Radiocarbon dates of perimortem tool marks reveal human presence in Madagascar 6000 years earlier than previously thought.
Abstract
Previous research suggests that people first arrived on Madagascar by ~2500 years before present (years B.P.). This hypothesis is consistent with butchery marks on extinct lemur bones from ~2400 years B.P. and perhaps with archaeological evidence of human presence from ~4000 years B.P. We report >10,500-year-old human-modified bones for the extinct elephant birds Aepyornis and Mullerornis, which show perimortem chop marks, cut marks, and depression fractures consistent with immobilization and dismemberment. Our evidence for anthropogenic perimortem modification of directly dated bones represents the earliest indication of humans in Madagascar, predating all other archaeological and genetic evidence by >6000 years and changing our understanding of the history of human colonization of Madagascar. This revision of Madagascar’s prehistory suggests prolonged human-faunal coexistence with limited biodiversity loss.
INTRODUCTION
Madagascar’s Holocene vertebrate megafauna included giant lemurs, hippopotami, giant tortoises, and the world’s largest birds—the elephant birds [Aepyornithidae, ~500 kg (1)]. This megafauna is now completely extinct, with the largest surviving endemic vertebrates less than 10 kg in body mass (2). Representatives of all of Madagascar’s extinct megafauna are known to have survived into the Holocene (2), with last-occurrence dates for all genera between ~2400 and 500 years before present (B.P.), suggesting that human activities, rather than climatic shifts, were responsible for the extinction of these animals. However, the dynamics of the Malagasy faunal extinction process and the nature of human involvement in driving prehistoric biodiversity loss (for example, overkill versus population attrition, possibly through indirect processes such as habitat degradation or natural climatic change) remain poorly understood due to limited data on human-faunal interactions and the duration of temporal overlap between humans and now-extinct species.
Researchers have sought to understand the process of Holocene biodiversity loss in Madagascar by comparing pre- and post-human eras (2). Archaeological evidence for settled villages dates from 1300 years B.P. onward, with occupation of most of Madagascar’s coasts by 900 years B.P. (3). Archaeological, genetic, and linguistic data all indicate that these colonists were of both Austronesian and East African heritage (4–8). Lake sediment cores indicate substantial ecological change associated with Madagascar’s known late Holocene archaeological period; precipitous drops of the dung fungus Sporormiella demonstrate a significant loss of endemic megafaunal biomass (9), followed by the expansion of grassland savannah evidenced by pollen shifts from C3 to C4 plants and sharp rises in charcoal microparticulates (10–13).
Evidence for the timing of first human arrival in Madagascar during the late Holocene informs how researchers define pre-human or “pristine” ecosystems, frameworks for understanding ecological succession and resilience, and natural baselines for conservation objectives for Madagascar’s threatened biodiversity (13–15). Evidence available in the 1980s to 2010s suggested a first human arrival about 1500 years B.P. However, several lines of evidence have been proposed to suggest a longer period of prehistoric human occupation of Madagascar across the middle to late Holocene. Western coastal rock shelters provide support for regional human presence from ~3000 years B.P. onward, through evidence of protracted subsistence on endemic coastal and marine fauna (16). Butchery traces have been used to understand global human impacts on naïve faunas and to document the spread of prehistoric humans (17–20). Bones of Madagascar’s extinct megafaunal mammals with butchery cut marks but lacking any associated artifacts are also known to predate the widely accepted archaeological settlement period. A Palaeopropithecus ingens radius with cut marks from Taolambiby, southwest Madagascar, has been dated to ~2400 years B.P. (21), and bones of Hippopotamus lemerlei from northwest Madagascar with calibrated radiocarbon dates of 4288 to 4035 years B.P. are reported to show cut marks (22). The paradigm of late human arrival in Madagascar has recently been further challenged by discovery of small assemblages of microlithic tools at sites indicating transient occupation in northern Madagascar (Lakaton’i Anja, Ambohiposa), which have also been dated to up to >4000 years B.P. (23). These microlithic tools are similar to those used in composite projectiles, and their morphology is consistent with designs from southern and eastern Africa. These two independent lines of evidence suggest a considerably older but poorly understood period of human presence in Madagascar, with important implications for understanding the resilience of the island’s fauna to prehistoric human activity. However, the age of the microlithic tools from Lakaton’i Anja is based on accelerator mass spectrometry (AMS) and optically stimulated luminescence (OSL) dating on associated substrate rather than direct dates on the artifacts themselves, giving an inferred date from their context rather than direct dating of their organic carbon. There are also discrepancies between OSL and radiocarbon dates from the same strata at Lakaton’i Anja, and supposed cut marks on the H. lemerlei bones do not exhibit a pattern associated with butchery, which has led to the strength of this evidence being questioned (2, 24).
