Abstract
Monogamous pair bonds helped solve ancestral problems pertinent to our survival as a species. In order for these pair bonds to succeed, biological systems were co-opted to support and reinforce attachment bonds through feelings of pleasure and reward. One of the major biological systems that may play an important role in the formation of romantic attachments is the stress response system (autonomic nervous system and hypothalamic–pituitary–adrenal axis). Research suggests attraction, mate preference, and emotional connectedness may be supported by the activation or inhibition of the stress response system. Further, as romantic relationships progress, new findings suggest partners’ physiological patterns coalesce, potentially serving a regulatory function that reinforces the pair bond and affects overall well-being. Based on this evidence, the current paper puts forth the Physiology of Romantic Pair Bond Initiation and Maintenance Model, which will provide researchers with a new perspective on the function of the stress response system in romantic relationships.
1 | INTRODUCTION
Monogamous pair bonds are viewed as a vital building block of contemporary society and are thought to be linked to higher financial resources, greater relationship stability, and optimal child development. At the beginning of our ancestral history, the transition to monogamy solved a variety of evolutionary challenges but required the co-opting of biological systems for its conception and ongoing success. The current paper reviews empirical data that suggests one of those biological systems is the human stress response system (SRS), with activation of the SRS potentially playing a critical role in the formation and maintenance of romantic pair bonds. We will begin with a brief summary of the evolutionary origins of monogamous pair bonds and the ancestral pressures monogamy helped solve, in addition to biobehavioral correlates of early romantic relationships. Then we will introduce and discuss the role of stress physiology, with attention to ways in which the autonomic nervous system (ANS) and the hypothalamic–pituitary–adrenal (HPA) axis may have been rewired to support the formation and ongoing maintenance of romantic bonds. Based on the empirical evidence reviewed, we will introduce a conceptual model that depicts the contextually and developmentally dependent nature of stress physiology and relationship processes.
2 | THE EVOLUTION OF PAIR BONDS IN HUMANS
From an evolutionary perspective, organisms have the primary goal of passing on their genetic material to the next generation through successful mating and the production of viable offspring. Successful reproduction may have been largely facilitated by group living and the formation of intense social bonds. Research on nonhuman primates supports the survival mechanisms of group living, with females who were more integrated into their groups and had stronger emotional bonds also enjoying greater reproductive success and lower infant mortality (Silk, Alberts, & Altmann, 2003; Silk et al., 2010). For Homo sapiens, the evolution of monogamous pair bonds is thought to have largely increased reproductive success. The discovery of a common ancestor between ape-like descendants and the early Homo illuminated a divergence in physical characteristics (e.g., comparable male and female body size and smaller canine teeth), which suggests transitioning to monogamous pair bonds may have led to a reduction in male-to-male competition (Lovejoy, 2009). This alternative reproductive strategy likely evolved from a greater female preference for nonaggressive males who provided valuable foods in exchange for copulation, co-occurring with higher levels of female fidelity (Gavrilets, 2012). This life history transformation (coevolution of male provisioning and female faithfulness) is thought to have led to monogamous pair bonding as the preferred reproductive strategy (Gavrilets, 2012). While in modern society reproduction and raising offspring most often occur within the context of a monogamous relationship, the consequences of transitioning from a promiscuous to a pair bonding species, and the mechanisms that facilitate pair bond formation are not entirely clear.
The adaptation of monogamy as a pathway to reproductive success is thought to have facilitated other life history traits, namely, division of domestic tasks and increased paternal investment in childcare (Kaplan, Hill, Lancaster, & Hurtado, 2000). Monogamous pair bonds increased fitness by providing access to high caloric foods more easily, and offsetting the biological costs of navigating exposure to predators while foraging for food and caring for young at the same time (Beckes & Coan, 2011; Fletcher, Simpson, Campbell, & Overall, 2015). As seen in hunter–gatherer societies, men are able to hunt for game and fish whereas women gather foods more easily accessible while caring for offspring (Marlowe, 2007). In addition to greater access to food, romantic pair bonds encouraged males to share in the burden of postnatal parental investment (e.g., Dunbar & Shultz, 2007). The human infant is born underdeveloped and helpless, requiring a protective environment with intensive caregiving. Increased paternal involvement improved survival and optimal development for the immature human infant (Flinn, Ward, & Noone, 2005). The increase in reproductive success afforded by monogamy is hypothesized to have evolutionarily selected for the development of larger human brains with more complex emotional circuitry to support greater social intelligence (e.g., ability to read and interpret social cues; Fletcher et al., 2015). This evolutionary adaptation was necessary for the success of future pair bond formation given the unique behavioral and physiological correlates of falling in love.
2.1 | It was love at first sight: The behavioral and biological correlates of falling in love
Falling in love is one of the most widely recognized and universal experiences of human life; it has been documented across cultures and is a target of countless novels and films (Jankowiak & Fischer, 1992). During this early phase of a relationship, individuals are quick to report high levels of passion and commitment, often referred to as feelings of “romantic love,” in addition to displaying behaviors that promote both emotional and sexual intimacy (Aaron & Westbay, 1996; Clark, Shaver, & Abrahams, 1999; Fletcher et al., 2015). The sensations of romantic love have often been described as an addiction, with new lovers experiencing obsessive thoughts of one another, seeking constant proximity, and displaying self-sacrificing behaviors (e.g., neglecting work and friends) for the relationship (Burkett & Young, 2012). Along with the high levels of pleasure experienced during this time, new lovers also report preoccupation, worry, and insecurity, about the new relationship (García, 1998), illuminating the dynamic nature of this period of time.
