Abstract
Introduction:
Pediatric intestinal failure (IF) patients experience significant morbidity, including sepsis related to central line–associated bloodstream infections. Adult studies of sepsis demonstrate an association between time to antibiotic administration (TTA) and mortality. To overcome challenges in treating pediatric IF patients in an emergency department (ED), we appropriated an existing, reliable system for febrile immunocompromised oncology/bone marrow transplant children. We describe the translation of this process to febrile IF patients in the ED and steps toward sustained improvement.
Methods:
We formed a multidisciplinary team and used the Model for Improvement to define aims and identify key drivers. The goal was to use an existing improvement process to increase the percentage of patients with IF who receive antibiotics within 60 minutes of arrival to the ED from 46% to 90%. Key drivers included pre- and postarrival processes, staff and family engagement, and a preoccupation with failure. We performed Plan-Do-Study-Act cycles targeting family engagement, prearrival efficiency, and postarrival consistency.
Results:
Two hundred seventy-six encounters involving febrile IF patients between November 2012 and March 2017 were evaluated. There was a sustained reduction in the median time from arrival to antibiotic administration (71–45 minutes). We decreased TTA to less than 60 minutes for 77% of febrile IF patients.
Conclusions:
The basic tenets of process improvement for 1 high-risk population can be translated to another high-risk population but must be adjusted for variability in characteristics.
INTRODUCTION
Intestinal failure (IF) is an orphan disease afflicting a heterogeneous group of patients. Sepsis is a common cause of morbidity, arising from bacterial translocation across the gut barrier or central venous catheter (CVC) contamination by skin or fecal flora1,2 and frequently leads to hospitalization in IF patients.3–5 Miko et al.6 identified bloodstream infections, often enteric organisms, in 46% of children with short bowel syndrome. Among adult patients with septic shock, administering antibiotics within 1 hour of documented hypotension improved outcomes while delays resulted in increased mortality.7,8 Despite this evidence, Volpe et al.9 showed difficulty maintaining timely antibiotic administration for at-risk patients presenting to the emergency department (ED). In the ED, where patients with a wide range of diagnoses and acuity are cared for simultaneously, risk-stratification is essential to maximize outcomes.10
Rationale
Like the IF population, oncology/bone marrow transplant (BMT) patients are at high risk of sepsis.11,12 In 2016, members of this team successfully utilized quality improvement (QI) methodology to decrease time to antibiotics (TTA) in febrile immunocompromised (F&I) oncology/bone marrow transplant patients.13 Using prearrival notification, family engagement, and standardized ED postarrival processes, over 90% of F&I patients received antibiotics within 60 minutes.13
Available Knowledge
There are no studies that specifically address whether directly applying the key drivers from 1 improvement process to another high-risk population would be successful.
Specific Aims
Given the risk of morbidity in febrile IF (FIF) patients, this team attempted to spread key processes from the F&I protocol to FIF patients. The goal was to increase the percentage of patients receiving antibiotics within 60 minutes of arrival in the ED from < 50% to > 90%. We based this timeline on national TTA standards, derived from the adult literature for shock.7,8
METHODS
Setting and Context
The improvement project occurred in an urban level 1 trauma center ED at a children’s hospital with ~90,000 encounters per year. FIF patients constitute < 1% of the annual visits to the ED (average 65 visits per year).
Gastroenterology (GI) intestinal rehabilitation manages 223 patients and has a dedicated team (faculty, care managers, and data coordinators). Included IF patients require a CVC for parenteral nutrition due to inability to maintain nutrition through enteral feeding. Forty-four percentage are followed for short bowel syndrome (including necrotizing enterocolitis, gastroschisis with intestinal atresia, trauma, and meconium peritonitis); 26% have pseudo-obstruction; 4% have a congenital enteropathy; 26% are “other” (Crohn’s disease, mitochondrial myopathy, and autoimmune enteropathy). Recognizing that the ED successfully reduced TTA in F&I patients, ED providers approached the IF team in spring 2013 to determine if they were interested in spreading interventions to FIF patients. The team used the Model for Improvement, defined global and smart aims then developed a key driver diagram to represent the theory of improvement.14 For each driver, the team defined, tested, and refined interventions using Plan-Do-Study-Act (PDSA) cycles.14,15
The institution has a mature QI infrastructure within a center for health care excellence.16 Clinical pathways are created to address diagnoses with opportunity for improvement. These pathways are linked to order sets in the electronic medical record (EMR) to assist providers.
