI] [PubMed] [Google Scholar]
- 27.Ducic I, West J, Maxted W. Management of chronic postoperative groin pain. Annals of plastic surgery 2008;60:294–8. 10.1097/SAP.0b013e3180de600e [DOI] [PubMed] [Google Scholar]
- 28.Ducic I, Levin M, Larson EE, Al-Attar A. Management of chronic leg and knee pain following surgery or trauma related to saphenous nerve and knee neuromata. Ann Plast Surg 2010. January;64(1):35–40. 10.1097/SAP.0b013e31819b6c9c [DOI] [PubMed] [Google Scholar]
- 29.Evans GR, Dellon AL. Implantation of the palmar cutaneous branch of the median nerve into the pronator quadratus for treatment of painful neuroma. J Hand Surg Am 1994. March;19(2):203–6. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira KS, Dach F, Speciali JG. Scar neuromas as triggers for headache after craniotomy: clinical evidence. Arq Neuropsiquiatr 2012. March;70(3):206–9. [DOI] [PubMed] [Google Scholar]
- 31.Follmar KE, Williams EH, Dellon AL. Rectal pain of neural origin: Resection of sensory rectal branches of pudendal nerve. Journal of Reconstructive Microsurgery 2015. February;31(2):119–23. 10.1055/s-0034-1384821 [DOI] [PubMed] [Google Scholar]
- 32.Geraghty TJ, Jones LE. Painful neuromata following upper limb amputation. Prosthet Orthot Int 1996. December;20(3):176–81. 10.3109/03093649609164440 [DOI] [PubMed] [Google Scholar]
- 33.Hazari A, Elliot D. Treatment of end-neuromas, neuromas-in-continuity and scarred nerves of the digits by proximal relocation. J Hand Surg Br 2004. August;29(4):338–50. 10.1016/j.jhsb.2004.01.005 [DOI] [PubMed] [Google Scholar]
- 34.Kandenwein JA, Richter HP, Antoniadis G. [Is surgery likely to be successful as a treatment for traumatic lesions of the superficial radial nerve?]. Nervenarzt 2006. February;77(2):175–80. 10.1007/s00115-005-1993-7 [DOI] [PubMed] [Google Scholar]
- 35.Kitagawa R, Kim D, Reid N, Kline D. Surgical management of obturator nerve lesions. Neurosurgery 2009. October;65(4 Suppl):A24–A28. [DOI] [PubMed] [Google Scholar]
- 36.Koch H, Haas F, Hubmer M, Rappl T, Scharnagl E. Treatment of painful neuroma by resection and nerve stump transplantation into a vein. Ann Plast Surg 2003. July;51(1):45–50. 10.1097/01.SAP.0000054187.72439.57 [DOI] [PubMed] [Google Scholar]
- 37.Koch H, Hubmer M, Welkerling H, Sandner-Kiesling A, Scharnagl E. The treatment of painful neuroma on the lower extremity by resection and nerve stump transplantation into a vein. Foot Ankle Int 2004. July;25(7):476–81. 10.1177/107110070402500706 [DOI] [PubMed] [Google Scholar]
- 38.Koch H. Painful neuroma—Mid-term results of resection and nerve stump transposition into veins. European Surgery—Acta Chirurgica Austriaca 2011. December;43(6):378–81. [Google Scholar]
- 39.Lanzetta M, Nolli R. Nerve stripping: new treatment for neuromas of the palmar cutaneous branch of the median nerve. J Hand Surg Br 2000. April;25(2):151–3. 10.1054/jhsb.1999.0355 [DOI] [PubMed] [Google Scholar]
- 40.Loh YC, Stanley JK, Jari S, Trail IA. Neuroma of the distal posterior interosseous nerve. A cause of iatrogenic wrist pain. J Bone Joint Surg Br 1998. July;80(4):629–30. [DOI] [PubMed] [Google Scholar]
- 41.Mackinnon SE, Dellon AL. Results of treatment of recurrent dorsoradial wrist neuromas. Ann Plast Surg 1987. July;19(1):54–61. [DOI] [PubMed] [Google Scholar]
- 42.Martins RS, Siqueira MG, Heise CO, Yeng LT, de Andrade DC, Teixeira MJ. Interdigital direct neurorrhaphy for treatment of painful neuroma due to finger amputation. Acta Neurochir (Wien) 2015. April;157(4):667–71. [DOI] [PubMed] [Google Scholar]
- 43.Mass DP, Ciano MC, Tortosa R, Newmeyer WL, Kilgore ES Jr. Treatment of painful hand neuromas by their transfer into bone. Plast Reconstr Surg 1984. August;74(2):182–5. [DOI] [PubMed] [Google Scholar]
- 44.Melendez MM, Patel A, Dellon AL. The Diagnosis and Treatment of Joplin's Neuroma. J Foot Ankle Surg 2014. December 5. [DOI] [PubMed] [Google Scholar]