Past research comparing romantic pair bonds to other close relationships, namely, the bond between a mother and an infant, have found remarkable behavioral similarities suggesting the potential for activation of similar neural pathways during the formation of both pair bonds (Bartels & Zeki, 2004; Corter & Fleming, 2002; Hazan & Shaver, 1987). For one, they are both characterized by a strong desire for proximity to each other, both partners’ display hyper awareness and sensitivity to each other’s cues, and there is a marked increase in distress when partners are separated for prolonged lengths of time (Sbarra & Hazan, 2008; Shaver, Hazan, & Bradshaw, 1988). Thus, it has been suggested that ancient biological mechanisms that facilitate the formation of mother–infant bonds were co-opted to facilitate monogamous pair bonds between adult partners, which in turn facilitate the survival of the human infant (Fletcher et al., 2015; Sbarra & Hazan, 2008). Specifically, neuroendocrine systems are thought to have been recalibrated to facilitate approach to novel partners, preference for a specific partner, and ongoing rewarding effects of solidified pair bonds (DeVries, DeVries, Taymans, & Carter, 1995).
Forming and maintaining close emotional bonds is not a trivial task, requiring complex cognitive functions to perceive, decipher, and respond to social cues in ways that maintain cohesion and defuse conflict (e.g., Byrne & Whiten, 1992). To facilitate harmonious and cooperative cohabitation with others, primitive biological systems likely evolved a heightened sensitivity to social threats (Silk, Cheney, & Seyfarth, 2013). Human and animal studies have found brain regions that underlie reward and motivation (e.g., ventral tegmental area), and molecules implicated in parent–infant bonds (e.g., oxytocin and dopamine), may also be important for romantic pair bonds (e.g., Acevedo, Aron, Fisher, & Brown, 2011; Aron et al., 2005; Bales & Carter, 2003; Ditzen et al., 2009). However, aside from animal research utilizing monogamous rodent species (e.g., prairie voles; DeVries et al., 1995), few human studies have examined how stress physiology may also directly support the formation of romantic pair bonds, a dynamic and important phase for the longevity of a relationship (e.g., Weisman, Schneiderman, Zagoory-Sharon, & Feldman, 2015).
2.2 | The “stress” of love, how the human stress response may support pair bonding
The term “stress” in the context that it is most widely used was first coined by the physician Hans Selye, the father of stress research, to refer to a “non-specific response of the body to any demand for change” (Selye, 1973). Within Selye’s framework, stress reflects the body’s reaction to a wide range of stimuli (termed “stressors”) from normative events such as a passionate kiss between new lovers to adverse circumstances such as being chased by a predator. Although today the term stress is almost exclusively used to refer to negative situations with the potential to tax individuals’ mental and physical resources, this is one side of a complex coin in which a stressor can be both a positive or negative event (Koolhaas et al., 2011; Shirtcliff, Peres, Dismukes, Lee, & Phan, 2014). This notion was depicted by Selye’s inverted u-shaped curve that identified “eustress” or positive stress as a performance enhancer. In fact, the SRS, made up of the ANS and the HPA axis, plays a critical role in facilitating behavior through metabolic and cardiovascular preparation of the body, mediating openness of the individual to environmental inputs (Sapolsky, Romero, & Munck, 2000).
The ANS is a faster acting system consisting of the parasympathetic nervous system (PNS) and sympathetic nervous system (SNS), activating both respiratory and cardiovascular processes, and resulting in the release of catecholamines (Chrousos & Gold, 1992). In the most simplistic view, a perceived stressor leads to PNS withdraw of its inhibitory control over the heart (i.e., the vagal brake), while the SNS increases heart rate, sweat secretion, and the release of epinephrine and norepinephrine (Kemeny, 2003; Levenson, 2014). In contrast, the HPA axis is a slower acting system beginning with the release of the corticotrophin-releasing hormone from the hypothalamus and culminating in the release of the steroid hormone, cortisol, from the adrenal cortex (Gunnar, Doom, & Esposito, 2015). The release of cortisol helps direct glucose and other resources to various areas of the body, necessary for mounting a response to an environmental stimulus while simultaneously reducing the activation of less critical processes such as digestion and immune regulation (Sapolsky, 2005).
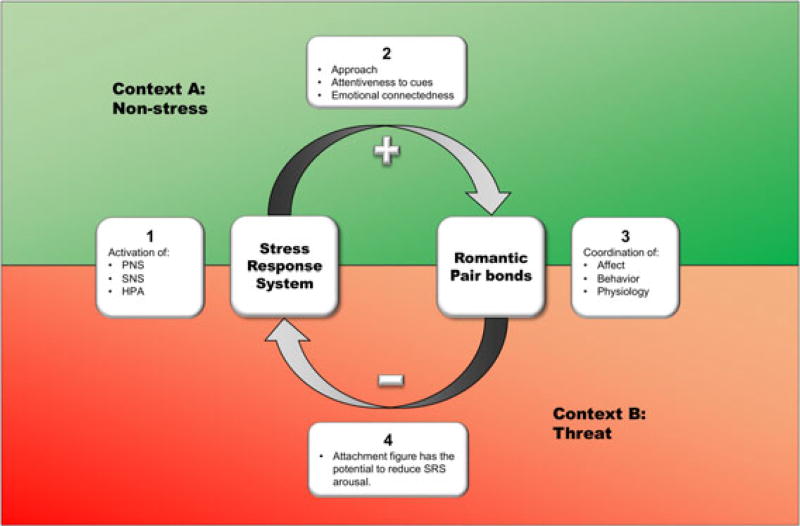
Several theoretical perspectives such as the polyvagal theory (Porges, 1995) and the “tend and befriend” hypothesis (Taylor, 2006) illustrate the potential for stress physiology to underlie affiliative processes, in addition to frameworks such as social baseline theory that suggest affiliation also regulates stress physiology (Beckes & Coan, 2011). The current paper proposes the Physiology of Romantic Pair Bond Initiation and Maintenance Model referred to as Physiology of Pair Bonding for short (PPB) (see Figure 1), which expands on this view and suggests stress physiology, may also play an important role in normative (i.e., non-stress) relationship functioning by facilitating an openness to input from partners, and the interpretation and encoding of affective cues. The PPB model highlights biobehavioral relationships as contextually dependent (Sapolsky et al., 2000; Schachter & Singer, 1962), with the potential for stress physiology to support engagement in affiliative behaviors if it is not overridden by contextual stressors. In other words, the SRS only facilitates romantic relationships in non-stress/normative environments, labeled context A in the diagram (Figure 1). In this model, we suggest activation of the SRS is important during the initial stage of a romantic relationship because it facilitates approach processes and helps organize behavior and attentiveness to social cues. However, once romantic pair bonds are formed, partners seek each other in times of duress, and likely play an important role in dampening activation of the SRS under conditions of threat and challenge (context B). In the following sections, we discuss the empirical evidence that supports the central tenets of the conceptual model presented (Figure 1), thus illustrating the ways in which the SRS functions to facilitate relationship initiation and maintenance.
