Abstract
Lignin micro- and nanoparticles (LMNPs) synthesized from side-streams of pulp and paper and biorefinery operations have been proposed for the generation of new, high-value materials. As sustainable alternatives to particles of synthetic or mineral origins, LMNPs viability depends on scale-up, manufacturing cost, and applications. By using experimental data as primary source of information, along with industrial know-how, we analyze dry and spherical LMNPs obtained by our recently reported aerosol/atomization method. First, a preliminary evaluation toward the commercial production of LMNPs from industrial lignin precursors is presented. Following, we introduce potential LMNPs applications from a financial perspective. Mass and energy balances, operating costs, and capital investment are estimated and discussed in view of LMNPs scalability prospects. The main potential market segments identified (from a financial perspective) include composite nanofillers, solid foams, emulsion stabilizers, chelating agents, and UV protection. Our technical, financial, and market assessment represent the basis for R&D planning and efforts to lower the risk related to expected industrialization efforts. Manufacturing costs were estimated between 870 and 1170 USD/t; also, minimum selling prices varied from 1240 and 1560 USD/t, depending on raw materials used. Sensitivity analysis indicated that manufacturing cost can be as low as 600 USD/t, depending on the process conditions considered. Finally, based on the financial assessment, potential applications were identified.
Keywords: Lignin, Organic colloids, Microparticles, Nanoparticles, Biorefinery, Mass and energy balance, Techno-economic analysis
Short abstract
Industrial production and potential applications of lignin micro- and nanoparticles guiding R&D efforts to generate value from forest industry byproducts.
Introduction
Lignin naturally occurs in woody and nonwoody plants, representing from 15% to 30% by weight of the total dry mass, depending on the source.1 Lignin has a complex structure originating from hydrogenative polymerization of three basic precursors, namely, p-hydroxyphenyl (H), coniferyl (G), and sinapyl (S) alcohols. The resulting macromolecule contains various functional groups and its monomeric phenylpropane units (or C9-unints) are linked by different ether and C–C linkages (Figure 1a).2−5 The huge opportunity for securing vast quantities of lignins from the forest products industry, economically and at large scale, is concurrent with developments in biorefineries and bioproduct mills that integrate biomass conversion processes. At present, the primary production sources of industrial lignin are the organic-rich streams generated during biomass digestion (wood pulping), which contains mainly dissolved lignin, carbohydrates and their degradation products and extractives, as well as cooking inorganics. The latter components are usually recovered from solution while the remaining material, the ligninic fraction, is normally used for energy generation.6 This current low-end use of lignin can be seen as a starting point for the future bioeconomy, as an alternative to fossil resources that do not compete with food supply.
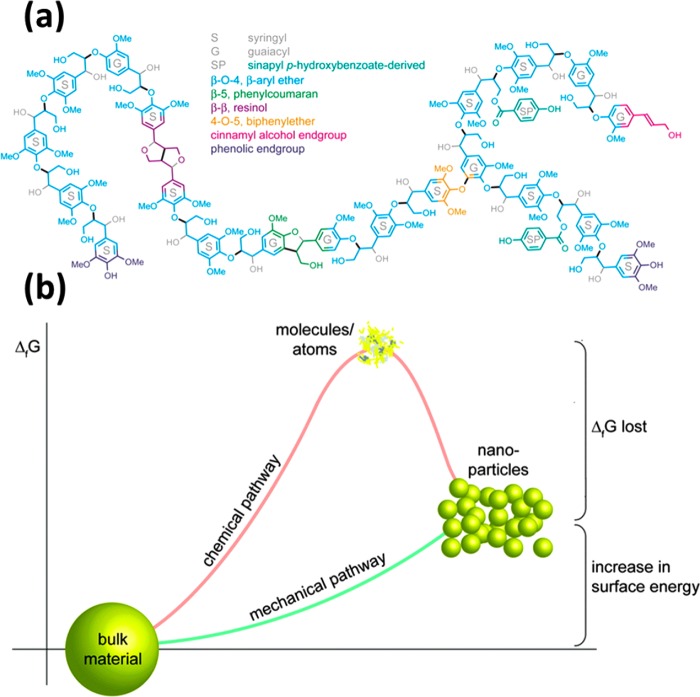
Figure 1.
(a) Plausible structural model of a lignin fragment showing various functional groups and interunit linkages. Reprinted in part with permission from ref (26) (Copyright 2010 American Society of Plant Biologists). (b) Energy profile (Gibbs free energy) along with chemical or mechanical nanoparticle preparation. The chemical pathway presents an unnecessary diminution step (high intermediate ΔG) since the atomic/ionic or molecular intermediates are then again combined to nanosized particles. Reprinted in part with permission from ref (22) (Copyright 2015 The Royal Society of Chemistry).
Due to its diverse functional groups, lignin can find other uses,7 such as absorbents, emulsifiers, dispersants, and as chelating agents.8−11 Additionally, a wide range of chemicals and products, such as adhesives, can be produced from lignin.12−14 Moreover, it can be used for the production of bioplastics, as well as for controlled and slow release of fertilizers.15 Carbon fibers can be obtained from lignin,16 which in turn can be used as component in supercapacitors and energy storage devices.4,16,17 Nevertheless, several challenges exist to facilitate value-added applications of lignin. They include relatively low reactivity, low compatibility in many polymer blends, the chemical structure variability (which depends on the feedstock and extraction conditions), broad molecular weight distributions, inconsistency in composition profiles, and tendency to repolymerize after fragmentation, among others.14,18 In addition to the complexity of the lignin structure in the plant biomasses, all biorefinery processes result in dramatic increase in complexity and chemical heterogeneity of the structures of technical lignins.14,19
Lignin Micro- and Nanoparticles (LMNPs)
A remarkable behavior of lignins, when subjected to specific conditions, is their tendency to form small particles within the plant cell walls.6,20 In this work, lignins from pulp and paper processes are used as feedstock for the manufacture of colloidal lignin or lignin micro- and nanoparticles (LMNPs). LMNPs represent a toolbox toward property development that can be quite attractive for practical applications. These can be exemplified in current efforts to supply materials with color, thermal, and light stability as well as strength.21 Moreover, improved or new functions can be achieved from multiple components integrated in the form of particles.22 In general, the isolation of particles via mechanical pathways is less energy demanding than chemical routes. This is a direct result of the surface energy that is lower compared with typical chemical manufacturing enthalpies, such as dissolution, reduction, and others (Figure 1b).22 Thus, small and chemically inert particles can be used, for example, in pigments, as polymer fillers or in surface finishing.22 In the specific case of lignin particles, there is great promise as emulsion stabilizers, antioxidants, and agents for UV protection.6,23−25
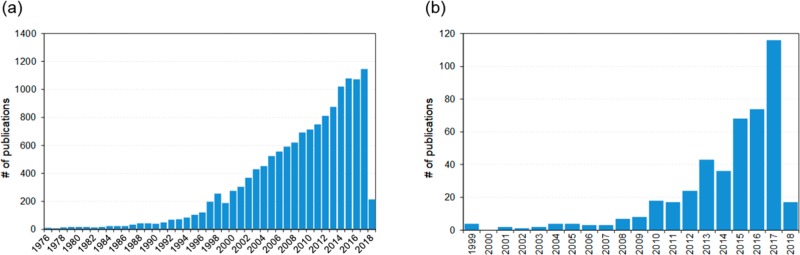
The field of polymer nanoparticles is quickly expanding and playing a pivotal role in a broad spectrum of areas.27 This fact can be realized from the ever-increasing number of publications, as depicted in Figure 2a. This trend is based on the properties of polymer particles, which meet a wide range of applications and market needs. To some extent, the same can be said for those derived from lignin, a subset of polymer particles, Figure 2b. The use of lignin micro- and nanoparticles to produce added value materials can open an important pathway for the development of new applications and consequently contribute to fostering the future biobased industry.6,28
Figure 2.
(a) Number of publications per year according to a search with Web of Science (March 2018) using the search terms “TI = (polymer* or biopolym* or biomacrom*) and TI = (particle* or nanoparticle* or microparticle* or bead* or microbead* or nanobead* or capsule* or microcapsule* or nanocapsule* or microsphere* or nanosphere*) and refined by TOPIC: (production or isolation or synthesis or development or separation). (b) Number of publications per year according to Web of Science (March 2018) using the search terms “TI = (Lignin*) and TI = (particle* or nanoparticle* or microparticle* or bead* or microbead* or nanobead* or capsule* or microcapsule* or nanocapsule* or microsphere* or nanosphere*) NOT TS = (particleboard* or board*). Field tags: TI = title, TS = topic.