Madagascar’s elephant birds have been the focus of remarkably little modern research in comparison to the island’s extinct endemic mammals, beyond recent attempts to extract ancient DNA (25–28), and little is known about prehistoric human interactions with these giant birds. Two proposed examples of human modification of elephant bird bones have both been rejected as evidence of anthropogenic exploitation. An undated, unidentified leg element of Mullerornis sp. recovered from an archaeological context from Ampasambazimba (29, 30) exhibits modification that may represent natural processes (31, 32), and an Aepyornis sp. tibiotarsus from Itampolo, dated to the pre-agriculture period [1297 to 1590 years B.P. (33)], exhibits postmortem rather than perimortem modification. Reworked elephant bird eggshell fragments have been reported from archaeological contexts in coastal rock shelters and settlements, but direct radiometric dates on eggshell are substantially older than dates on charcoal predicted by human activity at these sites (6, 16).
RESULTS
Here, we present new evidence of prehistoric human modification of multiple elephant bird postcranial elements, representing both currently recognized genera. We distinguish pathological and taphonomic damage from anthropogenic marks using the empirical classification of Corron et al. (34), comparison to experimental frameworks of Galán and Domínguez-Rodrigo (35), and the conservative criteria of Pérez et al. (21) and Godfrey et al. (36). Anthropogenic marks described here as clefts or kerfs differ in morphology and orientation from traces resulting from natural processes (34). Clefts and kerfs are consistent with patterns of butchery in ratites through disarticulation, as evidenced by marks at interarticular epiphyses of long bones (35) and phalanges (37), associated with hyperextension of limb joints followed by chopping and cutting through connective tissues on their exposed fascia, leaving comparatively few anthropogenic marks on the surfaces of the diaphysis (38).
The Christmas River (Ilaka) site (Fig. 1) is a wetland ecosystem from early Holocene Madagascar containing a well-preserved faunal assemblage (39). It is located on the east of the southernmost region of the Isalo sandstone massif near a tributary of the Ihazofotsy River and Ilakabe village (22°46′257″ S, 45°21′802″ E). The bedrock of the region is Permian and Triassic in age and belongs to the Karroo group of the Morondava basin. The bedrock is overlain by recent sediments that include layers of beige sandy soil, black clay, and a highly fossiliferous 13- to 15-m-deep layer of slate gray clay. The vertebrate fauna documented from the fossiliferous “bone bed” in the slate gray clay layer comprises ~600 vertebrate specimens, including Aepyornis and other extinct megafaunal taxa (crocodiles; tortoises; the carnivoran Cryptoprocta spelea; the giant lemurs Archaeolemur sp., Pachylemur insignis, and Megaladapis edwardsi; and the dwarf hippopotamus H. lemerlei) (39). No lithic tools or other human artifacts or remains have been reported from the single excavation so far conducted at the site. Previously published AMS dates from multiple vertebrate taxa and from wood present in the bone bed indicate a wet phase between ~11,000 and 9000 years B.P., and strata directly above the bone bed are consistent with increased regional aridity later in the Holocene (39). Two skeletal elements from a single Aepyornis maximus individual (collected by E. Simons as an articulated pair) from the bone bed show perimortem anthropogenic modification (Fig. 2 and Table 1). Bone collagen samples from USNM A605209 were directly dated at two separate AMS radiocarbon facilities, with a combined calibrated date range of 10,721 to 10,511 years B.P. (Table 2).
Fig. 1. Vegetation map of late Holocene (pre-industrial) Madagascar, showing sites with butchered elephant bird bones and calibrated AMS radiocarbon dates.
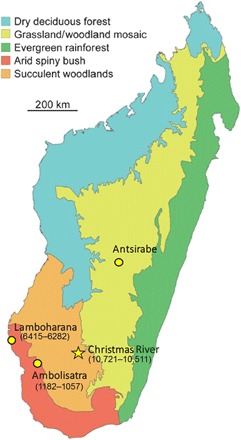
Fig. 2. A. maximus skeletal reconstruction.
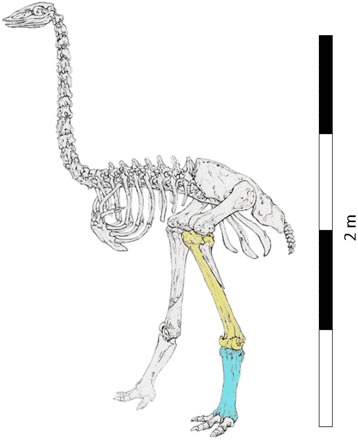
Highlighted elements correspond to Figs. 3 (blue) and 4 (yellow). Adapted from original drawing by A. Rasolao.