Evaluation of Existing Process for FIF Patients
Failures related to the prearrival process for the ED were inconsistent referral process; lack of communication between families and GI; and delayed referral communication between the ED and GI. Failures related to the postarrival process were unreliable identification of FIF patients at presentation; delayed CVC access; inconsistent team approach to patient evaluation; communication about care that began after patient evaluation in the ED; and variable laboratory and antibiotic selection depending on GI provider.
Interventions
Development of Key Drivers.
We used a successful, fully operational process that had reduced TTA for F&I patients as a template to create key drivers to address TTA for FIF patients. Key drivers included rapid identification of eligible patients, standardizing the pre/postarrival processes, staff/family engagement, preoccupation with failure,17 and timely vascular access. Secondary key drivers included accurate identification of IF patients; proper communication between families, GI and the ED; prearrival preparation of the patient room with equipment for CVC access; and standardized antibiotic administration (Fig. 1).
Fig. 1.
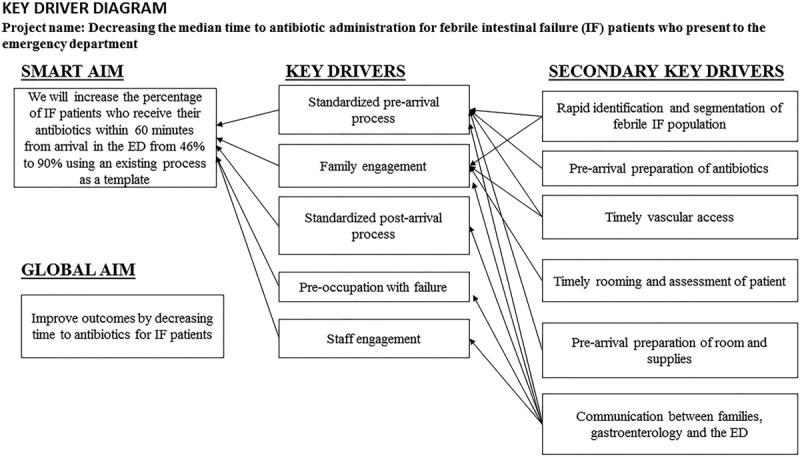
Key driver diagram for IF process improvement.
Interventions based on the Key Drivers
Prearrival and Family Engagement.
The team informed families about the improvement through letters and clinic visits that reviewed the process, the reasoning for the change, and potential benefits for their child. Parents of children with IF were instructed to notify GI for any temperature ≥ 100.4F. GI then contacted the ED to initiate a referral. The ED provider completed a referral then activated an order-set including standard labs, antibiotics, and supplies, which were delivered to the ED and placed in the room before patient arrival. The charge nurse prepared a room so the patient could be immediately assessed and the CVC accessed.
The ED referral coordinator confirmed orders were placed within 10 minutes of referral and completed a reminder card with the patient’s name for the ED greeter to facilitate rapid identification of the patient.
Postarrival.
Upon patient arrival, the ED referral coordinator sent an arrival page. If CVC issues were anticipated based on prior experience or parental report, the charge nurse informed the vascular access team (specialists in complex vascular access) before and upon patient arrival.
After ED evaluation with standard labs, and administration of intravenous fluids and an empiric, anti–Gram-negative bacteria antibiotic, all patients were admitted. If there was difficulty using the CVC, nursing placed a peripheral IV to obtain cultures and give antibiotics. Even if cultures were delayed, patients received antibiotics expediently (Fig. 2).