- 45.Nahabedian MY, Johnson CA. Operative management of neuromatous knee pain: patient selection and outcome. Ann Plast Surg 2001. January;46(1):15–22. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen JT, Buchanan IA, Patel PP, Aljinovic N, Lee BT. Intercostal neuroma as a source of pain after aesthetic and reconstructive breast implant surgery. J Plast Reconstr Aesthet Surg 2012. September;65(9):1199–203. 10.1016/j.bjps.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 47.Novak CB, van VD, Mackinnon SE. Subjective outcome following surgical management of lower-extremity neuromas. J Reconstr Microsurg 1995. May;11(3):175–7. 10.1055/s-2007-1006527 [DOI] [PubMed] [Google Scholar]
- 48.Rosson GD, Rodriguez ED, George P, Dellon AL. Surgical algorithm for treatment of post-traumatic trigeminal nerve pain. Microsurgery 2010. November;30(8):614–21. 10.1002/micr.20793 [DOI] [PubMed] [Google Scholar]
- 49.Schon LC, Anderson CD, Easley ME, Lam PWC, Trnka HJ, Lumsden DB, et al. Surgical treatment of chronic lower extremity neuropathic pain. Clinical Orthopaedics and Related Research 2001;(389):156–64. [DOI] [PubMed] [Google Scholar]
- 50.Souza JM, Nystrom AN, Dumanian GA. Patient-guided peripheral nerve exploration for the management of chronic localized pain. Plast Reconstr Surg 2012. January;129(1):221–5. 10.1097/PRS.0b013e318221dc12 [DOI] [PubMed] [Google Scholar]
- 51.Stokvis A, Ruijs AC, van Neck JW, Coert JH. Cold intolerance in surgically treated neuroma patients: a prospective follow-up study. J Hand Surg Am 2009. November;34(9):1689–95. 10.1016/j.jhsa.2009.06.003 [DOI] [PubMed] [Google Scholar]
- 52.Stokvis A, van der Avoort DJ, van Neck JW, Hovius SE, Coert JH. Surgical management of neuroma pain: a prospective follow-up study. Pain 2010. December;151(3):862–9. 10.1016/j.pain.2010.09.032 [DOI] [PubMed] [Google Scholar]
- 53.Tennent TD, Birch NC, Holmes MJ, Birch R, Goddard NJ. Knee pain and the infrapatellar branch of the saphenous nerve. J R Soc Med 1998. November;91(11):573–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas AJ, Bull MJ, Howard AC, Saleh M. Peri operative ultrasound guided needle localisation of amputation stump neuroma. Injury 1999. December;30(10):689–91. [DOI] [PubMed] [Google Scholar]
- 55.Vaienti L, Gazzola R, Villani F, Parodi PC. Perineural fat grafting in the treatment of painful neuromas. Tech Hand Up Extrem Surg 2012. March;16(1):52–5. 10.1097/BTH.0b013e31823cd218 [DOI] [PubMed] [Google Scholar]
- 56.Calcagni M, Zimmermann S, Scaglioni MF, Giesen T, Giovanoli P, Fakin RM. The novel treatment of SVF-enriched fat grafting for painful end-neuromas of superficial radial nerve. Microsurgery 2016. October 12. [DOI] [PubMed] [Google Scholar]
- 57.Hanna SA, Catapano J, Borschel GH. Painful pediatric traumatic neuroma: surgical management and clinical outcomes. Childs Nerv Syst 2016. July;32(7):1191–4. 10.1007/s00381-016-3109-z [DOI] [PubMed] [Google Scholar]
- 58.Dellon AL, Coady D, Harris D. Pelvic pain of pudendal nerve origin: Surgical outcomes and learning curve lessons. Journal of Reconstructive Microsurgery 2015. May 1;31(4):283–90. 10.1055/s-0034-1396896 [DOI] [PubMed] [Google Scholar]
- 59.Lidor G, Hall RL, Nunley JA. Centrocentral anastomosis with autologous nerve graft treatment of foot and ankle neuromas. Foot and Ankle International 1996;17(2):85–8. 10.1177/107110079601700205 [DOI] [PubMed] [Google Scholar]
- 60.Williams EH, Williams CG, Rosson GD, Heitmiller RF, Dellon AL. Neurectomy for treatment of intercostal neuralgia. Ann Thorac Surg 2008. May;85(5):1766–70. 10.1016/j.athoracsur.2007.11.058 [DOI] [PubMed] [Google Scholar]
- 61.Wong L. Intercostal neuromas: a treatable cause of postoperative breast surgery pain. Ann Plast Surg 2001. May;46(5):481–4. [DOI] [PubMed] [Google Scholar]
- 62.Zacest AC, Magill ST, Anderson VC, Burchiel KJ. Long-term outcome following ilioinguinal neurectomy for chronic pain. Journal of Neurosurgery 2010. April;112(4):784–9. 10.3171/2009.8.JNS09533 [DOI] [PubMed] [Google Scholar]
- 63.Fischer JE, Bachmann LM, Jaeschke R. A readers' guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med 2003. July;29(7):1043–51. 10.1007/s00134-003-1761-8 [DOI] [PubMed] [Google Scholar]
- 64.Streiner DL, Cairney J. What's under the ROC? An introduction to receiver operating characteristics curves. Can J Psychiatry 2007. February;52(2):121–8. 10.1177/070674370705200210 [DOI] [PubMed] [Google Scholar]