Table 1. Dimensions of tool marks on A. maximus USNM A605209 (TT: Tibiotarsus) and USNM AS05208 (TM: Tarsometatarsus).
| Mark number | Modification | Maximum length (mm) | Maximum width (mm) | Maximum depth (mm) |
| TM-1 | Cut mark | 16.7 | 5.3 | 1.6 |
| TM-2 | Cut mark | 11.0 | 4.7 | 1.3 |
| TM-3 | Cut mark | 12.4 | 3.3 | 1.3 |
| TM-4 | Cut mark | 14.7 | 5.4 | 3.3 |
| TM-5 | Cut mark | 5.7 | 4.4 | 3.8 |
| TT-1 | Depression fracture | 18.4 | 17.3 | 6.8 |
| TT-2 | Depression fracture | 52.1 | 16.6 | 7.6 |
| TT-3 | Chop mark | 44.5 | 7.8 | 5.1 |
| TT-4 | Cut mark | 18.1 | 4.2 | 2.0 |
Table 2. List of newly recognized elephant bird bones with perimortem anthropogenic modification and associated AMS radiocarbon dates.
USNM, National Museum of Natural History/Smithsonian Institution; MNHN, Muséum National d’Histoire Naturelle (Paris); N/A, not available.
| Specimen number | Species | Element | Location | Sample number |
14C age (years B.P.) |
Calibrated date (years B.P.), ±2σ |
| USNM A605209* | A. maximus | Tibiotarsus | Christmas River | UBA-31590 | 9428 ± 53 | 10,721–10,511 |
| USNM A605209* | A. maximus | Tibiotarsus | Christmas River | Hela-1774 | 9535 ± 70 | 10,721–10,511 |
| USNM A605208* | A. maximus | Tarsometatarsus | Christmas River | N/A | See USNM A605209 | See USNM A605209 |
| MNHN MAD6768 |
Mullerornis sp. | Tibiotarsus | Lamboharana | UBA-29726 | 5597 ± 40 | 6,415–6,282 (93.6%) |
| MNHN MAD1906-16-67 |
A. maximus | Tibiotarsus | Ambolisatra | OxA-33535 | 1297 ± 24 | 1,182–1,057 (93.7%) |
| MNHN MAD6662 |
Mullerornis sp. | Tarsometatarsus | Unknown | UBA-19725 | 1296 ± 32 | 1,270–1,074 (95.4%) |
| MNHN MAD384 | Aepyornis hildebrandti |
Tarsometatarsus | Antsirabe | N/A | Failed | Failed |
*Same individual.
A tarsometatarsus (USNM A605208; Fig. 3) exhibits two linear grooves on the distal aspect of the lateral condyle of the central trochlea. A third groove is present on the medial condyle of the central trochlea (posterior to the previous two marks), and a fourth groove is present on the medial condyle of the medial trochlea. A fifth is more centrally located on the lateral trochlea. All of these grooves have centrally oriented bevels and v-shaped floors. While the penetrating marks are intact and well defined, the edges are irregular and undefined at their centers, with portions of the bone surface absent. These marks are consistent with kerfs made by single-bladed, sharp lithic tools and multiple cutting actions intended to disarticulate the central phalanges (35).
Fig. 3. A. maximus tarsometatarsus (USNM A605208).
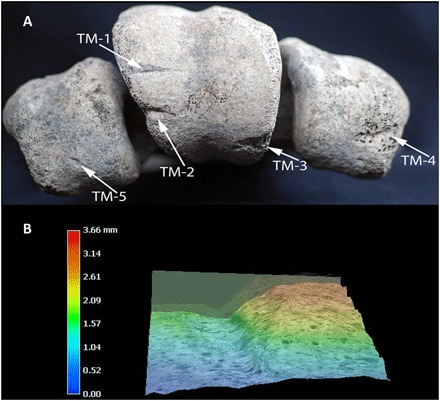
(A) Distal aspect of A. maximus tarsometatarsus (USNM A605208) from Christmas River (USNM A605208), showing five cut marks: three (TM-1 to TM-3) on the central trochlea (digit III), one (TM-4) on the medial trochlea (digit II), and one (TM-5) on the lateral trochlea (digit IV). (B) Cross section of TM-1 at ×30 magnification, illustrating depth using a topographic height color scale. Photo credit: V. R. Pérez, University of Massachusetts Amherst.