FIGURE 1.
The Physiology of Romantic Pair Bond Initiation and Maintenance Model depicts reciprocal effects of the stress response system (SRS) and romantic pair bonds, and their dependence on the context in which they occur. In a non-stress environment (context A), activation (+) of the SRS (Box 1) may serve a necessary function for pair bond formation by facilitating approach, attention, and connectedness (Box 2). During the solidification of a pair bond, partners affect, behavior, and physiology become coordinated over time, facilitating cohesion and perspective taking among partners (Box 3). Therefore, in the presence of a threat or challenge (context B) the presence of a romantic partner helps regulate or dampen (−) activation of the SRS (Box 4)
2.2.1 | SRS and relationship initiation
Social communication is largely enabled by the expression, interpretation, and response to emotional cues, functions that are all largely synonymous with activation of the ANS, and in particular the PNS (Levenson, 2014). The PNS regulates a wide range of bodily activities, from heart rate, salivary flow, stomach and intestinal activity (“gut feelings”), to the release of tears; all with the capacity to influence the experience and display of emotional arousal (Levenson, 2014; Vianna & Tranel, 2006). It is no surprise that the ANS is thought to play a central role in the formation of attachment bonds and feelings of romantic love, an effect that is in part mediated by oxytocin which contains receptors on the myelinated vagus (Carter, 1998). The vagus is the 10th and largest cranial nerve, it facilitates emotional expression and shared eye gazing, important components of positive social engagement (Carter, 2014; Porges, 2007). PNS control (specifically dampening) of heart rate through the vagus nerve (coined the vagal “brake”) during times of distress or conflict allow individuals to remain calm and engaged, facilitating healthy emotion regulation and positive social interactions. Conversely, decreases in vagal activation are associated with increased heart rate and potentially negative mood states (Beauchaine, 2001; Thayer & Lane, 2000). Regulation of emotional states, and in particular, the display of daily brief positive emotions (e.g., amusement), may be crucial during the initiation of a romantic bond and for the longevity of an established romantic relationship (Gottman & Levenson, 2000).
During the initial stage of a romantic relationship, partners must be willing to socially engage and reciprocate affective cues to encourage future communication, emphasizing the importance of ANS-mediated effects on emotion regulation. Differences in emotion regulation due to vagal influences have been largely documented through the examination of respiratory sinus arrhythmia (RSA), fluctuations in heart rate linked to breathing (Porges, 1995). Emotion regulation strategies have been associated with positive changes in RSA and greater emotional expressivity during social interactions (Butler, Wilhelm, & Gross, 2006). Further, PNS activation has been found to contribute to feelings of greater emotional connectedness (Grewen & Light, 2011), with studies in romantic couples suggesting high vagal regulation of the heart leads to higher levels of positive affect and a greater frequency of positive interactions (e.g., Diamond, Hicks, & Otter-Henderson, 2011). The empirical evidence presented in support of the hypothesized role of heightened activation of the ANS, and in particular the PNS, in facilitating pair bond formation, fits within the PPB model introduced above (see Figure 1, Box 2). While limited, these findings suggest autonomic functioning is responsive to social interactions and during the formation of a new bond has the capability to serve important emotion regulatory functions.
In addition to the ANS, the HPA axis is a slower acting and more valanced arm of the SRS. HPA activation was designed to assist in mounting a response in the face of predatory threats, but has also evolved sensitivities to social threats helping support and reinforce the human need for interpersonal belonging and thus the “social self” (Dickerson & Kemeny, 2004). Contexts that are perceived as socially evaluative, unpredictable, or uncontrollable (i.e., a threat context depicted in Figure 1 Context B) produce the largest increases in cortisol (Dickerson & Kemeny, 2004). However, past findings also suggest increases in HPA activity may not only occur in response to duress but may also play a normative function in conditions of non-stress (Context A) through the facilitation of mental processes necessary to engage in social interaction (e.g., increased mind reading skills or accuracy in interpretation of social cues) (Corter & Fleming, 2002; Flinn, Nepomnaschy, Muehlenbein, & Ponzi, 2011; Shirtcliff et al., 2014; Smeets, Dziobek, & Wolf, 2009).