Fig. 2.
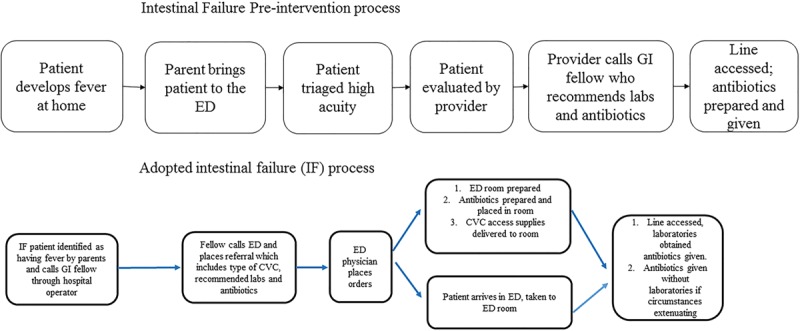
Pre- versus postintervention process.
Staff Engagement and Preoccupation with Failure.
Before implementation of the improvement, members of the improvement team educated ED and GI providers during meetings and conferences. Interval education followed via clinical updates and quarterly at staff meetings. Providers also received individualized reeducation for any process failures. Nursing education occurred during meetings and education councils. ED leadership and QI teams reviewed process run charts. Providers were informed about performance. Providers educated families during clinic visits and with each hospitalization. All failures were evaluated in real time.
Study of the Improvement
The study period was November 2012 through March 2017. We collected preintervention baseline data November 5, 2012, through October 13, 2013, when the improvement process was implemented. All FIF patients receive antibiotics in the ED and are admitted to the hospital (none are directly admitted from the GI clinic, primary care offices or outside hospitals), so the IF team accessed the admission logs each day to determine if any FIF patients had presented to the ED. TTA (from arrival) for FIF patients was measured in all patients. ED LOS (from arrival) was also measured to evaluate if ED LOS decreased during this time. We collected data from the EMR for all FIF patient encounters. The data included a medical record number, TTA, ED LOS, and where the patient was admitted (intensive care unit or general service). Data were reviewed for completeness.
Measures
Starting October 13, 2013, in our first PDSA, we sought to determine if the existing process could be directly translated to a different population while avoiding the use of additional ED resources beyond those used for the F&I process. We tested implementation of the existing F&I process without making concessions for differences in the IF population.
The second PDSA, beginning in January 2014, was intensive family follow-up by the case manager and provider follow-up by the improvement team to improve family and provider engagement. During this PDSA, the case manager explored reasons for the process failure with the family, reminded them of the potential advantages for the patient, and reviewed the algorithm. The QI team discussed the same with the ED and GI providers involved with the care. During this time, based on the observation that many process failures occurred with the first fever after CVC placement, case managers rigorously educated patients who had a newly placed CVC before discharge.
In January 2015, for the third PDSA, all GI providers agreed to standardize laboratories and antibiotic choice and timing. While there is a risk of drug-resistant organisms in this population, the decision was made to give an initial, standard antibiotic to all nonallergic patients at presentation then expand or narrow antibiotic choice as needed after admission. This decision allowed the ED provider to place orders for patients who arrived in the ED without a referral. Previously, these arrivals necessitated a phone call to GI and resulted in care delays.
Analysis
We evaluated the process through a quantitative time series study design. We used statistical process control methods to monitor changes. Annotated run-charts, created in excel, were developed and updated after every FIF patient ED visit. We also determined the median ED LOS. We established a median, illustrated as the center line on run charts. Standard industry criteria were used to determine whether observed changes in measures were due to random variation (common cause variation) or to a specific assignable cause (special cause variation18,19), in this case, the intervention. The intervention included implementation of the existing process; real-time follow-up with families and providers; GI internally standardizing their process; and moving the second antibiotic to the inpatient unit. A p-chart was also created for every 5 encounters, allowing for a timely response to the data. To ensure all encounters were included, we search the EMR for each IF patient in the GI database for visits during the study period, and any inadvertent exclusions that qualified were sent to the data specialist for inclusion.