A tibiotarsus from the same individual (USNM A605209; Fig. 4) contains ossified medullary bone in the cortex, indicating that the individual was a gravid adult female. The diaphysis exhibits two depression fractures, one on the anterior fascia of the proximal surface and another on the lateral portion of the posterior fascia of the distal surface, which may be hobbling impact marks from immobilizing the animal. A large, laterally oriented linear anthropogenic mark is also present on the medial condyle of the distal process, ending in a large undefined fragmentation of the anterior medial portion of the condyle and exposing a rough and uneven trabecular surface. Bevels are oriented centrally with an off-center v-shaped floor biased toward the anterior. The mark penetrates through cortical tissue, leaving exposed trabeculae forming both wall aspects. Groove edges are defined at the medial limit, becoming undefined at the center. The groove is rugose with varying relief in posterior aspect, characteristic of perimortem damage caused by a lithic tool (35), and is smooth and straight in anterior aspect. The lack of undefined cracking extending away from the central extremity of the mark indicates that this kerf was made upon fresh bone, and the homogeneous coloration of the bone surface and exposed fascia also indicates that it was made before deposition. A secondary anthropogenic linear groove is present off-center of the medial fascia, oriented toward the missing anterior medial condyle and with similar kerf morphology. The posterior-lateral bevel edge is defined at the anterior-medial end and undefined from the center to the posterior-lateral end. The morphology and orientation of the cleft and kerf are consistent with disarticulation at the intertarsal joint, including high-impact chopping actions associated with disarticulation of large animals (35, 38).
Fig. 4. A. maximus tibiotarsus (USNM A605209).
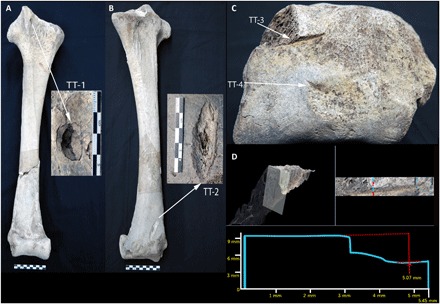
(A) Depression fracture on the anterior fascia of the proximal end of A. maximus tibiotarsus (USNM A605209) from Christmas River (USNM A605209). (B) Depression fracture on the lateral aspect of the posterior fascia. (C) Distal aspect of tibiotarsus, showing two cut marks (TajT-3 and TT-4). (D) Close-up and profile of cut mark TT-3 on the medial condyle of the distal articular process (digital thin section shows the wall and kerf floor of the mark). Photo credit: V. R. Pérez, University of Massachusetts Amherst.
Additional evidence of ancient Holocene exploitation of elephant birds is also available in historical museum collections from Madagascar that have been reexamined. A Mullerornis sp. tibiotarsus from Lamboharana (MNHN MAD6768) that has been directly AMS-dated to 6415 to 6282 years B.P. exhibits a shallow, laterally oriented 20 mm × 0.2 mm linear anthropogenic mark on the distal end of the posterior fascia of the diaphysis. Bevels are oriented centrally with a central v-shaped floor. The distal aspect of the groove is less regular and has more crenellations along the margin than the proximal aspect. This mark is again consistent with a kerf made by a lithic tool, with the orientation and morphology indicative of butchery (fig. S1). Other elephant bird specimens exhibiting distinct anthropogenic marks are dated to within Madagascar’s late Holocene human settlement period or could not be dated directly (figs. S2 to S4).
DISCUSSION
This evidence for anthropogenic perimortem modification of directly dated elephant bird bones from multiple taxa and geographical localities represents the earliest known evidence of human presence in Madagascar, predating all other archaeological evidence of regional anthropogenic activity by approximately 6000 years and greatly extending the island’s known archaeological period. Our study therefore reveals a previously unrecognized period of human presence and coexistence with now-extinct fauna on Madagascar, which is now documented through intermittent records of butchery of aepyornithids across almost the entirety of the Holocene.
These findings pose major archaeological and paleontological questions, of crucial importance for understanding early human migrations and Quaternary faunal extinction dynamics. Fundamentally, evidence for long-term coexistence of humans and megafauna in Madagascar demonstrates that a radically different extinction paradigm is required to understand biodiversity loss in this island ecosystem. In contrast, the elephant birds’ closest ecological analog, the moa (Dinornithiformes) of New Zealand, probably became extinct <150 years after Polynesian settlement (40).