Results stemming from rodent and human studies provide support for the role of cortisol in the initiation and formation of romantic relationships (Weisman et al., 2015). Studies on prairie voles (a monogamous species) suggest that the inhibition of endogenous corticosterone (primary rodent glucocorticoid similar to cortisol in humans) results in a faster romantic pair bond formation, while injections of corticosterone result in preferences for novel over partnered male voles (DeVries et al., 1995). A more recent study examined the effect of cortisol reactivity to a stressor on self-reported levels of perceived closeness to a stranger. The findings suggested men who exhibited cortisol reactivity reported greater levels of perceived social closeness relative to men who did not experience cortisol elevations (Berger, Heinrichs, von Dawans, Way, & Chen, 2016). These results highlight the possibility for cortisol fluctuations to underlie attachment formation by acting as a catalyst, with high cortisol levels increasing the need and desire for social contact. During the initial stages of romantic love, increases in cortisol may support approach to novel individuals and indicate a state of hypervigilance due to the uncertainty and novelty during this transient stage (Loving, Crockett, & Paxson, 2009; Marazziti & Canale, 2004; Weisman et al., 2015). Specifically, individuals who self-report high levels of passionate love displayed high levels of neurotrophin nerve growth factor that can upregulate HPA activity by increasing levels of circulating cortisol (Emanuele et al., 2006). Further, women in new romantic relationships who reported higher levels of relationship focused thinking had greater cortisol reactivity when asked to think about their romantic partner compared to women asked to think of a friend of the opposite sex (Loving et al., 2009).
2.2.2 | SRS and relationship maintenance
Once a romantic relationship is established, the intensity and unique pressures associated with maintaining cooperative relationships and their ongoing success continues to necessitate psychological and physiological resources (Fletcher et al., 2015). Romantic partners must continually negotiate their individual needs within the context of their partners’ needs and navigate numerous day-to-day hassles. The need for continual collaboration calls for heightened attention to cognitions and affective cues expressed by partners (Finkel, Hui, Carswell, & Larson, 2014; Flinn et al., 2011). It is no surprise that disputes and disagreements are commonplace among romantic partners, potentially threatening an individual’s identity within the relationship and thus the “social self.” Therefore, in established couples, activation of the SRS may function in different ways relative to new relationships.
First, as depicted in Box 3 of the PPB model (Figure 1), recent investigations have uncovered a pattern of linked or correlated stress physiology between romantic partners (e.g., Liu, Rovine, Cousino Klein, & Almeida, 2013; Saxbe & Repetti, 2010). We propose that this physiological linkage facilitates maintenance of pair bonds by promoting cohesion and emotional connectedness. Harmonious group living requires individuals to closely coordinate their behavior and effectively communicate to convey their motives and intensions. These unique social pressures are thought to have contributed to social animals’ evolution of unique sensitivities to others’ emotions and internal states (Barsäde & Gibson, 1998; Nesse & Ellsworth, 2009). It is this sensitivity that may allow partners’ emotional channels (subjective experience, behavior, and physiology; Butler, 2011) to, over time, become uniquely calibrated to the presence of their romantic partner and fluctuate in a reciprocal pattern (for a review, see Butler & Randall, 2013; Timmons, Margolin, & Saxbe, 2015). The display of synchronous behavioral interactions that involve reciprocity in positive affect and affectionate touch are thought to be critical to the success of pair bonds, both parent–child and romantic (e.g., Schoebi, 2008). Further, this coordination in behavior may be underlined by the presence of physiological linkage, which has been hypothesized to contribute to the dyad’s shared homeostasis (i.e., stability and constancy in biological functioning), higher relationship connectedness, and increased well-being (Butler, 2011; Butler & Randall, 2013; Sbarra & Hazan, 2008). Likewise, greater physiological linkage has been related to greater relationship satisfaction (Helm, Sbarra, & Ferrer, 2014; Saxbe & Repetti, 2010) and physical closeness (Papp, Pendry, Simon, & Adam, 2013). Compared to strangers, romantic partners are more likely to display synced heart rates while in close proximity and during active engagement (Helm, Sbarra, & Ferrer, 2012), and greater linkage has been associated with higher empathetic accuracy suggesting emotional connectedness (Levenson & Ruef, 1992).
However, as can be seen in the PPB model (Figure 1) with Box 3 situated in both the green “non-stress” and red “threat” areas, being physiologically linked is not unilaterally related to positive couple characteristics and outcomes. For example, just as the transmission and reception of physiology within the context of positive emotions may coordinate group behaviors and mutual understanding of group needs (Butler, 2011; Spoor & Kelly, 2004), the linkage of stress physiology within negative or threatening contexts may also contribute to escalation of hostile behavior patterns and relationship dissatisfaction (e.g., Levenson & Gottman, 1983). Thus, we also propose that in threatening contexts (Context B) physiological linkage may lead to escalation of negative mood states, preventing adaptive recovery for romantic partners. Specifically, some research indicates an association between increased physiological linkage and relationship dissatisfaction, disagreement, and conflict behaviors (Levenson & Gottman, 1983; Liu et al., 2013). Similarly, in a sample of married couples, both higher marital satisfaction and increased negative mood were related with higher linkage in cortisol levels across the day (Saxbe & Repetti, 2010). While still novel, future research should continue to examine ways in which being physiologically linked may reinforce romantic pair bonds versus deter them, depending on relationship dynamics.
Second, as depicted in Figure 1, Box 4, the PPB model builds off of the consistently documented phenomenon of social regulation/social buffering (e.g., Ditzen et al., 2007; Eisenberger, Taylor, Gable, Hilmert, & Lieberman, 2007; Hostinar, Sullivan, & Gunnar, 2014). Specifically, numerous studies have described the physiological and psychological stress reduction that occurs in the presence of attachment figures (see Hostinar et al., 2014). As a social species, humans are hardwired to seek resources from social partners in the face of threat, and function optimally in the presence of others compared to being in isolation (Beckes & Coan, 2011). A defining characteristic of a securely attached pair bond is the association of “psychological safety and physiological calmness” felt between partners (Sbarra & Hazan, 2008). Thus, marital partners function as external regulators of each other’s physiological arousal (e.g., Feeney & Kirkpatrick, 1996; Sbarra & Hazan, 2008). Specifically, past studies suggest satisfied marital partners demonstrate reductions in cortisol output across the day and in the presence of a partner, demonstrate reduced reactivity to stress (e.g., Ditzen et al., 2007; Kirschbaum, Klauer, Filipp, & Hellhammer, 1995). In a more recent study examining how autonomic reactivity functions differently in new lovers compared to single individuals, new lovers were found to display greater RSA activation (lower stress reactivity) while being exposed to a negative relationship vignette relative to single individuals (Schneiderman, Zilberstein-Kra, Leckman, & Feldman, 2011). However, social buffering effects of romantic partners are not exclusive to new lovers and have been extensively studied in established couples, making social buffering an important but familiar part of the current conceptual model.