Ethical Considerations
This initiative fell within the institutional review board’s guidance for QI projects that did not constitute human subjects research.
RESULTS
During the study period, 276 FIF encounters occurred. All encounters during this time frame were included based on an exhaustive chart review of IF patients.
Baseline data were obtained November 5, 2012, through October 13, 2013: there were 50 encounters before process implementation. During this period, the median time to antibiotics was 71 minutes, and 46% of the patients (23 of 50 encounters) received parenteral antibiotics within 60 minutes. Of the 50 encounters, 7 patients (14%) were admitted to the pediatric intensive care unit. The study period was October 13, 2013, through March 19, 2017. The initial PDSA, attempting to translate the existing F&I process directly to IF patients, showed no change in the median TTA. Analysis of these data showed that most failures occurred either with the first febrile illness after CVC placement or in a subset of families who consistently did not call the GI provider before arrival in the ED. The second PDSA, real-time analysis of failures with case manager follow-up with the families and provider follow-up by the QI team, did demonstrate an improvement in TTA but was not sustained enough to shift the process central line toward the goal. After the third PDSA, when GI providers agreed to standardize care for IF patients, the median TTA consistently improved. In April 2015, the median TTA decreased to 45 minutes. During this time, the percentage of patients who met the TTA goal increased from 46% at baseline to 68% after the initial intervention and 77% after the sustained decrease in median TTA. We also maintained a p-chart to allow for a timely response to shifts in the data. As this is a small population who does not often present to the ED, we used a sampling strategy of n = 5 for the p chart so we would not miss subtle shifts in the process. Following interventions, 14 of 226 encounters (6%) were admitted to the pediatric intensive care unit. There were no measured unintended consequences (Figs. 3–5).
Fig. 3.
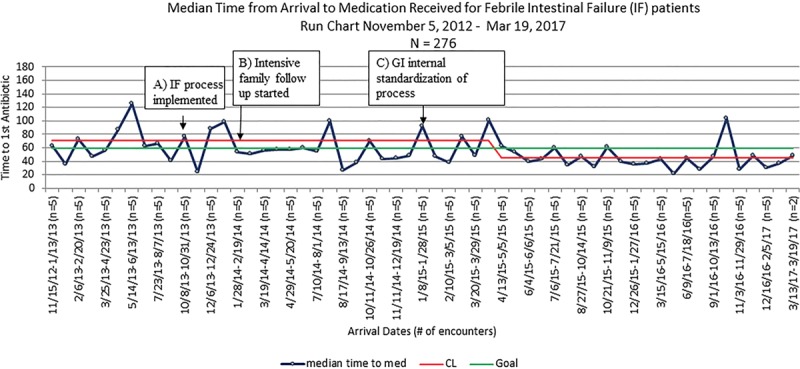
Run-chart demonstrating the median time to antibiotic for FIF patients in the ED.
Fig. 5.
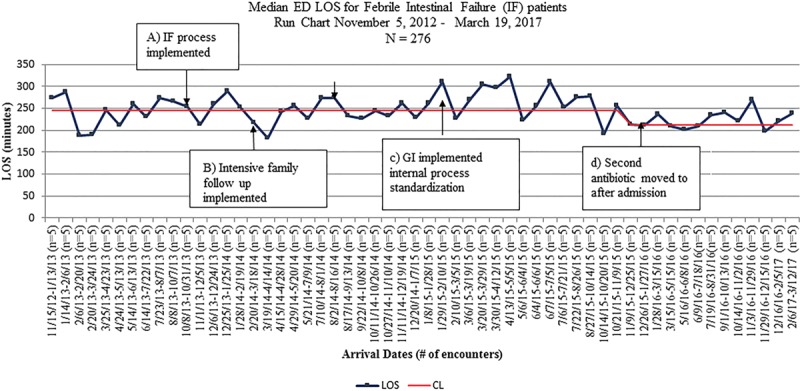
Run-chart demonstrating the median ED length of stay for FIF patients.