This discovery of early Holocene evidence of human presence on Madagascar also raises the important question of why, if the island was occupied by prehistoric migrants continually throughout the Holocene, direct archaeological evidence of human settlement predating the late Holocene has not yet been detected. Archaeological research in Madagascar has largely concentrated on relatively recent open-air village sites and early Holocene sediments have rarely been examined (23) and so it is possible that evidence of older human presence has so far been missed. Alternatively, early-mid Holocene human presence on Madagascar may have been restricted to transient Late Stone Age migration(s), presumably across the Mozambique Channel, rather than permanent island-wide settlement. New well-described excavations are required to test between these alternative potential hypotheses for prehistoric human colonization, and provide further insights into human-megafaunal interactions in Late Quaternary Madagascar.
MATERIALS AND METHODS
Aepyornithid pelvic limb specimens held in museum collections in Europe, United States, and Madagascar were investigated for anthropogenic marks. Length and width measurements of each mark were taken by hand using digital calipers. Length was defined using two points for each mark including origin and termination of the mark, following standards in cut mark morphology at multiple magnifications. Width is defined as the widest point of modified bone, with termination points at the unmodified bone surface perpendicular to the long axis of the cut mark.
When possible, measurements were taken using a Keyence VHX5000 microscope with built-in visualization software, allowing measurements to be taken without molding. Cut mark depth can be measured at the deepest point of the mark directly from the scan. We assessed cut mark depth at magnifications between ×20 and ×150. If cut mark length extended beyond the field of view at this magnification, Keyence’s stitch function was used to combine measurements along the mark’s length axis. The stitch function creates a three-dimensional composite image from several image planes and overlapping focus levels. The visualization software then creates a true focus reproduction of the scanned bone surface. When access to the Keyence microscope was not possible, Xantopren L blue putty was used to generate casts of affected areas (41), where there would be no possibility of causing further damage. These casts were observed and measured using a Hitachi TM3000 tabletop scanning electron microscope.
Impact marks were compared to the morphology and position of tool marks previously reported from late Holocene Madagascar (21, 42), modern assessments of meat utility and butchery of emu, archaeological records of tool marks on rhea, and modern frameworks of archaeological exploitation analysis (35, 38).
Here, the conservative frameworks of Corron et al. (34) and Pérez et al. (21) were applied in determining evidence for butchery practices: (i) patterning or redundancy through multiple marks in the same region and (ii) purposefulness, a bioarchaeological explanation of why the cut marks are present. The authors recognized that this framework underestimates butchery practices, as signs of exploitation through tool marks are rare due to false-negative or type II errors, where flesh is sufficiently thick that tools do not cut all the way through it.
New AMS radiocarbon dates were obtained by extracting 0.5-g aliquots of bone using a Dremel 4000 rotary tool with a diamond cutting wheel, with analysis at the 14CHRONO Centre (Belfast, UK) and the Oxford Radiocarbon Accelerator Unit (Oxford, UK) through an NRCF [Natural Environment Research Council (NERC) Radiocarbon Facility] grant (NF/2015/1/4). All radiocarbon data used were calibrated using OxCal version 4.2 (43) and the southern hemisphere curve SHCal13 (44).
Supplementary Material
Acknowledgments
Funding: This study was supported by an NRCF grant (NF/2015/1/4). Author contributions: J.H., D.E., T.T., V.R.P., and S.T.T. designed the study. J.H., D.E., T.T., and V.R.P. analyzed the tool mark data. J.H. collected samples for new radiocarbon dates. J.H., S.T.T., P.C.W., and L.R.G. wrote and edited the manuscript. A.R. and P.C.W. were responsible for accessing material into the Smithsonian National Museum of Natural History. V.R.P. and J.H. created images for photographic figures. Figure 2 was adapted from A. Rasolao’s original drawing with permission. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/9/eaat6925/DC1
Fig. S1. Mullerornis sp. tibiotarsus from Lamboharana (MNHN MAD6768) dated to 6415 to 6282 years B.P., exhibiting a shallow, laterally oriented linear anthropogenic mark on the distal end of the posterior fascia of the diaphysis.
Fig. S2. A. maximus tibiotarsus from Ambolisatra (MNHN 1906-16-67) directly dated to 1182 to 1057 years B.P., exhibiting four linear anthropogenic marks disseminated across the proximal epiphysis.
Fig. S3. Mullerornis sp. tarsometatarsus from an unknown locality on Madagascar (MNHN MAD6662) directly dated to 1270 to 1054 years B.P., exhibiting an open-ended linear anthropogenic groove on the lateral portion of the distal epiphysis (16 mm length, 2.5 mm maximum depth, 3 mm maximum width), oriented laterally across the articular surface and angled toward the posterior distal epiphysis of the central condyle.