2.2.3 | The physiology of romantic pair bond initiation and maintenance model
In order to synthesize the empirical evidence presented above, we will summarize and reiterate the points presented by the Physiology of Romantic Pair Bond Initiation and Maintenance Model, referred to as PPB. The PPB model emphasizes the role of the SRS (Box 1) in supporting an individual’s openness to the environment (Box 2); as such, activation of the SRS is dependent on the context at hand. We demonstrate two general contexts, a normative (non-stress) environment depicted in green, and a threatening environment presented in red. Boxes overlap both contexts to illuminate the diversity in functionality of these systems, and how accurate interpretation of biobehavioral relationships depends on the context in which activation unfolds. Within a non-stress environment, activation of the SRS is thought to support approach, attentiveness, and emotional connectedness (Box 2), which are critical for the initiation and maintenance of romantic pair bonds. Thus, in a non-stress environment, increases in SRS reactivity may facilitate important pair bond processes. Romantic partners in turn are thought to become more behaviorally and physiologically similar over time, which may facilitate cohesion and promote the longevity of a bond (Box 3). Within the context of a perceived threat, the presence of a close partner is thought to dampen physiological reactivity facilitating an adaptive and quick recovery (see Box 4). However, it is also possible that similarity in physiological and behavioral patterns occurring in the context of threat may also adversely affect the pair bond and prevent adaptive regulation, and social buffering. The presentation of the PPB model reflects a cyclical pattern highlighting the potential for continuity between these processes. Specifically, SRS activation has the potential to continually facilitate relationship behavior, and close partners have the ongoing capability to regulate each other’s physiological arousal. Similarly, coordination between partner’s emotional and physiological states has the potential to facilitate both SRS regulation and dysregulation as they engage in the ebb and flow of their daily lives.
2.3 | In sickness and in health: An evolutionary paradox
While the PPB model illuminates how activation of the SRS adaptively leads to the initiation and continuation of pair bonds, it is also important to acknowledge constant activation of biological systems and their chemical mediators may give rise to harmful effects on normative functioning (McEwen, 2008). The acute activation of the SRS facilitates important behavioral adaptations needed to regain emotional and biological stability, a process referred to as “allostasis” (Sterling & Eyer, 1988). However, the chronic activation of these systems over time produces significant wear and tear on the body, and jeopardizes future functioning, a consequence coined “allostatic load” (McEwen, 2008). Allostatic load may dysregulate normative functioning manifesting as hyper (toomuch) or hypo (not enough) activation, and increase susceptibility to negative physical and mental health outcomes (McEwen, 2008).While both arms of the SRS are activated by the presence of social threat, they are also dampened by the presence of close partners, indicating attachment partners’ ability to both buffer and heighten the effects of stress on later disease and health outcomes. Thus, it is not surprising that the past half century of research on the health implications of social relationships has consistently demonstrated a link between strong social bonds and lower mortality rates (Holt-Lunstad, Smith, & Layton, 2010;House, Landis, & Umberson, 1988; Kessler, 1979; Robles et al., 2014). The quality and quantity of social relationships in an individual’s life predict their survival, and rival the magnitude of effects other public health concerns such as smoking and obesity have on longevity (Holt-Lunstad et al., 2010). The link between close relationships and health outcomes underscores the importance of research on relationship processes and their biological correlates.
3 | FUTURE DIRECTIONS AND CONCLUSIONS
The purpose of the current review was to illuminate the evolutionary and modern-day importance of romantic human pair bonds. Building off of this evolutionary framework, we put forth a working conceptual model highlighting the critical role of the human stress response in the formation of romantic relationships, and their ongoing maintenance. This model extends past models that suggest SRS activation may underlie affiliative processes in the context of threat, by suggesting these functions are not exclusive to threatening situations and may provide critical support for the cognitive and affective processes that unfold during daily social interactions. The PPB model provides a new lens by which to interpret mixed findings that exist in the current literature, with activation of the SRS occurring in both positive and negative contexts, and physiological linkage relating to both positive and negative relationship outcomes. In modern society, attachment partners continue to play an important role in an individual’s physical and mental health; however, the micro-level biological processes that unfold during romantic interactions are still not entirely clear. Past human research has yielded numerous discrepancies in the direction of effects and the adaptive significance of heightened physiological arousal, leaving relationship scientists to attempt to disentangle mixed findings regarding physiological processes in the context of social relationships (see Shirtcliff et al., 2014).
As depicted in the current paper, more research on the activation of the SRS during the initial stages of romantic bonds, and how these biological profiles might predict successful or failed encounters, will contribute to our growing knowledge of the implications of relationships on health. While numerous studies conducted on rodent models have mapped various biological systems that may enable the solidification of a pair bond (see Carter, Devries, & Getz, 1995; Young, Gobrogge, Liu, & Wang, 2011), replication with human relationships has only recently gone underway (e.g., Weisman et al., 2015). Past studies have solely focused on the regulatory functions of established relationships (e.g., Ditzen, Hoppmann, & Klumb, 2008), and almost exclusively examined relationship processes in stressful contexts (e.g., Brooks, Robles, & Schetter, 2011; Kiecolt-Glaser et al., 1996; Pietromonaco, DeBuse, & Powers, 2013). The PPB model helps guide future studies by illuminating potential pathways by which physiological activation may facilitate important relationship functions. Currently the top half of the model (Context A) is largely theoretical given the limited amount of research on physiological changes during the initial stages of a romance. Studies that incorporate “baseline” or “comparison” measures before relationship initiation and in the months that follow are needed to support the role the SRS plays during this time. Numerous obstacles may arise for research in this area, such as the ambiguity in the definition of what an “early” stage romance is temporally (1, 2, or 3 months?) and when nonexclusive dating transitions to a monogamous relationship. In addition, whether individual differences exist in the rate of progression within a relationship, and in turn, impact biological changes during this time, is a research question of interest.