Fig. 4.
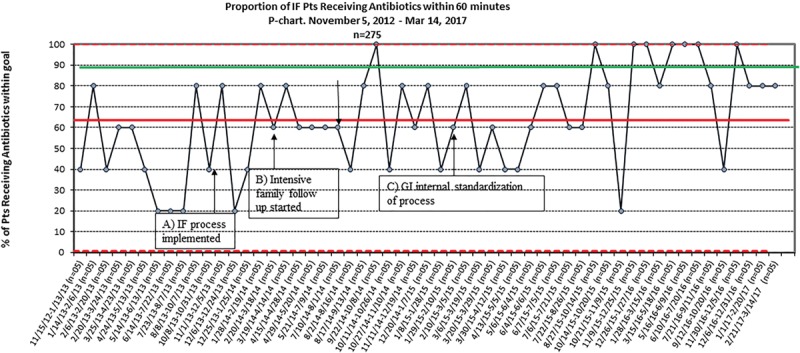
Proportion of IF patients receiving antibiotics within 60 minutes.
DISCUSSION
Summary
This study tests the spread of an existing process (timely antibiotic delivery in F&I patients) to another population (IF patients). Although there have not been any studies specifically analyzing whether TTA is important in IF patients, the increased risk of bacteremia and mortality in the group, speculated to be related to a relative immune deficient state from nutritional deficiencies and an impaired intestinal barrier function, has been well described.20–24 We were able to successfully reduce median TTA for the IF population presenting to our ED from 71 to 45 minutes, without requiring additional ED or hospital resources.
Interpretation
The most important factor in our initial success was convincing all GI providers to accept an internal, standard approach to FIF patients who present to the ED. Before this improvement, each provider had a unique plan for his/her patients, and the families were accustomed to that plan of care. Once the improvement team developed a workflow that emphasized internal consistency and antibiotic choice, the median TTA consistently improved. This change also allowed the ED provider to enter orders for patients who presented to the ED without having to call the consult service first.
The second factor that contributed to success was the focused effort on family education and engagement. While this is ongoing, when case managers and providers began real-time follow-up with families and discussed the rationale for the process and possible benefits for the child, the TTA began to improve, though the results were not initially sustained.
At our institution, we observe all IF patients for 1 hour after administration of antibiotics directed at Gram-negative organisms for potential decompensation. As a result of reducing time to initial antibiotics in the ED, we were able to reduce the ED length of stay by 30 minutes. Theoretically, this allows us to increase throughput in our ED, though we did not directly measure the effect of this decreased length of stay on other patient populations in the ED.
Despite our success at improving the median TTA to 45 minutes, we still have not met our additional goal of antibiotics within 60 minutes of arrival for 90% of the encounters. Further work is needed to address gaps. One key learning was trying to directly apply an existing process to a different population without modification does not result in identical improvement. Many process failures occurred with the first febrile illness after discharge from the hospital with the CVC. Although we did intensify education before discharge for families with a newly placed CVC, we did not analyze the data as a discrete PDSA. Second, many IF patients received a CVC in infancy and had the line for a prolonged period, which is different than F&I patients who have implanted central access for a defined period while being treated for a specific diagnosis. As a result, at the time of process implementation, IF families were accustomed to a specific manner of care delivery, including methods to access the CVC and antibiotic choice. During data analysis, we noticed that the same groups consistently experienced a process failure. Despite education and acceptance by their primary IF provider, some families do not notify GI before presenting to the ED. Additionally, many families wanted the CVC accessed in a certain fashion or presented during their parental nutrition infusion and were reluctant to have the infusion stopped for antibiotics. Future work must center on family engagement as the key driver.