LMNPs Formation Mechanism and Synthesis
At given conditions, lignin tends to form spherical particles.6,20 Prior studies have proposed that LMNPs are formed in situ when biomass is subjected to temperatures higher than the lignin phase transition (∼175 °C).6,20 Formation of particles with different shapes and sizes, between 5 nm to 10 μm, have been reported. The mechanisms proposed for the formation of these particles includes: (i) a coalescence phase, when temperature is above lignin softening point, allowing it to expand and move within the cell wall matrix; (ii) a migration phase, when lignin migrates to the cell surface, and finally (iii) particle formation and extrusion from the cell wall, as cellulose microfibrils are exposed and consequently attached to each other by hydrogen bonding.6,20 Lignin can be separated from other wood components by well-known processes (such as kraft pulping), although particular conditions are needed for the generation of respective particles at the micro- and nanoscale.23
Biomass pretreatments may indeed change the lignin structure and generate different types of materials.18,29 For lignin characterization purposes, various analytical procedures including ball milling (followed or not by enzymatic treatments) have been used to isolate lignins from biomass without significantly modifying its structure.5 The drawbacks of these processes include the very high energy demand and limitations in scaling up, making them industrially unrealistic. At the industrial scale, lignin is isolated from the wood by different processes, such as kraft, soda, and sulfite pulping, generating kraft lignin, alkali lignin, and lignosulfonates, respectively.14,23,30 On the basis of pulp production, the kraft process is the most commonly used to separate lignin from wood, generating about 78 million metric tons of lignin worldwide.14,30−32 While this lignin is used in energy cogeneration, there is a potential to recover between 5% to 10% of the total kraft lignin production without negative impacts on mills’ operations and finance (depending on pulp mill production limitations). The current recovery of pulping lignins is only 2% of the industrial production, where the majority of commercial lignin is sourced from the sulfite process, with a total production close to 1.1 million metric tons/year of lignosulfonates as of 2015.14,23,30,33,34
Lignin particles with various sizes and shapes, hollow or solid, have been reported by using different processes, such as solvent or pH shifting, cross-linking/polymerization, mechanical treatment, ice-segregation, template-based synthesis, electrospinning, CO2 antisolvent, and aerosol processing.23 A description of these processes can be found in recently published reviews.6,18,23 In the solvent shifting process, the lignin is dissolved in a water-miscible organic solvent. The solution is then mixed with excess water, generating the LMNPs due to the reduced solvency.23 In pH shifting, lignin is diluted in a solvent (e.g., ethylene glycol) or water at a high pH, followed by addition of an acidic solution (e.g., HCl), allowing the formation of LMNPs.35 Cross-linking and polymerization have been used for the synthesis of LMNPs, which may include the use of surfactants and cross-linking agents.23 Mechanical treatments, such as homogenization and sonication, were applied to reduce particle size of lignin to the nanoscale.36,37 In the ice-segregation method, an aqueous lignin solution was rapidly frozen, followed by ice sublimation, thus generating a network of lignin nanofibers.38 Template-based synthesis, as suggested by its name, uses a template (e.g., alumina membrane) to shape the lignin into a desired form, such as nanotubes.39,40 Electrospinning of lignin solutions has been demonstrated in the presence of poly(ethylene oxide) or poly(vinyl alcohol) in the solution.41 Carbon dioxide antisolvent is a technique that uses CO2 at supercritical conditions for lignin precipitation.42 Finally, LMNPs have been produced from kraft lignin solution (tetrahydrofuran and water) by evaporating the solvents and redispersing the solids obtained in water.43
Solvent shifting, CO2 antisolvent, aerosol, and dissolution/evaporation pathways have presented the highest yields reported. The main drawbacks of solvent shifting and CO2 antisolvent are the low solids concentration in final product and high operating pressures, respectively. The dissolution/evaporation process, despite the high yields, needs multiple steps. Mechanical treatment for the manufacturing of LMNPs seems promising, given the similarities to existing pulp and paper operations and the absence of hazardous solvents; however, the broad range of particle sizes obtained may prevent applications of the final product (due to nonuniformity). Most relevant for any practical use, most of the above methods are highly intensive in solvent use. In contrast, previous efforts demonstrated the possibility of aerosol processing, in one step, to obtain dry lignin particles with sizes ranging from 30 nm to 2 μm and with various surface energies (wettability), depending on the source and process conditions.28 Moreover, in situ development of surface features on the particles is possible, such as wrinkling, which is attractive in advanced applications.44 A comparison of the processes previously discussed including the types of lignin used, yields, advantages, and disadvantages is presented in Table 1. Due to ease of scale-up and relatively high yields, the aerosol process was selected in our techno-economic study.
Table 1. Comparison of Different LMNPs Isolation Methods (See Also Beisl et al., 2017).23.
| technology | lignin precursor | reported yield | advantages | disadvantages |
|---|---|---|---|---|
| solvent shifting | AL, KL, OS, EHL | 33%–90.9% | · simple process; yields solid and hollow particles | · low solids content (∼1%) |
| pH shifting | AL,KL, EHL | 10% | · high solvent demand; low yields | |
| cross-linking/polymerization | KL, AL, LS, | · controlled size of particles45 | ||
| mechanical treatment | AL, KL | · process known in the pulp and paper industry; simple process; do not use hazardous solvents18 | · nonuniformity of particle size; broad particle distribution range | |
| ice segregation | AL | |||
| template-based synthesis | AL, other lignins | · controlled shape characteristics39,40 | ||
| electrospinning | KL, OS, LS, PL | · low solvent usage | ||
| CO2 antisolvent | KL, OS | 51–88% | · controllability of morphology, size and size distribution; CO2 is nonflammable and nontoxic | · high operating pressure |
| aerosol processing | AL, KL, OS | higher than 60% | · single step process; the absence of liquid byproducts; high yields | |
| dissolution followed by solvent evaporation and redispersion43 | KL | 85% | · possibility to scale-up; simple process | · several steps needed; final product is dispersed in water |
AL = alkali lignin, KL = kraft lignin, OS = organosolv lignin, EHL = enzymatic hydrolysis lignin, LS = lignosulfonate, and PL = pyrolytic lignin.
Due to the relative novelty of LMNPs and the recent dates associated with the reports and advances in the manufacturing processes, to our knowledge, no techno-economic studies on LMNPs manufacturing process are available, although some studies indicate potential for scale-up.28,43 We hereby propose a preliminary assessment for LMNPs manufacturing at industrial scale. The selected process (aerosol method) is described and discussed along with estimated capital and operating costs. Additionally, the impact of different process conditions on project financials is evaluated through sensitivity analyses. Finally, an evaluation of potential applications is provided. The collective information and methodologies used are expected to help in directing LMNPs development efforts to the most promising opportunities.
Materials and Methods
Synthesis of LMNPs
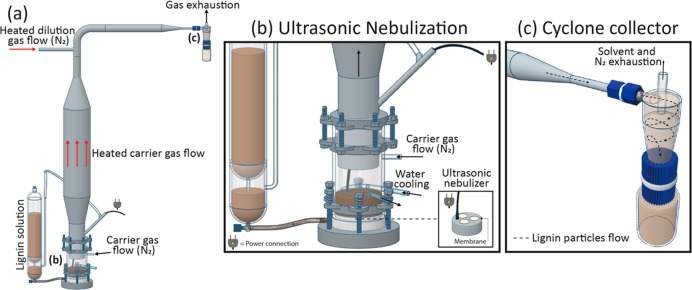
Different types of lignins were used at the laboratory scale for the aerosol flow process. The precursors included soda, organosolv, and kraft lignins.28 In the first generation of this process, a lignin solution (e.g., alkaline solution for alkali lignin, and dimethylformamide for organosolv or kraft lignin) was prepared in an agitated vessel. The solution was then sent to a collison-type jet atomizer with nitrogen that generated small droplets. The droplets were carried to a heated reactor at a given temperature (e.g., 100 °C for water or 153 °C for DMF), and the solvent was evaporated. The reactor outflow consisted of a mixture of lignin particles and solvent in the vapor phase. This outflow was sent to a Berner-type low-pressure impactor, where LMNPs were collected and the vapor was exhausted. A second, larger version of the unit was developed for the benefit of this study to increase the capacity, which included a nebulizer and a cyclone collector (Figure 3). In this larger version, the cooling water in the solution source is necessary to prevent overheating of the ultrasonic nebulizer during extended operation periods. Figure 4 illustrates LMNPs that can be produced with this system.
Figure 3.
Laboratory-scale setup used for LMNPs synthesis in this study: (a) general scheme for the aerosol flow reactor; (b) ultrasonic nebulizer assembly; and (c) cyclone collector assembly.
Figure 4.
Example of LMPNP produced by the aerosol flow system shown in Figure 3. On the left, spherical, dry, and smooth lignin particles with sizes in the micrometer range (scale bar = 3 μm). On the right, a SEM image of LMNPs (scale bar = 200 nm) showing surface wrinkling, which can be produced by adjusting the operation conditions in the unit. Adapted with permission from ref (44) (Copyright 2018 The Royal Society of Chemistry).
Techno-Economic Analysis of LMNPs Manufacturing at Industrial Scale
The steps used to perform the techno-economic analysis for LMNPs manufacturing are illustrated in Figure 5. Initially, information from the laboratory scale process was collected to identify the main streams and processes (e.g., reaction, separation, heating, etc.). Using this information, and based on industrial experience, a block diagram was constructed. Process areas and major equipment needs were defined, as well as main process streams. Process information from the laboratory scale was collected (such as production yields, raw materials, and energy requirements), and adjusted for mass and energy balances at the industrial scale. Production flow rates were estimated, and the main equipment (tanks, pumps, atomizer and separator) were designed.
Figure 5.