Fig. S4. A. hildebrandti tarsometatarsus from Antsirabe (MNHN MAD384), which failed AMS dating due to low collagen yield.
REFERENCES AND NOTES
- 1.Campbell K. Jr, Marcus L., The relationship of hindlimb bone dimensions to body weight in birds. Nat. Hist. Mus. Los Angeles Cty. Sci. Ser. 36, 395–412 (1992). [Google Scholar]
- 2.S. Goodman, W. Jungers, Extinct Madagascar (University of Chicago Press, 2014). [Google Scholar]
- 3.Crowther A., Lucas L., Helm R., Horton M., Shipton C., Wright H. T., Walshaw S., Pawlowicz M., Radimilahy C., Douka K., Picornell-Gelabert L., Fuller D. Q., Boivin N. L., Ancient crops provide first archaeological signature of the westward Austronesian expansion. Proc. Natl. Acad. Sci. U.S.A. 113, 6635–6640 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierron D., Heiske M., Razafindrazaka H., Rakoto I., Rabetokotany N., Ravololomanga B., Rakotozafy L. M.-A., Rakotomalala M. M., Razafiarivony M., Rasoarifetra B., Raharijesy M. A., Razafindralambo L., Ramilisonina, Fanony F., Lejamble S., Thomas O., Mohamed Abdallah A., Rocher C., Arachiche A., Tonaso L., Pereda-Loth V., Schiavinato S., Brucato N., Ricaut F.-X., Kusuma P., Sudoyo H., Ni S., Boland A., Deleuze J.-F., Beaujard P., Grange P., Adelaar S., Stoneking M., Rakotoarisoa J.-A., Radimilahy C., Letellier T., Genomic landscape of human diversity across Madagascar. Proc. Natl. Acad. Sci. U.S.A. 114, E6498–E6506 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blench R., The Austronesians in Madagascar and their interaction with the Bantu of the East African coast: Surveying the linguistic evidence for domestic and translocated animals. Stud. Philipp. Lang. Cult. 18, 18–43 (2008). [Google Scholar]
- 6.M. Parker-Pearson, Pastoralists, Warriors and Colonists: The Archaeology of Southern Madagascar (Archaeopress, 2010). [Google Scholar]
- 7.Kusuma P., Cox M. P., Pierron D., Razafindrazaka H., Brucato N., Tonasso L., Suryadi H. L., Letellier T., Sudoyo H., Ricaut F.-X., Mitochondrial DNA and the Y chromosome suggest the settlement of Madagascar by Indonesian sea nomad populations. BMC Genomics 16, 191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brucato N., Kusuma P., Cox M. P., Pierron D., Purnomo G. A., Adelaar A., Kivisild T., Letellier T., Sudoyo H., Ricaut F.-X., Malagasy genetic ancestry comes from an historical Malay trading post in Southeast Borneo. Mol. Biol. Evol. 33, 2396–2400 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burney D. A., Robinson G. S., Burney L. P., Sporormiella and the late Holocene extinctions in Madagascar. Proc. Natl. Acad. Sci. U.S.A. 100, 10800–10805 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burney D., Burney L. P., Godfrey L. R., Jungers W. L., Goodman S. M., Wright H. T., Jull A. J., A chronology for late prehistoric Madagascar. J. Hum. Evol. 41, 25–63 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Crowley B. E., A refined chronology of prehistoric Madagascar and the demise of the megafauna. Quat. Sci. Rev. 29, 2591–2603 (2010). [Google Scholar]
- 12.Crowley B., Samonds K., Stable carbon isotope values confirm a recent increase in grasslands in northwestern Madagascar. Holocene 23, 1066–1073 (2013). [Google Scholar]
- 13.Burns S., Godfrey L. R., Faina P., McGee D., Hardt B., Ranivoharimanana L., Randrianasy J., Rapid human-induced landscape transformation in Madagascar at the end of the first millennium of the Common Era. Quat. Sci. Rev. 134, 92–99 (2016). [Google Scholar]
- 14.Bond W., Silander J. Jr, Ranaivonasy J., Ratsirarson J., The antiquity of Madagascar’s grasslands and the rise of C4 grassy biomes. J. Biogeogr. 35, 1743–1758 (2008). [Google Scholar]
- 15.Vorontsova M., Besnard G., Forest F., Malakasi P., Moat J., Clayton W. D., Ficinski P., Savva G. M., Nanjarisoa O. P., Razanatsoa J., Randriatsara F. O., Kimeu J. M., Luke W. R., Kayombo C., Linder H. P., Madagascar’s grasses and grasslands: Anthropogenic or natural? Proc. Biol. Sci. 283, 20152262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douglass K., The diversity of late Holocene shellfish exploitation in Velondriake, southwest Madagascar. J. Isl. Coast. Archaeol. 12, 333–359 (2017). [Google Scholar]