Another possible cause for failure is that there was a concurrent process to improve recognition and treatment of sepsis in chronic patients in the ED. This process involved administering normal saline using the CVC of these patients. On occasion, if there was concern about compatibility of an antibiotic with the fluids being given, the nurses opted to give the fluids before administering antibiotics. Consequently, a QI team has been created that prioritizes efforts and monitors collaboration to address the multiple, competing, QI initiatives in the ED.
Limitations
There are several limitations to this study. Our global aim was to decrease morbidity and mortality in IF patients; however, due to the constraints associated with a QI study, we did not evaluate these measures, though a future study is specifically analyzing these data. Second, we do not have routine quantitative data to know whether the process resulted in interruptions in care for other children in the ED. Finally, we do not know if the antibiotics we chose to give in the ED specifically treated the organisms infecting this population that is at risk for multi-drug resistant or Gram-positive bacteria.
Future Direction
The processes applied to this population are being generalized to other high-risk populations at our institution. The ED is establishing a “therapeutic reliability” algorithm for high-risk populations and the translated success of the F&I process to the IF population has provided insight into how to improve care. An institutional review board–approved statistical analysis comparing outcomes in patients who received antibiotics within 60 minutes to those who did not and outcomes pre- and postintervention is currently being completed.
CONCLUSIONS
We describe the translation of an existing improvement process to decrease TTA in the ED for a high risk IF population. Ultimately, we determined that while the QI concepts and framework from an existing process can be applied to a different, high-risk population, the most important predictors of success were internal standardization of the process and family engagement.
Key drivers of success for this project were dependent on acceptance of the process change by providers and families followed by adequate communication between the family, GI, and the ED. Verification of the process at all visits, and frequent discussions surrounding the IF process and the role of the family in preparation were also essential for success.
This work provides insight regarding the use of an existing improvement protocol to spread quality care to high-risk populations.
This report employed the Standards for QI Reporting Excellence (SQUIRE) 2.0 publication guidelines for reporting health care QI research.25
ACKNOWLEDGMENTS
The authors express their gratitude to the patients and their families. The authors also thank the physicians, nurses, PCA’s, paramedics, NPs, hospitalists, fellows, and staff at their institution.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online July 20, 2018.
To cite: Hariharan S, Mezoff EA, Dandoy CE, Zhang Y, Chiarenzelli J, Troutt ML, Simpkins J, Dewald M, Klotz K, Mezoff AG, Cole CR. A Quality Improvement Initiative to Decrease Time to Antibiotics for Children with Intestinal Failure, Fever, and a Central Line. Pediatr Qual Saf 2018;3:090.
Table of Contents Summary: We describe use of an existing, mature process for oncology patients to improve time to antibiotics for an intestinal failure population at risk for infection.