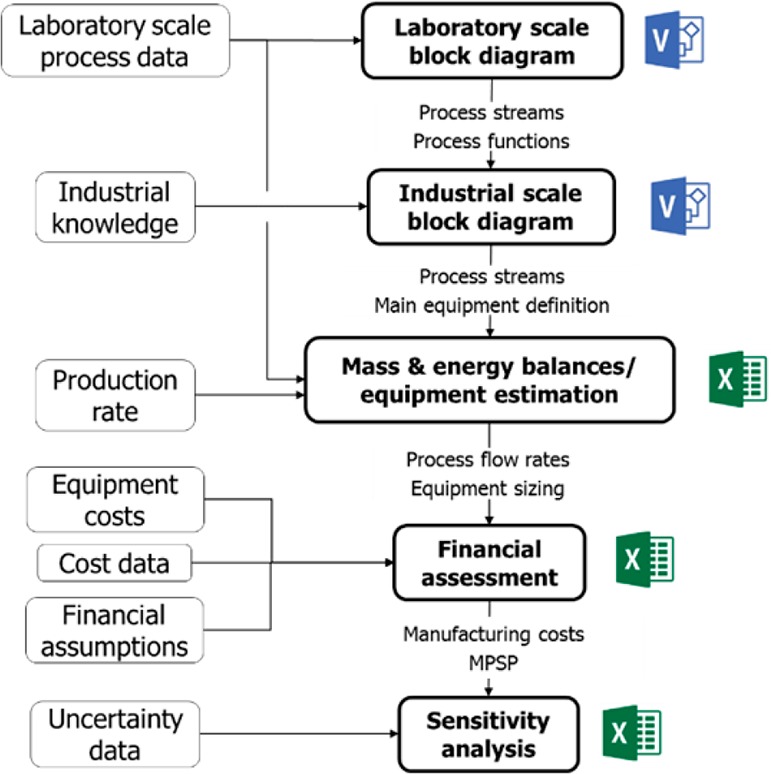
Steps to perform techno-economic analysis of LMNPs manufacturing.
The following step aimed to link the technical and financial models. Ordinary equipment costs were estimated using Peters and Timmerhaus’ cost estimation tool and Matches’ databases.46,47 The cost for atomization and separation equipment was based on previous quotations from equipment suppliers. The overall capital investment was estimated by multiplying the sum of equipment costs by factors that account for equipment erection, piping, electrical systems, instrumentation and controls, buildings, yard improvements, service facilities, land, as well as indirect costs (engineering, construction and legal expenses, contractor fees, and contingency); factors used were based on information available in literature.48 To estimate direct operating costs, calculated process flow rates were combined with unitary costs of feedstocks and utilities. Other operating costs (maintenance, labor, depreciation, overheads, and other fixed costs) were based on direct operating costs and capital investment values, in addition to wages information used in previous assessments.49,50 Finally, a simplified cash flow modeling was executed and the minimum product selling price (MPSP) was estimated for a set of financial assumptions that will be described later. MPSP is the estimated product price for the investment to achieve Net Present Value (NPV) zero at a specified hurdle rate (the minimum rate expected when investing in a project). The assessment was then supplemented by a sensitivity analysis, where main process and financial parameters were varied and their impact on the project financials were evaluated.51 Microsoft Visio was used to create block and process diagrams at the laboratory and industrial scales. Microsoft Excel was used for mass and energy balance calculations, as well as financial assessment and sensitivity analysis of the industrial process.
Feedstock
Kraft lignin and lignosulfonate were selected as the precursors for the industrial scale process, due to their commercial availability. For experiments at the laboratory scale process, kraft lignin, DMF and ammonium hydroxide (25% aqueous) were purchased from Sigma-Aldrich; they were used without further purification. For the industrial process, bulk-scale feedstocks and chemicals were assumed. Suitable solvents for lignin precursors were defined: water for the lignosulfonate and dimethylformamide (DMF) and ammonium hydroxide solution (14% wt.) for kraft lignin. DMF was used in the initial experiments with kraft lignin28 and ammonium hydroxide was considered given its lower cost. The three solvents were selected to establish a comparison of their effect in the economic feasibility and considering that they easily dissolve the respective lignin for the aerosol flow process, at 5% solids concentration.
Technology Selected and Scenarios Assessed
As indicated previously, the techno-economic analysis of the aerosol technology was performed. It is worth noting that the atomizer and separation technologies were used for the cost modeling of LMNPs manufacturing at the industrial scale. Additional bench scale analyses, pilot testing, as well as discussions with equipment suppliers were carried out to confirm the adequacy of this technology from a technical perspective. Three scenarios were selected (Table 2). All scenarios considered the LMNPs facility colocated with a pulp mill, which was assumed to supply dried lignin to the new facility. Note, however, the possibility of using lignin solutions generated directly in the process, avoiding drying, which would entail a considerable benefit in the economics of the process. In our calculation we assumed that lignin was delivered in dry form, making it more cost-demanding. Despite this added cost, it is reasonable to still expect a lower cost compared to alternative processes to obtain lignin particles, such as solvent or pH shifting. Not only they require the lignin solution feed but also other solutions (anti-solvent or acid solutions) that ultimately are to be removed by drying.
Table 2. Summary of Scenarios Assessed.
| scenario | lignin precursor | solvent |
|---|---|---|
| 1 | kraft lignin | dimethylformamide (DMF) |
| 2 | kraft lignin | ammonium hydroxide (14% wt. in water) |
| 3 | lignosulfonate | water |
Description of Simulated Industrial Scale Process
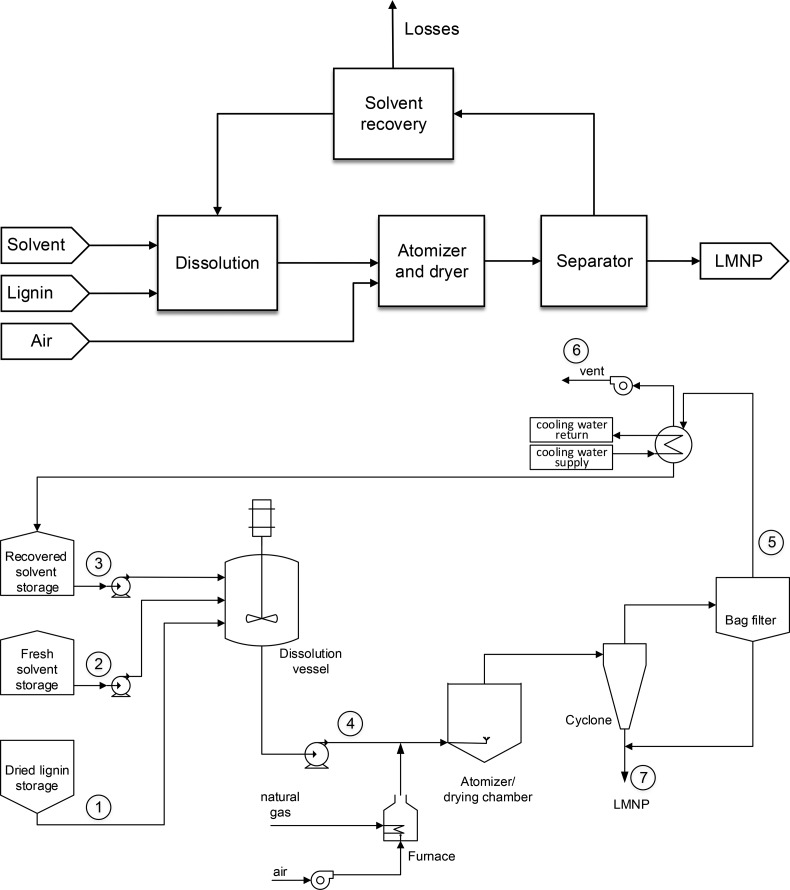
The first step to simulate the nth plant for LMNPs manufacturing was to construct a block diagram of the laboratory-scale process, and to adapt it to the industrial scale. For the laboratory scale facility (Figure 3), four main unit operations were identified (dissolution, atomization, drying, and vapor–solid separation). The block diagram of the proposed industrial scale process for LMNPs manufacturing is illustrated in Figure 6, top. While one can assume that the lignin is sourced as a solution from the actual process, in situ, here we adopt the most challenging situation where the lignin feed is obtained as a dry powder. Such dried lignin was then diluted in the given solvent using an agitated vessel. Afterward the solution was sent to the atomizer and separation systems. This equipment was able to generate small solution droplets, followed by the evaporation of the solvent using hot air that flowed countercurrent in a drying chamber. The stream consisting of dried particles and the vapor phase of the solvent was sent to a device where the vapor–solid separation was performed. A cyclone and bag filter were assumed for separation. The particles were collected at the bottom of the cyclone, and the vapor phase passed through a bag filter to collect the remaining particles. The solids collected from cyclone and bag filter are the final product, LMNPs. The vaporized solvent in the separation step was drafted from the system using a fan and recovered in the solvent recovery section. In the solvent recovery section, the solvent was condensed in a heat exchanger. The recovered solvent was mixed with fresh solvent and fed back to the dissolution step. Solvent losses were accounted in the simulation, due to possible residual solvent within the final product (LMNPs) and the very small losses through venting systems, which were calculated using EPA guidelines.52 The impact of solvent losses is further evaluated in this work. A flowsheet for the proposed industrial process is presented in Figure 6, bottom.
Figure 6.
Simplified block (top) and process (bottom) diagrams for industrial scale manufacturing of LMNPs.
Process Assumptions
The main process assumptions for the industrial process are included in Table 3. The production rate was defined based on Culbertson (2017),53 which assumed that 5.2% of lignin liquor can be extracted as lignin product from a kraft pulp mill with a capacity of 440 000 air-dry metric ton/yr. The yields for LMNPs reported by Ago et al.28 were >60%; such relatively lower yields at the laboratory scale, in absence of a bag filter, are mainly attributed to particles retained in the drying chamber and losses in the solvent exhaustion system. It is expected that for the industrial facility these issues would be controlled, generating no product losses. Thus, we assumed negligible lignin losses in the industrial scale manufacturing. A separator efficiency was defined for the industrial process, which means that the solids that could not be separated in the cyclone and bag filter were sent to the solvent recovery system together with the solvent and then reprocessed. A typical mass balance is presented in Table 4.