- 65.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011. March 17;12:77 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atherton DD, Elliot D. Relocation of neuromas of the lateral antebrachial cutaneous nerve of the forearm into the brachialis muscle. J Hand Surg Eur Vol 2007. June;32(3):311–5. 10.1016/J.JHSB.2006.10.012 [DOI] [PubMed] [Google Scholar]
- 67.Tagliafico A, Bignotti B, Cadoni A, Perez MM, Martinoli C. Anatomical study of the iliohypogastric, ilioinguinal, and genitofemoral nerves using high-resolution ultrasound. Muscle Nerve 2015. January;51(1):42–8. 10.1002/mus.24277 [DOI] [PubMed] [Google Scholar]
- 68.Gofeld M, Bristow SJ, Chiu S, Kliot M. Preoperative ultrasound-guided mapping of peripheral nerves. J Neurosurg 2013. September;119(3):709–13. 10.3171/2013.5.JNS122243 [DOI] [PubMed] [Google Scholar]
- 69.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006. October 5;52(1):77–92. 10.1016/j.neuron.2006.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elliot D. Commentary on Vaienti et al. Perineural fat grafting in the treatment of painful end-neuromas of the upper limb. J Hand Surg Eur Vol 2013. January;38(1):43 10.1177/1753193412466203 [DOI] [PubMed] [Google Scholar]
- 71.Anand P. Nerve growth factor regulates nociception in human health and disease. Br J Anaesth 1995. August;75(2):201–8. [DOI] [PubMed] [Google Scholar]
- 72.Bullo M, Peeraully MR, Trayhurn P, Folch J, Salas-Salvado J. Circulating nerve growth factor levels in relation to obesity and the metabolic syndrome in women. Eur J Endocrinol 2007. September;157(3):303–10. 10.1530/EJE-06-0716 [DOI] [PubMed] [Google Scholar]
- 73.Freund V, Pons F, Joly V, Mathieu E, Martinet N, Frossard N. Upregulation of nerve growth factor expression by human airway smooth muscle cells in inflammatory conditions. Eur Respir J 2002. August;20(2):458–63. [DOI] [PubMed] [Google Scholar]
- 74.Vaienti L, Merle M, Battiston B, Villani F, Gazzola R. Perineural fat grafting in the treatment of painful end-neuromas of the upper limb: a pilot study. J Hand Surg Eur Vol 2013. January;38(1):36–42. 10.1177/1753193412441122 [DOI] [PubMed] [Google Scholar]
- 75.Souza JM, Cheesborough JE, Ko JH, Cho MS, Kuiken TA, Dumanian GA. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop Relat Res 2014. October;472(10):2984–90. 10.1007/s11999-014-3528-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wager TD. Expectations and anxiety as mediators of placebo effects in pain. Pain 2005. June;115(3):225–6. 10.1016/j.pain.2005.03.018 [DOI] [PubMed] [Google Scholar]
- 77.Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain 2006. January;7(1 Suppl 1):S3–S12. [DOI] [PubMed] [Google Scholar]
- 78.Strichartz G. Molecular mechanisms of nerve block by local anesthetics. Anesthesiology 1976. October;45(4):421–41. [DOI] [PubMed] [Google Scholar]
- 79.Strichartz GR, Berde CB. Local Anesthetics In: Miller RD, editor. Anesthesia. 4th ed. New York, Churchill Livingstone; 1994. p. 489–521. [Google Scholar]
- 80.Loos MJ, Scheltinga MR, Roumen RM. Surgical management of inguinal neuralgia after a low transverse Pfannenstiel incision. Ann Surg 2008. November;248(5):880–5. 10.1097/SLA.0b013e318185da2e [DOI] [PubMed] [Google Scholar]
- 81.Blumberg H, Janig W. Discharge pattern of afferent fibers from a neuroma. Pain 1984. December;20(4):335–53. [DOI] [PubMed] [Google Scholar]
- 82.Moseley JB, O'Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002. July 11;347(2):81–8. 10.1056/NEJMoa013259 [DOI] [PubMed] [Google Scholar]
- 83.de Ruiter GC, Malessy MJ, Yaszemski MJ, Windebank AJ, Spinner RJ. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus 2009. February;26(2):E5 10.3171/FOC.2009.26.2.E5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, et al. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery 2012. January;32(1):1–14. 10.1002/micr.20975 [DOI] [PubMed] [Google Scholar]
- 85.Sunderland S. Nerve injuries and their repair: A critical appraisal. Edingburgh, London, Melbourne, New York: Churchill Livingstone; 1991. [Google Scholar]
- 86.Bourke J. The Story of Pain: From Prayer to Painkillers. 1 ed. Oxford University Press; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.