- 17.Pitulko V., Tikhonov A. N., Pavlova E. Y., Nikolskiy P. A., Kuper K. E., Polozov R. N., Early human presence in the Arctic: Evidence from 45,000-year-old mammoth remains. Science 351, 260–263 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Dowd M., Carden R., First evidence of a Late Upper Palaeolithic human presence in Ireland. Quat. Sci. Rev. 139, 158–163 (2016). [Google Scholar]
- 19.Hockett B., Jenkins D., Identifying stone tool cut marks and the pre-Clovis occupation of the Paisley Caves. Am. Antiq. 78, 762–778 (2013). [Google Scholar]
- 20.McPherron S., Alemseged Z., Marean C. W., Wynn J. G., Reed D., Geraads D., Bobe R., Béarat H. A., Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature 466, 857–860 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Pérez V., Godfrey L. R., Nowak-Kemp M., Burney D. A., Ratsimbazafy J., Vasey N., Evidence of early butchery of giant lemurs in Madagascar. J. Hum. Evol. 49, 722–742 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Gommery D., Ramanivosoa B., Faure M., Guérin C., Kerloc’h P., Sénégas F., Randrianantenaina H., Les plus anciennes traces d’activités anthropiques de Madagascar sur des ossements d’hippopotâmes subfossiles d’Anjohibe (Province de Mahajanga). C. R. Palevol 10, 271–278 (2011). [Google Scholar]
- 23.Dewar R., Radimilahy C., Wright H. T., Jacobs Z., Kelly G. O., Berna F., Stone tools and foraging in northern Madagascar challenge Holocene extinction models. Proc. Natl. Acad. Sci. U.S.A. 110, 12583–12588 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A. Ekblom, P. Lane, C. Radimilahy, J.-A. Rakotoarisoa, P. Sinclair, M. Virah-Sawmy, in Early Exchange Between Africa and the Wider Indian Ocean World, G. Campbell, Ed. (Springer International Publishing, 2016), pp. 195–230. [Google Scholar]
- 25.Grealy A., Phillips M., Miller G., Gilbert M. T. P., Rouillard J.-M., Lambert D., Bunce M., Haile J., Eggshell palaeogenomics: Palaeognath evolutionary history revealed through ancient nuclear and mitochondrial DNA from Madagascan elephant bird (Aepyornis sp.) eggshell. Mol. Phylogenet. Evol. 109, 151–163 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Yonezawa T., Segawa T., Mori H., Campos P. F., Hongoh Y., Endo H., Akiyoshi A., Kohno N., Nishida S., Wu J., Jin H., Adachi J., Kishino H., Kurokawa K., Nogi Y., Tanabe H., Mukoyama H., Yoshida K., Rasoamiaramanana A., Yamagishi S., Hayashi Y., Yoshida A., Koike H., Akishinonomiya F., Willerslev E., Hasegawa M., Phylogenomics and morphology of extinct paleognaths reveal the origin and evolution of the ratites. Curr. Biol. 27, 68–77 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Oskam C. L., Haile J., McLay E., Rigby P., Allentoft M. E., Olsen M. E., Bengtsson C., Miller G. H., Schwenninger J.-L., Jacomb C., Walter R., Baynes A., Dortch J., Parker-Pearson M., Gilbert M. T., Holdaway R. N., Willerslev E., Bunce M., Fossil avian eggshell preserves ancient DNA. Proc. Biol. Sci. 277, 1991–2000 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell K. J., Llamas B., Soubrier J., Rawlence N. J., Worthy T. H., Wood J., Lee M. S., Cooper A., Ancient DNA reveals elephant birds and kiwi are sister taxa and clarifies ratite bird evolution. Science 344, 898–900 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Fontoynont M., Les gisements fossilifères d’Ampasambazimba. Bull. Acad. Malgache 6, 3–8 (1909). [Google Scholar]
- 30.R. E. Dewar, Extinctions in Madagascar: The loss of the subfossil fauna, in Pleistocene Extinctions: The Search for a Cause, P. Martin, R. Klein, Eds. (Yale Univ. Press, 1984), pp. 574–593. [Google Scholar]