REFERENCES
- 1.Sigalet DL.Short bowel syndrome in infants and children: an overview. Semin Pediatr Surg. 2001;10:49–55.. [DOI] [PubMed] [Google Scholar]
- 2.Buchman AL.Complications of long-term home total parenteral nutrition: their identification, prevention and treatment. Dig Dis Sci. 2001;46:1–18.. [DOI] [PubMed] [Google Scholar]
- 3.Howard L, Ament M, Fleming CR, et al. Current use and clinical outcome of home parenteral and enteral nutrition therapies in the United States. Gastroenterology. 1995;109:355–365.. [DOI] [PubMed] [Google Scholar]
- 4.Spencer AU, Neaga A, West B, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg. 2005;242:403–409; discussion 409.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marra AR, Opilla M, Edmond MB, et al. Epidemiology of bloodstream infections in patients receiving long-term total parenteral nutrition. J Clin Gastroenterol. 2007;41:19–28.. [DOI] [PubMed] [Google Scholar]
- 6.Miko BA, Kamath SS, Cohen BA, et al. Epidemiologic associations between short-bowel syndrome and bloodstream infection among hospitalized children. J Pediatric Infect Dis Soc. 2015;4:192–197.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596.. [DOI] [PubMed] [Google Scholar]
- 8.Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045–1053.. [DOI] [PubMed] [Google Scholar]
- 9.Volpe D, Harrison S, Damian F, et al. Improving timeliness of antibiotic delivery for patients with fever and suspected neutropenia in a pediatric emergency department. Pediatrics. 2012;130:e201–e210.. [DOI] [PubMed] [Google Scholar]
- 10.Iyer SRS, Varadarajan K, Alessandrini E.The Acute Care Model: a new framework for quality care in emergency medicine. Clin Pediat Emerg Med. 2011;12:91–101.. [Google Scholar]
- 11.Freire MP, Pierrotti LC, Zerati AE, et al. Infection related to implantable central venous access devices in cancer patients: epidemiology and risk factors. Infect Control Hosp Epidemiol. 2013;34:671–677.. [DOI] [PubMed] [Google Scholar]
- 12.Dandoy CE, Haslam D, Lane A, et al. Healthcare burden, risk factors, and outcomes of mucosal barrier injury laboratory-confirmed bloodstream infections after stem cell transplantation. Biol Blood Marrow Transplant. 2016;22:1671–1677.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dandoy CE, Hariharan S, Weiss B, et al. Sustained reductions in time to antibiotic delivery in febrile immunocompromised children: results of a quality improvement collaborative. BMJ Qual Saf. 2016;25:100–109.. doi: 10.1136/bmjqs-2015–004451. [DOI] [PubMed] [Google Scholar]
- 14.Berwick DM.A primer on leading the improvement of systems. BMJ. 1996;312:619–622.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pronovost PJ, Berenholtz SM, Goeschel CA, et al. Creating high reliability in health care organizations. Health Serv Res. 2006;41(4 Pt 2):1599–1617.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaminski GM, Schoettker PJ, Alessandrini EA, et al. A comprehensive model to build improvement capability in a pediatric academic medical center. Acad Pediatr. 2014;14:29–39.. [DOI] [PubMed] [Google Scholar]
- 17.Bowermaster R, Miller M, Ashcraft T, et al. Application of the aviation black box principle in pediatric cardiac surgery: tracking all failures in the pediatric cardiac operating room. J Am Coll Surg. 2015;220:149–55.e3.. [DOI] [PubMed] [Google Scholar]
- 18.Benneyan JC, Lloyd RC, Plsek PE.Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458–464.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amin SG.Control charts 101: a guide to health care applications. Qual Manag Health Care. 2001;9:1–27.. [DOI] [PubMed] [Google Scholar]
- 20.Dudeck MA, Horan TC, Peterson KD, et al. National Healthcare Safety Network report, data summary for 2011, device-associated module. Am J Infect Control. 2013;41:286–300.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wales PW, Kosar C, Carricato M, et al. Ethanol lock therapy to reduce the incidence of catheter-related bloodstream infections in home parenteral nutrition patients with intestinal failure: preliminary experience. J Pediatr Surg. 2011;46:951–956.. [DOI] [PubMed] [Google Scholar]
- 22.Cober MP, Kovacevich DS, Teitelbaum DH.Ethanol-lock therapy for the prevention of central venous access device infections in pediatric patients with intestinal failure. JPEN J Parenter Enteral Nutr. 2011;35:67–73.. [DOI] [PubMed] [Google Scholar]
- 23.Squires RH, Duggan C, Teitelbaum DH, et al. Pediatric Intestinal Failure Consortium. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr. 2012;161:723–8.e2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole CR, Frem JC, Schmotzer B, et al. The rate of bloodstream infection is high in infants with short bowel syndrome: relationship with small bowel bacterial overgrowth, enteral feeding, and inflammatory and immune responses. J Pediatr. 2010;156:941–947, 947.e1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25:986–992.. [DOI] [PMC free article] [PubMed] [Google Scholar]