Table 3. Main Process Assumptions for LMNP Manufacturing at Industrial Scale.
| input | value | unit | reference |
|---|---|---|---|
| production rate | 150 | metric ton/day | Culbertson (2017)53 |
| facility type | colocated with a pulp mill | assumed | |
| lignin concentration in solution | 5% | % wt. | Ago et al. (2016)28 |
| lignin losses to the environment | 0% | % wt. of input flow | assumed |
| losses of solvent to atmosphere | 0.001% | % wt. of recirculating solvent | based on EPA52 |
| solvent concentration in final product | 0.5% | % wt. | assumed |
| separation efficiency | 99% | wt. solid out/wt. solid in | assumed |
| cooling tower temperature difference | 10 | °C | assumed |
| cooling water makeup | 2% | based on recirculating flow | assumed |
| overall equipment efficiency | 95% | assumed |
Table 4. Typical Mass Balance for Industrial Manufacturing of LMNPs (for stream numbers refer to Figure 6, bottom).
| stream | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| description | lignin, t/day | fresh solvent, t/day | recovered solvent, t/day | solution to atomizer, t/day | vapors to recovery, t/day | losses, t/day | LMNPs, t/day |
| lignin | 150.0 | 1.5 | 151.5 | 1.5 | 150.0 | ||
| solvent | 0.8 | 2878.0 | 2878.8 | 2878.0 | 0.03 | 0.8 | |
| total | 150.0 | 0.8 | 2879.5 | 3030.3 | 2879.5 | 0.03 | 150.8 |
Scalability Assessment
Feedstock
For the proposed techno-economic analysis, we assumed that the kraft and lignosulfonate lignins used were not of high purity. Lignin may contain ash and carbohydrates, which are not soluble in organic solvents. Although not considered in the current analysis, additional filtration equipment might be necessary for separating insoluble impurities from the lignin solution. In addition, it was assumed that a less pure lignin does not affect negatively the LMNPs quality for the intended applications. The consequences of using an industrial grade feedstock should be assessed by laboratory and pilot trials before scaling up the process.
Process Configuration and Equipment
The production of LMNPs at the laboratory scale process was performed in semibatch mode. The dissolution phase was in batch, and atomizing and drying steps were performed continuously. To ease operation and consequently produce LMNPs in a cost-effective manner, the industrial process was assumed to run in continuous mode; this prevents equipment idle time and ensures their efficient utilization. Nevertheless, it was assumed that the facility would operate 95% of the year (Overall Equipment Efficiency—OEE—of 95%) to account for maintenance and facility downtime (Table 3).
The nebulizer in the laboratory scale process was defined as an atomizer and separator system, which can be easily designed and scaled up for capital cost estimation. In addition, the particle drying system at the laboratory scale (oven) used electrical resistance to evaporate the gases. In the industrial scale, air was assumed to be heated in a furnace using natural gas as fuel (Figure 6, bottom).
Utilities and Infrastructure
Considerations about infrastructure and utilities are necessary for the simulation of an industrial facility. For example, the laboratory scale process used nitrogen as carrying gas between process equipment. The transport of particles at industrial scale considered a fan to draft air and consequently carry the particles. It was assumed that the solvents were recovered by a condensing system and recirculated back to the dissolution step.
Major Costs and Financial Assumptions
Major raw materials and energy costs are presented in Table 5. Reported Kraft lignin value vary between 250 and 500 USD/t.34,53 For the current assessment, we considered USD 250/t based on Culbertson (2017).53 Lignosulfonates values range from 300 to as high as 2700 USD/t, depending on purity.7,34 For LMNPs, we assumed that there is no requirement on lignosulfonate quality. Using the literature review and industry quotations, we adopted the value reported of 300 USD/t. DMF costs were retrieved from ICIS database (2000) and adjusted to current values using the PPI (producer price index) reported by the Bureau of Labor Statistics.54,55 The PPI used was for crude petroleum and natural gas extraction–primary products, since DMF is manufactured from methanol. Ammonium hydroxide costs were based on average anhydrous ammonia prices reported by Fertecon,56 and calculated as indicated by Airgas, an ammonium hydroxide supplier.57 Water was assumed as tap water. Natural gas and electricity costs were retrieved from FisherSolve and EIA (U.S. Energy Information Administration)58 databases, respectively. Table 6 illustrates financial assumptions for the techno-economic analysis of LMNPs industrial manufacturing. These assumptions are similar to the ones considered in previous bionanomaterial assessments.49,50 The hurdle rate considered (16%) is approximately twice the average cost of capital reported for pulp and paper and chemicals (specialty) industry segments.63 The construction time considered for the LMNPs facility is two years, although we acknowledge that additional savings are possible if the construction time is reduced to 1.5 years.
Table 5. Major Costs Considered for LMNPs Process.
| input | value (2019) | unit | reference |
|---|---|---|---|
| Kraft lignin | 250 | USD/t | Culbertson (2017)53 |
| lignosulfonate | 300 | USD/t | Holladay et. al (2007);7 Miller et. al (2016);34 industry contacts |
| dimethylformamide (DMF) | 1530 | USD/t | ICIS (2000)54 |
| ammonium hydroxide (14 wt %) | 72.7 | USD/t | Fertecon56 and AirGas57 |
| tap water | 0.66 | USD/m3 | EPAa (2004)60 |
| natural gas | 4.5 | USD/MMBTU | FisherSolve |
| electricity | 69.5 | USD/MWh | EIAb (2016)58 |
EPA – Environmental Protection Agency, USA.
EIA – Energy Information Administration, USA.
Table 6. Financial Assumptions.
| input | value | unit | reference |
|---|---|---|---|
| project start | 2017 | assumed | |
| production year 0 (2019) | 80% | % of plant capacity | assumed |
| production year 1 (2020) | 100% | % of plant capacity | assumed |
| project life (after start-up) | 10 | years | assumed |
| % of CAPEX spent in year −2 | 50% | assumed | |
| % of CAPEX spent in year −1 | 50% | assumed | |
| depreciation schedule, straight line | 10 | years | assumed |
| working capital | 10% | % of sales next year | assumed |
| maintenance cost | 2% | % of RAVa | assumed |
| capital reinvestment | 1% | % of RAVa | assumed |
| hourly and administrative staff | 12 | employees | assumed |
| overhead costs | 3% | % of sales | assumed |
| other fixed costs (insurance, property taxes, and emissions) | 1.5% | % of RAVa | assumed |
| inflation | 1.2 | %/yr. | U.S. Treasury61 |
| taxes on EBIT | 35% | % | NREL62 |
| hurdle rate | 16 | % | Damodaran (2017)63 |
| project terminal value (at year 10) | 5 | times EBITDA | assumed |
RAV—Replacement Asset Value.
Assessment of LMNPs Market Value and Potential Applications
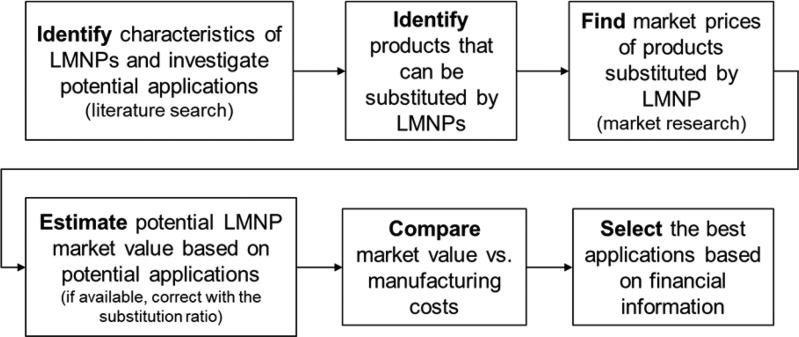
A product price may be defined by its manufacturing cost, as well as by the market, which is driven by supply and demand. Additional analyses were proposed to assess the potential market value of LMNPs. The market value of LMNP was assessed systematically (Figure 7). In brief, a literature search was carried out to identify characteristics of LMNPs and investigate potential applications. Following, a preliminary evaluation of products in current use was performed, identifying potential ones where LMNPs can be used as a substitute. Estimated manufacturing costs for LMNPs were compared to potential market prices of materials that can be substituted by LMNPs. Applications where the manufacturing costs were higher than market prices were screened out. The most promising applications were the ones with higher market prices, in addition to the ones with broad market availabilities. When available, the use of a substitution ratio is recommended (i.e., the amount of lignin needed for a given application divided by the amount of existing product used for the same application). In the absence of information, a ratio of 1:1 was considered. The information generated are proposed as a guide to screen the most promising applications, aiming commercialization.
Figure 7.
Steps for preliminary assessment of LMNPs economic potential.