- 31.Barthère F.-M., Observations sur une hache en os, provenant des fouilles exéctuées par l’Académie Malgache, à Ampasambazimba en 1908 (Madagascar). Bull. Soc. Préhist. Fr. 12, 358–361 (1915). [Google Scholar]
- 32.MacPhee R. D. E., Burney D. A., Wells, N. A. Early Holocene chronology and environment of Ampasambazimba, a Malagasy subfossil lemur site. Int. J. Primatol. 6, 463–489 (1985). [Google Scholar]
- 33.R. MacPhee, Extinctions in Near Time (Kluwer Academic/Plenum Publishers, 1999). [Google Scholar]
- 34.Corron L., Huchet J.-B., Santos F., Dutour O., Using classifications to identify pathological and taphonomic modifications on ancient bones: Do “taphognomonic” criteria exist? Bull. Mém. Soc. Anthropol. Paris 29, 1–18 (2017). [Google Scholar]
- 35.Galán A., Domínguez-Rodrigo M., An experimental study of the anatomical distribution of cut marks created by filleting and disarticulation on long bone ends. Archaeometry 55, 1132–1149 (2013). [Google Scholar]
- 36.Godfrey L., Perez V. R., Meador L. R., Crowley B. E., Bankoff R. J., Culleton B. J., Kennett D. J., Perry G. H., New evidence of widespread hunting of giant lemurs on Madagascar. Am. J. Phys. Anthropol. 159, 156–157 (2016). [Google Scholar]
- 37.Salemme M., Frontini R., The exploitation of Rheidae in Pampa and Patagonia (Argentina) as recorded by chroniclers, naturalists and voyagers. J. Anthropol. Archaeol. 30, 473–483 (2011). [Google Scholar]
- 38.Garvey J., Cochrane B., Field J., Boney C., Modern emu (Dromaius novaehollandiae) butchery, economic utility and analogues for the Australian archaeological record. Environ. Archaeol. 16, 97–112 (2011). [Google Scholar]
- 39.Muldoon K., Crowley B. E., Godfrey L. R., Rasoamiaramanana A., Aronson A., Jernvall J., Wright P. C., Simons E. L., Early Holocene fauna from a new subfossil site: A first assessment from Christmas River, south central Madagascar. Madagascar Conserv. Dev. 7, 23–29 (2012). [Google Scholar]
- 40.Holdaway R. N., Jacomb C., Rapid extinction of the moas (Aves: Dinornithiformes): Model, test, and implications. Science 287, 2250–2254 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Dittmar J., Errickson D., Caffell A., The comparison and application of silicone casting material for trauma analysis on well preserved archaeological skeletal remains. J. Archaeol. Sci. Rep. 4, 559–564 (2015). [Google Scholar]
- 42.MacPhee R. D. E., Burney D. A., Dating of modified femora of extinct dwarf Hippopotamus from Southern Madagascar: Implications for constraining human colonization and vertebrate extinction events. J. Archaeol. Sci. 18, 695–706 (1991). [Google Scholar]
- 43.Bronk Ramsey C., Bayesian analysis of radiocarbon dates. Radiocarbon 51, 337–360 (2009). [Google Scholar]
- 44.Hogg A., Hua Q., Blackwell P. G., Niu M., Buck C. E., Guilderson T. P., Heaton T. J., Palmer J. G., Reimer P. J., Reimer R. W., Turney C. S. M., Zimmerman S. R. H., SHCal13 Southern Hemisphere calibration, 0–50,000 years cal BP. Radiocarbon 55, 1889–1903 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/9/eaat6925/DC1
Fig. S1. Mullerornis sp. tibiotarsus from Lamboharana (MNHN MAD6768) dated to 6415 to 6282 years B.P., exhibiting a shallow, laterally oriented linear anthropogenic mark on the distal end of the posterior fascia of the diaphysis.
Fig. S2. A. maximus tibiotarsus from Ambolisatra (MNHN 1906-16-67) directly dated to 1182 to 1057 years B.P., exhibiting four linear anthropogenic marks disseminated across the proximal epiphysis.
Fig. S3. Mullerornis sp. tarsometatarsus from an unknown locality on Madagascar (MNHN MAD6662) directly dated to 1270 to 1054 years B.P., exhibiting an open-ended linear anthropogenic groove on the lateral portion of the distal epiphysis (16 mm length, 2.5 mm maximum depth, 3 mm maximum width), oriented laterally across the articular surface and angled toward the posterior distal epiphysis of the central condyle.
Fig. S4. A. hildebrandti tarsometatarsus from Antsirabe (MNHN MAD384), which failed AMS dating due to low collagen yield.