The PPB model has several caveats; its singular focus on the SRS, lack of discrimination among subsystems, and temporal ambiguity make it a “working” model. Specifically, it is likely that interactions across biological systems are occurring during this time, with neuropeptides such as oxytocin and vasopressin (Carter & Porges, 2013), and hormones such as estrogen and testosterone (e.g., Schneiderman, Kanat-Maymon, Zagoory-Sharon, & Feldman, 2014), all playing a meaningful role. Secondly, the subsystems underlying the SRS may also have differential effects during this time, an area worthy of further study. Nevertheless, the PPB model provides a valuable starting point for future research by highlighting the adaptability of stress physiology. The model posits activation is not always indicative of distressful and threatening situations, and both deactivation and hyperactivation have the ability to support vital relationship processes, an idea previously discussed by Shirtcliff et al. (2014). The limited but growing findings surrounding the biological correlates of early romantic relationships leave ample room for future research in this exciting and important area of study.
Acknowledgments
Funding information
National Institute of Child Health and Development, Grant/Award Number: HD066269-01A1. National Institute of Mental Health, Grant/Award Number: T32MH015750.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biographies
Evelyn Mercado is a National Institute of Mental Health postdoctoral trainee in Health Psychology at UCLA. Her research focuses on understanding how both stressful and positive family interactions (romantic and parent–child) impact indices of allostatic load (adrenocortical, autonomic, and immune functioning), rendering individuals more or less susceptible to adverse health outcomes. Her dissertation work focused on the physiological correlates of marital relationships in both positive and negative contexts. She received her BA in Psychology from California State University, San Marcos, and went on to complete her MS in Human Development and Family Studies at Purdue University. In 2016, she received her PhD in Human Development and Family Studies from the University of California, Davis.
Dr. Leah Hibel is an Associate Professor in the Department of Human Ecology. Dr. Hibel’s research focuses on maternal and child health particularly in the context of family stress. She takes a biopsychosocial approach to studying the impact of stress on parent–child relationships, and parents’ and children’s mental and physical well-being. Her research is currently funded by a grant from the National Institute of Child Health & Human Development. Dr. Hibel received her BS in Behavioral Biology from Johns Hopkins University. In 2009, she received her PhD in Biobehavioral Health from Penn State University, with a minor in Human Development and Family Studies.
References
- Aaron A, Westbay L. Dimensions of the prototype of love. Journal of Personality and Social Psychology. 1996;70(3):535. [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;94(1):327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Acevedo BP, Aron A, Fisher HE, Brown LL. Neural correlates of long-term intense romantic love. Social Cognitive & Affective Neuroscience. 2011;7:145–159. doi: 10.1093/scan/nsq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales K, Carter CS. Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 2003;117(4):854–859. doi: 10.1037/0735-7044.117.4.854. [DOI] [PubMed] [Google Scholar]
- Barsäde SG, Gibson DE. Group emotion: A view from top and bottom. Research on Managing Groups and Teams. 1998;1:81–102. [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21(3):1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beckes L, Coan JA. Social baseline theory: The role of social proximity in emotion and economy of action. Social and Personality Psychology Compass. 2011;5(12):976–988. [Google Scholar]
- Berger J, Heinrichs M, von Dawans B, Way BM, Chen FS. Cortisol modulates men’s affiliative responses to acute social stress. Psychoneuroendocrinology. 2016;63:1–9. doi: 10.1016/j.psyneuen.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Brooks KP, Robles TF, Schetter CD. Adult attachment and cortisol responses to discussions with a romantic partner. Personal Relationships. 2011;18(2):302–320. [Google Scholar]
- Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology. 2012;224(1):1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA. Temporal interpersonal emotion systems the “TIES” that form relationships. Personality and Social Psychology Review. 2011;15(4):367–393. doi: 10.1177/1088868311411164. [DOI] [PubMed] [Google Scholar]
- Butler EA, Randall AK. Emotional coregulation in close relationships. Emotion Review. 2013;5(2):202–210. [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43(6):612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Byrne RW, Whiten A. Cognitive evolution in primates: Evidence from tactical deception. Man. 1992:609–627. [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23(8):779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS. Oxytocin pathways and the evolution of human behavior. Annual Review of Psychology. 2014;65:17–39. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- Carter CS, Devries AC, Getz LL. Physiological substrates of mammalian monogamy: The prairie vole model. Neuroscience & Biobehavioral Reviews. 1995;19(2):303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS, Porges SW. The biochemistry of love: An oxytocin hypothesis. EMBO Reports. 2013;14(1):12–16. doi: 10.1038/embor.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- Clark CL, Shaver PR, Abrahams MF. Strategic behaviors in romantic relationship initiation. Personality and Social Psychology Bulletin. 1999;25(6):709–722. [Google Scholar]
- Corter C, Fleming AS. Psychobiology of maternal behavior in human beings. In: Bornstein MH, editor. Handbook of parenting: Biology and ecology of parenting. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. pp. 141–182. [Google Scholar]