Results and Discussion
Capital Investment for LMNPs Manufacturing
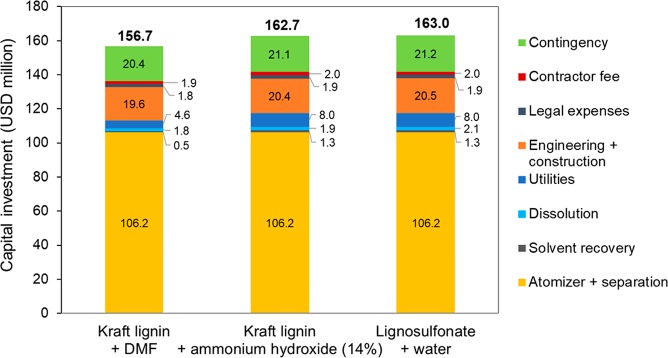
The estimated capital investment for LMNPs manufacturing is shown in Figure 8. For the three scenarios assessed, the total investment was estimated at about USD 160 million, with atomizer and separation system representing approximately 65% of the capital investment, followed by engineering and construction expenses. A similar capital investment was expected for the three scenarios, since the atomizer and separation systems costs are based on the flow rate of the liquid evaporated. The scenarios considering water-based solvents present higher solvent recovery capital investment due to the higher energy demand (the heat of vaporization of water is approximately four times that of DMF) and consequently require larger heat exchanger areas.
Figure 8.
Estimated capital investment for LMNPs manufacturing.
LMNPs Manufacturing Cost
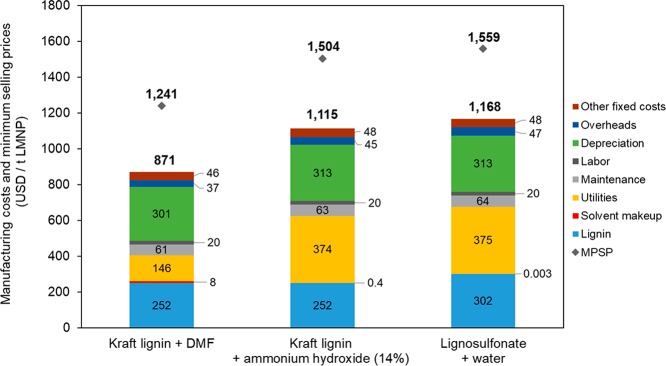
The estimated manufacturing cost for LMNPs using the aerosol technology are illustrated in Figure 9. The manufacturing costs vary between 871 and USD 1168 USD/t, depending on the scenario selected. In addition, the estimated minimum product selling prices to achieve NPV zero at 16% hurdle rates vary from 1241 to 1559 USD/t.
Figure 9.
Estimated costs for LMNPs manufacturing.
For all scenarios, major costs are related to lignin, utilities, and depreciation. The scenario where DMF was used as a solvent presented significant lower utility costs. As the heat of vaporization of DMF is approximately four times lower than water (137 kcal/kg vs 540 kcal/kg),64,65 less energy is needed to vaporize the DMF used as solvent and consequently lower costs related to natural gas are expected. However, solvent makeup costs for the scenario that uses DMF are higher due to its relatively higher cost, although solvent makeup is not a significant portion of manufacturing cost.
On the basis of the manufacturing cost information and knowledge of the process, changes in process conditions were proposed to reduce costs. For instance, a higher concentration of lignin in the solution that feeds the atomizer would allow for less solvent vaporization and recirculation in the system. Consequently, solvent makeup and natural gas consumptions can be reduced, as well as capital investment. Other aspects to be investigated are the impact of lignin costs, production rate, and solvent losses on manufacturing costs. In addition, the use of other (not yet commercial but promising) lignin/solvent combination (e.g., organosolv lignin and acetone) was preliminarily examined. Using sensitivity analysis, the impacts of these propositions were quantified and discussed in the following section.
Sensitivity Analysis
Impact of Initial Lignin Concentration on Project Financials
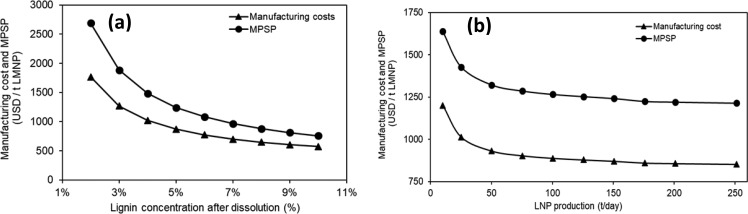
As previously discussed, lignin concentration in the precursor solution markedly affects energy and capital investment for LMNPs manufacturing. For scenario 1 (kraft lignin/DMF), the manufacturing cost significantly decreased when lignin concentration in the initial solution increased (Figure 10a). For low lignin concentrations, the difference between manufacturing cost and MPSP increased substantially due to the increase of evaporative capacity and higher capital investment. The manufacturing cost decreased by approximately 35% if lignin concentration was 10% (wt.) or more, when compared to the 5% (wt.) lignin concentration scenario. If the lignin concentration drops to 2%, then the manufacturing costs will be double of that determined for the base case scenario (5% wt.). Ultimately, the manufacturing cost of LMNPs are very sensitive to the initial lignin concentration in the precursor solution, and should be carefully evaluated for further work.
Figure 10.
(a) Impact of lignin concentration on manufacturing cost and MPSP. (b) Impact of production rate on manufacturing cost and MPSP. Both cases apply to scenario 1 (kraft lignin/DMF).
Impact of Production Rate in Project Financials
The production capacity selected for the current study considered a feasible scenario of a kraft lignin manufacturing facility colocated with a pulp mill, where the lignin is extracted from the black liquor (see Culbertson, 2017).53 Since LMNPs may be classified as a specialty material, which is added in small quantities to enhance product and material properties, the product demand may be lower than the reference scenario (150 t/day). The impact of facility scale on manufacturing cost is illustrated in Figure 10b (scenario 1 - kraft lignin/DMF). From a financial perspective, for LMNPs productions over 100 t/day, the impact of scale in manufacturing costs was minor: if the production is doubled, from 100 to 200 t/day, then the manufacturing costs and MPSP decrease by about 3.6%. However, higher production rates may imply better contracts for raw materials and energy supply, which were not accounted in this assessment and may benefit potential producers. It is worth noting that additional factors might be considered when investing in a LMNPs facility, such as the market demand and competition, which were not in the scope of this work. The most expensive equipment in the LMNPs manufacturing facility was the atomizer and separation system, which presents modular capital investment for capacities over the base case scenario (150 t/day), not extensively affected by economies of scale. If the LMNPs production rates were below 50 t/day, then the impact of economies of scale starts to take effect. For a 25 t/day production rate, for example, the estimated manufacturing costs and MPSP were nearly 15% higher than the base-case.
Impact of Lignin Cost on Manufacturing Cost and MPSP
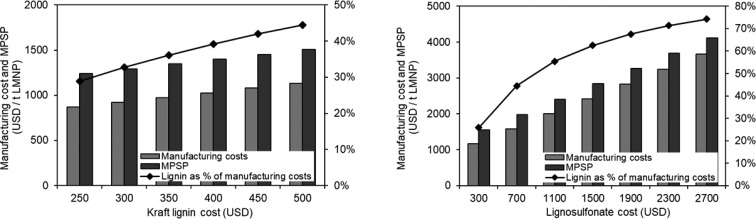
For the initial assessment, the lowest cost possible of lignin was considered for all scenarios. Nevertheless, as described previously, kraft lignin and lignosulfonate costs can vary widely. Figure 11 illustrates how lignin costs affect manufacturing cost and minimum product selling prices (MPSP) of LMNPs. For the scenarios assessed (scenario 1, kraft lignin/DMF and scenario 3, lignosulfonate/water), the range of costs considered for lignin precursor is between 250 USD/t to 500 USD/t for kraft lignin and 300 USD/t to 2700 USD/t for lignosulfonate. When the highest kraft lignin cost reported (500 USD/t) is considered, the LMNPs manufacturing cost is moderately increased by 30%, and MPSP by 20%. Lignin costs varies between 29% and 45% of manufacturing costs.
Figure 11.
Impact of lignin cost on LMNPs manufacturing cost and MPSP (left: scenario 1−kraft lignin/DMF, right: scenario 3 lignosulfonate/water).
Given the broader ranges of reported costs for lignosulfonates, it can be noticed that the MPSP can be as high as 4100 USD/t when higher-grade lignosulfonates are used. For the highest lignosulfonate cost situation, lignin accounts for 74% of manufacturing cost, versus 26% when lignosulfonate prices of 300 USD/t are considered. For an average cost of lignosulfonate (1500 USD/t), estimated LMNPs manufacturing cost is 2414 USD/t, which is about two times the manufacturing cost when lignin is 300 USD/t. As lignin costs are significant in LMNPs manufacturing, lower grades and cheaper lignin precursors are preferred from a financial perspective.
Impact of Solvent Losses in Project Financials
Solvent losses were considered minimal in the simulated scenarios (around 0.03% of recirculating flow rate). As the solvent losses during processing in industrial scale (e.g., as purge) or losses of solvent to final product have not been investigated in detail, we believe that an assessment on the impact of solvent losses in product manufacturing cost is valuable at this initial stage. Figure 12 illustrates the impact of solvent losses in manufacturing cost. The impact of solvent loss in LMNP manufacturing cost is more pronounced when DMF is used as a solvent (scenario 1). DMF prices are higher than ammonium hydroxide; consequently, increased solvent losses affect LMNP from kraft lignin and ammonium hydroxide to a lesser extent. An interesting outcome of this analysis is that either minimizing solvent losses or selecting a low cost solvent, when losses are inevitable, contribute to important reductions in LMNPs manufacturing cost.
Figure 12.
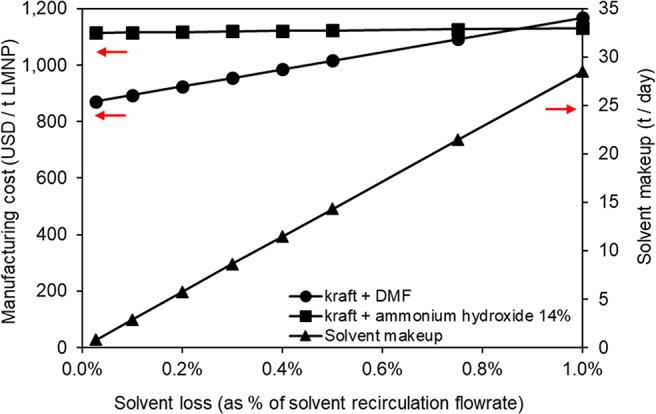
Impact of solvent losses on LMNPs manufacturing cost for kraft lignin/DMF (scenario 1) and kraft lignin/ammonium hydroxide 14% wt. (scenario 2).
Use of Acetone as a Solvent and a Biorefinery Lignin as a Feedstock
The manufacturing costs and minimum product selling prices previously calculated considered lignin precursors that are commercially available (kraft lignin and lignosulfonates). However, additional lignin precursors can be potentially used for LMNPs manufacturing. For instance, lignin can be efficiently extracted from the crude biorefinery residues with approximately 90% acetone solution in water66 and, after separation of residual cellulose-rich solids, directly sent to the atomizer without lignin isolation from the solution. This inexpensive feedstock should dramatically reduce manufacturing cost. The heat of vaporization for the acetone solution was calculated as 180 kcal/kg, using the software Aspen Plus v8.2, considering the thermodynamic model NRTL-HOC. The heat of vaporization of the acetone solution is slightly higher than the DMF value; indeed, there are no energy savings when compared with scenario 1 (kraft lignin/DMF). If, alternatively, pure acetone is used (heat of vaporization of 120 kcal/kg),67 then small cost reductions are possible. Assuming a lignin cost of 100 USD/dry t (fuel value),7,14 acetone cost of 1200 USD/t (based on ICIS and adjusted for current values),59 initial lignin concentration of 5% (wt.), and the same assumptions for previous scenarios, the calculated LMNPs manufacturing cost is 747 USD/t and the minimum selling price is 1119 USD/t (14% and 9% lower than kraft lignin/DMF scenario). Most of the savings are due to the reduced cost of lignin. The breakdown of manufacturing cost for the lignin biorefinery scenario is shown in Figure 13. As for previous scenarios, lignin feedstock, energy (natural gas and electricity), and depreciation are the major contributors to the manufacturing cost.
Figure 13.
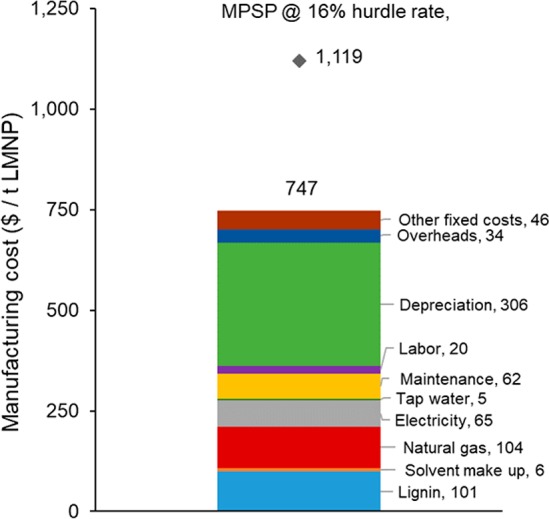
Breakdown of manufacturing cost and MPSP for biorefinery lignin and acetone as solvent.
Potential Applications of LMNPs
A preliminary assessment of LMNPs properties and potential applications was performed. Initially, the specific characteristics of LMNP and potential applications were identified by a literature search. It is worth noting the broad range of LMNPs applications, such as in the food industry, pharmaceutical, materials, and agriculture. Table 7 correlates the potential applications with distinct characteristics of lignin particles. Detailed information can be found in recently published reviews on LMNPs applications.21,68
Table 7. LMNPs Properties and Potential Applications.
| properties for specific applications | application | reference | comments |
|---|---|---|---|
| resistance to decay and biological attacks | veneer protection | (69) | use of derivatized lignin (epoxidation) |
| films for active packaging (drug delivery, tissue engineering, wound healing) | (70) | ||
| UV absorbance and antioxidant activity | cosmetics (skin care, sunscreens) | (25) | |
| UV absorbance | pesticide protection | (25) | lignin used for UV protection of microbial agents that act against insect pests |
| capsule formation | pesticide encapsulation | (71) | use of acetylated lignin to allow spheres formation |
| high stiffness | polymer reinforcement | (23) | |
| low toxicity, non-cytotoxicity, small size, capsule formation | drug-delivery | (23,71−78) | |
| stable behavior of nanoparticles | emulsion stabilizer | (79,80) | application in oil well drilling |
| pH dependent stability | Pickering emulsions for polymerization | (81) | polystyrene preparation |
| possible to electrospun | feedstock for carbon fiber production | (82) | diameter of carbon fibers between 400 nm and 1 μm |
| shape tunability, high interfacial area, UV absorbance, antioxidant effect, high stiffness, miscibility with polymer matrix, thermal stability | composite and polymer filler | (6,68,69,83) | lignin at nanoscale allows uniform distribution in composite |
| low density, non-conducting material, light color | natural rubber filler | (84) | |
| low density, reinforcing properties, large availability | phenolic foam reinforcement | (85) | |
| affinity of lignin with TNT (2,4,6-trinitrotoluene) | substrates for TNT detection | (86) |
A literature research on the materials in current use for the potential applications was performed. The purpose was to exemplify what type of materials LMNPs could substitute. As no information was available, a substitution ratio of 1:1 was considered. Note that additional assessments for the substitution of current materials are necessary, such as the LMNPs substitution ratio and the impacts on final product performance. The most promising applications were identified from a financial perspective, by comparing existing products prices and the estimated LMNPs manufacturing cost and MPSP. In addition, when available, the existing market volume for the materials is provided. A summary of the findings is illustrated in Table 8. Suggestions on potential market for LMNPs were done by comparing the lowest estimated manufacturing cost (871 USD/t) and MPSP (1241 USD/t) of LMNPs, specifically for kraft lignin and DMF as precursors, with prices of potential substitutes. In most cases, the use of spherical lignin nano- and microparticles for the given application bears a significant advantage compared to the respective (amorphous) powder. This assessment reveals that some uses of LMNPs seem unfeasible from a financial perspective (such as displacing carbon black). The relatively low price of carbon black used for these applications makes the adoption of LMNPs more difficult in these markets, if only a financial perspective is considered. Nevertheless, this scenario can change if the manufacturing costs are reduced, as illustrated by our previous sensitivity analyses. From this initial assessment, replacement of some emulsion stabilizers (gum arabic and Grinsted), UV protection products, chelating agents (such as nitriloacetic acid), nanofillers (other than carbon black), and composites reinforcements were economically attractive and therefore were identified as potential candidates for further technical investigation. These categories present substitutes with high prices, but relatively limited market sizes. Other categories of potential applications includes phenol-formaldehyde resins, foams, bactericides, other emulsion stabilizers, and other chelating applications. Some of these products represent a large market size, which makes it very attractive for lignin product commercialization. However, prices of potentially replaced products (from 1500 to 3000 USD/t) are close to the MPSP estimated for LMNPs. Therefore, further assessments should include market perspective and technical feasibility of each specific application.
Table 8. Preliminary Assessment of the Economic Potential of LMNPs.
| category | examples of material currently used | price (USD/t) | source for synthesis | volume (t/y) |
|---|---|---|---|---|
| emulsion stabilizers | gum arabic | 1500–300087 | acacia trees | 60 00088 |
| glycerol monostearate | 190089 | glycerol | ||
| Grinsted (DuPont) | 380089 | vegetable oils and algae | ||
| hydrogenated castor oil | 150090 | hydrogen + castor oil | 15 00090 | |
| cetyl alcohol | 200091 | palm oil | ||
| foams raw materials | polyols | 270092 | glycols (e.g., ethylene glycol, propylene glycols) | 7.5 million93 |
| chelating agents | ethylenediaminetetraacetic acid (EDTA) | 120058 | acetic acid + ethylene diamine | |
| nitriloacetic acid (NTA) | 10 00094 | ammonium, formaldehyde and sodium cyanide | ||
| diethylenetriaminepentaacetic acid (DTPA) | 2 50091 | |||
| UV protection | 2-ethylhexyl-4-methoxy cinnamate | 10 00091 | oil (propylene) | |
| benzophenones | 400091 | methane and benzene | ||
| phenolic benzotriazoles | 20 00091 | nitrobenzenes | 12 00095 | |
| bactericide & carrier | silver nanoparticle | 1 500 00091 | silver | |
| titanium dioxide (TiO2) | 1500–250096 | mineral | 14 million97 | |
| zinc oxide (ZnO) | 1500–200091 | mineral | 1.4 million98 | |
| carbon filler | carbon nanotubes | 10 000 00099 | carbon dioxide, acetylene | few hundreds99 |
| graphene | 200 000100 | carbon | ||
| carbon black | 500–700101 | petroleum | 12 million101 | |
| raw material for phenol-formaldehyde resins | PF resin | 1200102 | petroleum | 2.5 million (phenol used to resin production)103 |
| reinforcement for composites | glass fiber | 800–250091 | mineral | 4.5 million105 |
| aramid | 26 000104 | petroleum | 78 000106 |
Conclusions and Future Prospects
While the Life Cycle Assessment was not the focus of this work, converting lignocellulosic streams has been shown to be quite attractive. Particularly, valorization of lignin has been identified as a key factor to reach environmental benefits.53 With this in mind, the present study dealt with a techno-economic analysis and market assessment of LMNPs. Preliminary manufacturing costs using different types of lignins (kraft lignin and lignosulfonates) and solvents (DMF, water, and ammonium hydroxide) were estimated between 870 and 1170 USD/t, depending on raw materials used and processing conditions. Major cost drivers for LMNPs manufacturing include lignin, utilities, and depreciation. Estimated manufacturing costs can be as low as 600 USD/t if higher lignin concentration in the solution feed is used. Sensitivity assessment showed that if higher grades of lignin are utilized, the manufacturing cost can increase considerably, reaching 1100 USD/t (for kraft/DMF scenario) and 3660 USD/t (for lignosulfonate/water scenario).