- DeVries AC, DeVries MB, Taymans S, Carter CS. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proceedings of the National Academy of Sciences. 1995;92(17):7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond LM, Hicks AM, Otter-Henderson KD. Individual differences in vagal regulation moderate associations between daily affect and daily couple interactions. Personality and Social Psychology Bulletin. 2011;37(6):731–744. doi: 10.1177/0146167211400620. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Neumann ID, Bodenmann G, von Dawans B, Turner RA, Ehlert U, Heinrichs M. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 2007;32(5):565–574. doi: 10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Hoppmann C, Klumb P. Positive couple interactions and daily cortisol: On the stress-protecting role of intimacy. Psychosomatic Medicine. 2008;70(8):883–889. doi: 10.1097/PSY.0b013e318185c4fc. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65(9):728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Dunbar RI, Shultz S. Evolution in the social brain. Science. 2007;317(5843):1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage. 2007;35(4):1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele E, Politi P, Bianchi M, Minoretti P, Bertona M, Geroldi D. Raised plasma nerve growth factor levels associated with early-stage romantic love. Psychoneuroendocrinology. 2006;31(3):288–294. doi: 10.1016/j.psyneuen.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Feeney BC, Kirkpatrick LA. Effects of adult attachment and presence of romantic partners on physiological responses to stress. Journal of Personality and Social Psychology. 1996;70(2):255. doi: 10.1037//0022-3514.70.2.255. [DOI] [PubMed] [Google Scholar]
- Finkel EJ, Hui CM, Carswell KL, Larson GM. The suffocation of marriage: Climbing Mount Maslow without enough oxygen. Psychological Inquiry. 2014;25(1):1–41. [Google Scholar]
- Fletcher GJ, Simpson JA, Campbell L, Overall NC. Pair-bonding, romantic love, and evolution: The curious case of Homo sapiens. Perspectives on Psychological Science. 2015;10(1):20–36. doi: 10.1177/1745691614561683. [DOI] [PubMed] [Google Scholar]
- Flinn MV, Nepomnaschy PA, Muehlenbein MP, Ponzi D. Evolutionary functions of early social modulation of hypothalamic-pituitary-adrenal axis development in humans. Neuroscience & Biobehavioral Reviews. 2011;35(7):1611–1629. doi: 10.1016/j.neubiorev.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Flinn MV, Ward CV, Noone R. Hormones and the human family. Handbook of Evolutionary Psychology. 2005:552–580. [Google Scholar]
- García CY. Temporal course of the basic components of love throughout relationships. Psychology in Spain. 1998;2:76–86. [Google Scholar]
- Gavrilets S. Human origins and the transition from promiscuity to pair-bonding. Proceedings of the National Academy of Sciences. 2012;109(25):9923–9928. doi: 10.1073/pnas.1200717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottman JM, Levenson RW. The timing of divorce: predicting when a couple will divorce over a 14-year period. Journal of Marriage and Family. 2000;62(3):737–745. [Google Scholar]
- Grewen KM, Light KC. Plasma oxytocin is related to lower cardiovascular and sympathetic reactivity to stress. Biological Psychology. 2011;87(3):340–349. doi: 10.1016/j.biopsycho.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Doom JR, Esposito EA. Psychoneuroendocrinology of stress: Normative development and individual differences. In: Lerner RM, editor. Handbook of Child Psychology and Developmental Science. Florida: John Wiley & Sons, Inc; 2015. pp. 106–151. [Google Scholar]
- Hazan C, Shaver P. Romantic love conceptualized as an attachment process. Journal of Personality and Social Psychology. 1987;52(3):511. doi: 10.1037//0022-3514.52.3.511. [DOI] [PubMed] [Google Scholar]
- Helm JL, Sbarra D, Ferrer E. Assessing cross-partner associations in physiological responses via coupled oscillator models. Emotion. 2012;12(4):748. doi: 10.1037/a0025036. [DOI] [PubMed] [Google Scholar]
- Helm JL, Sbarra DA, Ferrer E. Coregulation of respiratory sinus arrhythmia in adult romantic partners. Emotion. 2014;14(3):522. doi: 10.1037/a0035960. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Medicine. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140(1):256. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241(4865):540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Jankowiak WR, Fischer EF. A cross-cultural perspective on romantic love. Ethnology. 1992;47:163–180. [Google Scholar]
- Kaplan H, Hill K, Lancaster A, Hurtado AM. A theory of human life history evolution: Diet, intelligence, and longevity. Evolutionary Anthropology. 2000;9:156–185. [Google Scholar]
- Kessler RC. Stress, social status, and psychological distress. Journal of Health and Social Behavior. 1979:259–272. [PubMed] [Google Scholar]
- Kemeny ME. The psychobiology of stress. Current Directions in Psychological Science. 2003;12(4):124–129. [Google Scholar]
- Kiecolt-Glaser JK, Newton T, Cacioppo JT, MacCallum RC, Glaser R, Malarkey WB. Marital conflict and endocrine function: Are men really more physiologically affected than women? Journal of Consulting and Clinical Psychology. 1996;64(2):324. doi: 10.1037//0022-006x.64.2.324. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine. 1995;57(1):23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda BD, De Boer SF, Flügge G, Korte SM, Richter-Levin G. Stress revisited: A critical evaluation of the stress concept. Neuroscience & Biobehavioral Reviews. 2011;35(5):1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM. Marital interaction: Physiological linkage and affective exchange. Journal of Personality and Social Psychology. 1983;45(3):587. doi: 10.1037//0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ruef AM. Empathy: A physiological substrate. Journal of Personality and Social Psychology. 1992;63(2):234. [PubMed] [Google Scholar]
- Levenson RW. The autonomic nervous system and emotion. Emotion Review. 2014;6(2):100–112. [Google Scholar]
- Liu S, Rovine MJ, Cousino Klein L, Almeida DM. Synchrony of diurnal cortisol pattern in couples. Journal of Family Psychology. 2013;27(4):579. doi: 10.1037/a0033735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy CO. Reexamining human origins in light of Ardipithecus ramidus. Science. 2009;326(5949):74–74e8. [PubMed] [Google Scholar]