The current work shows a potential feasibility for the applications of LMNPs in replacement of high value products, such as some emulsion stabilizers, UV protection products, chelating agents and polymer nanofillers. Additionally, there is the possibility of using LMNPs in applications targeting large size markets, such as composites and formaldehyde resins. Special mention is the case of particulate coatings, which can be quite promising given the excellent packing of LMNPs in coating layers, as demonstrated recently.81 In all cases, the utilization of LMNPs as biobased component is beneficial from the point of view of the inherent properties of spherical micro and nanoparticles, which are different than those in other forms as well as the fact that they are environmentally benign. Observing the difference between the estimated LMNPs minimum product selling prices and the market price of large-scale lignin substitutes, it is important to develop manufacturing pathways to reduce LMNPs manufacturing cost. Additionally, it is suggested a better understanding of the substitution ratios for specific applications, as well as the impact of LMNPs use in final product properties and performance. On the basis of the financial evaluation herein discussed, important elements contributing to LMNPs production costs are (i) the cost of the lignin feedstock, (ii) the energy required to evaporate the solvent, (iii) the lignin concentration in the solvent, and, likely, (iv) the solvent price. Thus, it is suggested to target future research on:
The technical feasibility of using potentially lower cost feedstock, such as lower grade industrial lignins and crude biorefinery lignins.
The use of low cost solvents with low heat of vaporization, such as aqueous acetone and ethanol, which are well suitable for dissolution of some grades of lignin.
The effect of higher concentration of lignin starting solution on LMNPs properties and application performance.
The reduction of solvent losses and improvement of solvent recovery, especially on studies aiming the scale-up of LMNPs manufacturing.
We believe the outcomes of this study are relevant for LMNPs industrialization and fast-track commercialization.
Acknowledgments
The authors acknowledge funding support by NordForsk Project 82214 “High-Value Products from Lignin” as well as the European Research Commission for the ERC Advanced Grant (O.J.R.). The authors are also thankful to the Academy of Finland through its Centers of Excellence Program (2014–2019) under Project 264677 “Molecular Engineering of Biosynthetic Hybrid Materials Research” (HYBER).
The authors declare no competing financial interest.
Figures 11−13 were renumbered in the version published ASAP August 2, 2018. The corrected version was published ASAP on August 6, 2018.
References
- Wurm F. R.; Weiss C. K. Nanoparticles from Renewable Polymers. Front. Chem. 2014, 2 (July), 1–13. 10.3389/fchem.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W.; Ralph J.; Baucher M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- Ragauskas A. J.; Beckham G. T.; Biddy M. J.; Chandra R.; Chen F.; Davis M. F.; Davison B. H.; Dixon R. A.; Gilna P.; Keller M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science (Washington, DC, U. S.) 2014, 344, 1246843. 10.1126/science.1246843. [DOI] [PubMed] [Google Scholar]
- Welker C. M.; Balasubramanian V. K.; Petti C.; Rai K. M.; De Bolt S.; Mendu V. Engineering Plant Biomass Lignin Content and Composition for Biofuels and Bioproducts. Energies 2015, 8, 7654–7676. 10.3390/en8087654. [DOI] [Google Scholar]
- Balakshin M. Y.; Capanema E. A.; Chang H.-m.. Recent Advances in Isolation and Analysis of Lignins and Lignin-Carbohydrate Complexes. In Characterization of Lignocellulosic Materials; Hu T. Q., Ed.; Blackwell Publishing Ltd: Oxford, U.K., 2008; pp 148–170. [Google Scholar]
- Ago M.; Tardy B. L.; Wang L.; Guo J.; Khakalo A.; Rojas O. J. Supramolecular Assemblies of Lignin into Nano- and Microparticles. MRS Bull. 2017, 42 (05), 371–378. 10.1557/mrs.2017.88. [DOI] [Google Scholar]
- Holladay J. E.; White J. F.; Bozell J. J.; Johnson D. Top Value-Added Chemicals from Biomass—Vol. II—Results of Screening for Potential Candidates from Biorefinery Lignin, U.S. Department of Energy, 2007.
- Mohan D.; Pittman C. U.; Steele P. H. Single, Binary and Multi-Component Adsorption of Copper and Cadmium from Aqueous Solutions on Kraft Lignin - a Biosorbent. J. Colloid Interface Sci. 2006, 297, 489–504. 10.1016/j.jcis.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Sena-Martins G.; Almeida-Vara E.; Duarte J. C. Eco-Friendly New Products from Enzymatically Modified Industrial Lignins. Ind. Crops Prod. 2008, 27, 189–195. 10.1016/j.indcrop.2007.07.016. [DOI] [Google Scholar]
- Boeriu C. G.; Bravo D.; Gosselink R. J. A.; Van Dam J. E. G. Characterisation of Structure-Dependent Functional Properties of Lignin with Infrared Spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. 10.1016/j.indcrop.2004.04.022. [DOI] [Google Scholar]
- Yang D.; Qiu X.; Zhou M.; Lou H. Properties of Sodium Lignosulfonate as Dispersant of Coal Water Slurry. Energy Convers. Manage. 2007, 48, 2433–2438. 10.1016/j.enconman.2007.04.007. [DOI] [Google Scholar]
- Toledano A.; García A.; Mondragon I.; Labidi J. Lignin Separation and Fractionation by Ultrafiltration. Sep. Purif. Technol. 2010, 71 (1), 38–43. 10.1016/j.seppur.2009.10.024. [DOI] [Google Scholar]
- Doherty W. O. S.; Mousavioun P.; Fellows C. M. Value-Adding to Cellulosic Ethanol: Lignin Polymers. Ind. Crops Prod. 2011, 33 (2), 259–276. 10.1016/j.indcrop.2010.10.022. [DOI] [Google Scholar]
- Berlin A.; Balakshin M. Y.. Industrial Lignins: Analysis, Properties, and Applications. In Bioenergy Research: Advances and Applications; Gupta V. K., Kubicek C. P., Saddler J., Xu F., Tuohy M., Eds.; Elsevier: Amsterdam, Nederlands, 2014; pp 315–336. [Google Scholar]
- Capanema E. A.; Balakshin M. Y.; Chen C. L.; Gratzl J. S.; Kirkman A. G. Oxidative Ammonolysis of Technical Lignins Part 1. Kinectics of the Reaction under Isothermal Condition at 130°C. Holzforschung 2001, 55, 397–404. 10.1515/HF.2001.066. [DOI] [Google Scholar]
- Hu S.; Zhang S.; Pan N.; Hsieh Y. High Energy Density Supercapacitors from Lignin Derived Submicron Activated Carbon Fibers in Aqueous Electrolytes. J. Power Sources 2014, 270, 106–112. 10.1016/j.jpowsour.2014.07.063. [DOI] [Google Scholar]
- Ago M.; Borghei M.; Haataja J. S.; Rojas O. J. Mesoporous Carbon Soft-Templated from Lignin Nanofiber Networks: Microphase Separation Boosts Supercapacitance in Conductive Electrodes. RSC Adv. 2016, 6, 85802–85810. 10.1039/C6RA17536H. [DOI] [Google Scholar]
- Zhao W.; Simmons B.; Singh S.; Ragauskas A.; Cheng G. From Lignin Association to Nano-/Micro-Particle Preparation: Extracting Higher Value of Lignin. Green Chem. 2016, 18 (21), 5693–5700. 10.1039/C6GC01813K. [DOI] [Google Scholar]
- Balakshin M. Y.; Capanema E. A. Comprehensive Structural Analysis of Biorefinery Lignins with a Quantitative 13C NMR Approach. RSC Adv. 2015, 5, 87187–87199. 10.1039/C5RA16649G. [DOI] [Google Scholar]
- Donohoe B. S.; Decker S. R.; Tucker M. P.; Himmel M. E.; Vinzant T. B. Visualizing Lignin Coalescence and Migration through Maize Cell Walls Following Thermochemical Pretreatment. Biotechnol. Bioeng. 2008, 101 (5), 913–925. 10.1002/bit.21959. [DOI] [PubMed] [Google Scholar]