- Loving TJ, Crockett EE, Paxson AA. Passionate love and relationship thinkers: Experimental evidence for acute cortisol elevations in women. Psychoneuroendocrinology. 2009;34(6):939–946. doi: 10.1016/j.psyneuen.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Canale D. Hormonal changes when falling in love. Psychoneuroendocrinology. 2004;29(7):931–936. doi: 10.1016/j.psyneuen.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Marlowe FW. Hunting and gathering: The human sexual division of foraging labor. Cross-Cultural Research. 2007;41(2):170–195. doi: 10.1177/1069397106297529. [DOI] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583(2):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM, Ellsworth PC. Evolution, emotions, and emotional disorders. American Psychologist. 2009;64(2):129. doi: 10.1037/a0013503. [DOI] [PubMed] [Google Scholar]
- Papp LM, Pendry P, Simon CD, Adam EK. Spouses’ cortisol associations and moderators: Testing physiological synchrony and connectedness in everyday life. Family Process. 2013;52(2):284–298. doi: 10.1111/j.1545-5300.2012.01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietromonaco PR, DeBuse CJ, Powers SI. Does attachment get under the skin? Adult romantic attachment and cortisol responses to stress. Current Directions in Psychological Science. 2013;22(1):63–68. doi: 10.1177/0963721412463229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modification of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32(4):301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, McGinn MM. Marital quality and health: A meta-analytic review. Psychological Bulletin. 2014;140(1):140. doi: 10.1037/a0031859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Saxbe D, Repetti RL. For better or worse? Coregulation of couples’ cortisol levels and mood states. Journal of Personality and Social Psychology. 2010;98(1):92. doi: 10.1037/a0016959. [DOI] [PubMed] [Google Scholar]
- Sbarra DA, Hazan C. Coregulation, dysregulation, self-regulation: An integrative analysis and empirical agenda for understanding adult attachment, separation, loss, and recovery. Personality and Social Psychology Review. 2008;12(2):141–167. doi: 10.1177/1088868308315702. [DOI] [PubMed] [Google Scholar]
- Schachter S, Singer J. Cognitive, social, and physiological determinants of emotional state. Psychological Review. 1962;69(5):379. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Schneiderman I, Kanat-Maymon Y, Zagoory-Sharon O, Feldman R. Mutual influences between partners’ hormones shape conflict dialog and relationship duration at the initiation of romantic love. Social Neuroscience. 2014;9(4):337–351. doi: 10.1080/17470919.2014.893925. [DOI] [PubMed] [Google Scholar]
- Schneiderman I, Zilberstein-Kra Y, Leckman JF, Feldman R. Love alters autonomic reactivity to emotions. Emotion. 2011;11(6):1314. doi: 10.1037/a0024090. [DOI] [PubMed] [Google Scholar]
- Schoebi D. The coregulation of daily affect in marital relationships. Journal of Family Psychology. 2008;22(4):595. doi: 10.1037/0893-3200.22.3.595. [DOI] [PubMed] [Google Scholar]
- Selye H. The evolution of the stress concept: The originator of the concept traces its development from the discovery in 1936 of the alarm reaction to modern therapeutic applications of syntoxic and catatoxic hormones. American Scientist. 1973;61(6):692–699. [PubMed] [Google Scholar]
- Shaver PR, Hazan C, Bradshaw D. The integration of three behavioral systems. The Psychology of Love. 1988:68–99. [Google Scholar]
- Shirtcliff EA, Peres JC, Dismukes AR, Lee Y, Phan JM. Hormones: Commentary: Riding the physiological roller coaster: Adaptive significance of cortisol stress reactivity to social contexts. Journal of Personality Disorders. 2014;28(1):40. doi: 10.1521/pedi.2014.28.1.40. [DOI] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302(5648):1231–1234. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Cheney DL. Strong and consistent social bonds enhance the longevity of female baboons. Current Biology. 2010;20(15):1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Silk J, Cheney D, Seyfarth R. A practical guide to the study of social relationships. Evolutionary Anthropology: Issues, News, and Reviews. 2013;22(5):213–225. doi: 10.1002/evan.21367. [DOI] [PubMed] [Google Scholar]
- Smeets T, Dziobek I, Wolf OT. Social cognition under stress: Differential effects of stress-induced cortisol elevations in healthy young men and women. Hormones and Behavior. 2009;55(4):507–513. doi: 10.1016/j.yhbeh.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Spoor JR, Kelly JR. The evolutionary significance of affect in groups: Communication and group bonding. Group Processes & Intergroup Relations. 2004;7(4):398–412. [Google Scholar]
- Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher J, Reason J, editors. Handbook of life stress, cognition, and health. New York: Wiley; 1988. pp. 629–649. [Google Scholar]
- Taylor SE. Tend and befriend biobehavioral bases of affiliation under stress. Current Directions in Psychological Science. 2006;15(6):273–277. [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Timmons AC, Margolin G, Saxbe DE. Physiological linkage in couples and its implications for individual and interpersonal functioning: A literature review. Journal of Family Psychology. 2015;29(5):720. doi: 10.1037/fam0000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna EP, Tranel D. Gastric myoelectrical activity as an index of emotional arousal. International Journal of Psychophysiology. 2006;61(1):70–76. doi: 10.1016/j.ijpsycho.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Weisman O, Schneiderman I, Zagoory-Sharon O, Feldman R. Early stage romantic love is associated with reduced daily cortisol production. Adaptive Human Behavior and Physiology. 2015;1(1):41–53. [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: Insights from a socially monogamous rodent. Frontiers in Neuroendocrinology. 2011;32(1):53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]