- Beisl S.; Friedl A.; Miltner A. Lignin from Micro- To Nanosize: Applications. Int. J. Mol. Sci. 2017, 18 (11), 2367. 10.3390/ijms18112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark W. J.; Stoessel P. R.; Wohlleben W.; Hafner A. Industrial Applications of Nanoparticles. Chem. Soc. Rev. 2015, 44 (16), 5793–5805. 10.1039/C4CS00362D. [DOI] [PubMed] [Google Scholar]
- Beisl S.; Miltner A.; Friedl A. Lignin from Micro- to Nanosize: Production Methods. Int. J. Mol. Sci. 2017, 18 (6), 1244. 10.3390/ijms18061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A. P.; Brown J. S.; Bharti B.; Wang A.; Gangwal S.; Houck K.; Cohen Hubal E. A.; Paunov V. N.; Stoyanov S. D.; Velev O. D. An Environmentally Benign Antimicrobial Nanoparticle Based on a Silver-Infused Lignin Core. Nat. Nanotechnol. 2015, 10 (9), 817–823. 10.1038/nnano.2015.141. [DOI] [PubMed] [Google Scholar]
- Yearla S. R.; Padmasree K. Preparation and Characterisation of Lignin Nanoparticles: Evaluation of Their Potential as Antioxidants and UV Protectants. J. Exp. Nanosci. 2016, 11 (4), 289–302. 10.1080/17458080.2015.1055842. [DOI] [Google Scholar]
- Vanholme R.; Demedts B.; Morreel K.; Ralph J.; Boerjan W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153 (3), 895–905. 10.1104/pp.110.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao J. P.; Geckeler K. E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36 (7), 887–913. 10.1016/j.progpolymsci.2011.01.001. [DOI] [Google Scholar]
- Ago M.; Huan S.; Borghei M.; Raula J.; Kauppinen E. I.; Rojas O. J. High-Throughput Synthesis of Lignin Particles (∼30 nm to ∼ 2 μm) via Aerosol Flow Reactor: Size Fractionation and Utilization in Pickering Emulsions. ACS Appl. Mater. Interfaces 2016, 8 (35), 23302–23310. 10.1021/acsami.6b07900. [DOI] [PubMed] [Google Scholar]
- Lievonen M.; Valle-Delgado J. J.; Mattinen M.-L.; Hult E.-L.; Lintinen K.; Kostiainen M. A.; Paananen A.; Szilvay G. R.; Setälä H.; Österberg M. A Simple Process for Lignin Nanoparticle Preparation. Green Chem. 2016, 18 (5), 1416–1422. 10.1039/C5GC01436K. [DOI] [Google Scholar]
- Duval A.; Lawoko M. A Review on Lignin-Based Polymeric, Micro- and Nano-Structured Materials. React. Funct. Polym. 2014, 85, 78–96. 10.1016/j.reactfunctpolym.2014.09.017. [DOI] [Google Scholar]
- Chakar F. S.; Ragauskas A. J. Review of Current and Future Softwood Kraft Lignin Process Chemistry. Ind. Crops Prod. 2004, 20 (2), 131–141. 10.1016/j.indcrop.2004.04.016. [DOI] [Google Scholar]
- Lake M.; Scouten C.. What Are We Going to Do with All This Lignin? In Frontiers in BioRefining Conference; St. Simons Island, GA, 2014.
- Gosselink R. J. A.Lignin as a Renewable Aromatic Resource for the Chemical Industry, Ph.D. Thesis, Wageningen University: Wageningen, Netherlands, 2011. [Google Scholar]
- Miller J.; Faleiros M.; Pilla L.; Bodart A. C.. Lignin: Technology, Applications and Markets; RISI, Inc.: Bedford, MA, 2016. [Google Scholar]
- Frangville C.; Rutkevičius M.; Richter A. P.; Velev O. D.; Stoyanov S. D.; Paunov V. N. Fabrication of Environmentally Biodegradable Lignin Nanoparticles. ChemPhysChem 2012, 13 (18), 4235–4243. 10.1002/cphc.201200537. [DOI] [PubMed] [Google Scholar]
- Nair S. S.; Sharma S.; Pu Y.; Sun Q.; Pan S.; Zhu J. Y.; Deng Y.; Ragauskas A. J. High Shear Homogenization of Lignin to Nanolignin and Thermal Stability of Nanolignin-Polyvinyl Alcohol Blends. ChemSusChem 2014, 7 (12), 3513–3520. 10.1002/cssc.201402314. [DOI] [PubMed] [Google Scholar]
- Gilca I. A.; Popa V. I.; Crestini C. Obtaining Lignin Nanoparticles by Sonication. Ultrason. Sonochem. 2015, 23, 369–375. 10.1016/j.ultsonch.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Spender J.; Demers A. L.; Xie X.; Cline A. E.; Earle M. A.; Ellis L. D.; Neivandt D. J. Method for Production of Polymer and Carbon Nanofibers from Water-Soluble Polymers. Nano Lett. 2012, 12 (7), 3857–3860. 10.1021/nl301983d. [DOI] [PubMed] [Google Scholar]
- Caicedo H. M.; Dempere L. A.; Vermerris W. Template-Mediated Synthesis and Bio-Functionalization of Flexible Lignin-Based Nanotubes and Nanowires. Nanotechnology 2012, 23 (10), 105605. 10.1088/0957-4484/23/10/105605. [DOI] [PubMed] [Google Scholar]
- Ten E.; Ling C.; Wang Y.; Srivastava A.; Dempere L. A.; Vermerris W. Lignin Nanotubes as Vehicles for Gene Delivery into Human Cells. Biomacromolecules 2014, 15, 327–338. 10.1021/bm401555p. [DOI] [PubMed] [Google Scholar]
- Dallmeyer I.; Ko F.; Kadla J. F. Electrospinning of Technical Lignins for the Production of Fibrous Networks. J. Wood Chem. Technol. 2010, 30 (4), 315–329. 10.1080/02773813.2010.527782. [DOI] [Google Scholar]
- Lu Q.; Zhu M.; Zu Y.; Liu W.; Yang L.; Zhang Y.; Zhao X.; Zhang X.; Zhang X.; Li W. Comparative Antioxidant Activity of Nanoscale Lignin Prepared by a Supercritical Antisolvent (SAS) Process with Non-Nanoscale Lignin. Food Chem. 2012, 135 (1), 63–67. 10.1016/j.foodchem.2012.04.070. [DOI] [Google Scholar]
- Leskinen T.; Smyth M.; Xiao Y.; Lintinen K.; Mattinen M.; Kostiainen M. A.; Oinas P.; Österberg M. Scaling Up Production of Colloidal Lignin Particles. Nord. Pulp Pap. Res. J. 2017, 32 (4), 586–596. 10.3183/NPPRJ-2017-32-04-p586-596. [DOI] [Google Scholar]
- Kämäräinen T.; Ago M.; Seitsonen J.; Raula J.; Kauppinen E. I.; Ruokolainen J.; Rojas O. J. Harmonic Analysis of Surface Instability Patterns on Colloidal Particles. Soft Matter 2018, 14, 3387–3396. 10.1039/C8SM00383A. [DOI] [PubMed] [Google Scholar]
- Nypelö T. E.; Carrillo C. A.; Rojas O. J. Lignin Supracolloids Synthesized from (W/O) Microemulsions: Use in the Interfacial Stabilization of Pickering Systems and Organic Carriers for Silver Metal. Soft Matter 2015, 11, 2046–2054. 10.1039/C4SM02851A. [DOI] [PubMed] [Google Scholar]
- Peters M. S.; Timmerhaus K. D.; West R. E. Cost Estimator Tool http://www.mhhe.com/engcs/chemical/peters/data/ce.html (accessed Mar 5, 2018).
- Matches’ Process Equipment Cost Estimates http://www.matche.com/equipcost/Default.html (accessed Mar 5, 2018).
- Peters M. S.; Timmerhaus K. D.. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw-Hill, 2003. [Google Scholar]
- de Assis C. A.; Houtman C.; Phillips R.; Bilek E.M. T.; Rojas O. J.; Pal L.; Peresin M. S.; Jameel H.; Gonzalez R. Conversion Economics of Forest Biomaterials: Risk and Financial Analysis of CNC Manufacturing. Biofuels, Bioprod. Biorefin. 2017, 11 (4), 682–700. 10.1002/bbb.1782. [DOI] [Google Scholar]
- de Assis C. A.; Iglesias M. C.; Bilodeau M.; Johnson D.; Phillips R. B.; Peresin M. S.; Bilek E. M. T.; Rojas O. J.; Venditti R. A.; Gonzalez R. Cellulose Micro- and Nanofibrils (CMNF) Manufacturing - Financial and Risk Assessment. Biofuels, Bioprod. Biorefin. 2018, 12 (2), 251–264. 10.1002/bbb.1835. [DOI] [Google Scholar]
- de Assis C. A.; Gonzalez R.; Kelley S.; Jameel H.; Bilek T.; Daystar J.; Handfield R.; Golden J.; Prestemon J.; Singh D. Risk Management Consideration in the Bioeconomy. Biofuels, Bioprod. Biorefin. 2017, 11, 549–566. 10.1002/bbb.1765. [DOI] [Google Scholar]
- U.S. EPA. Organic Liquid Storage Tanks. In AP 42, Fifth Edition Compilation of Air Pollutant Emission Factors; Raleigh, NC, 2006; p 7.1–1-7.1–123.
- Culbertson C. G., Jr.Commercialization of Sustainable Bio-Refinery Projects for the Pulp & Paper Industry, Ph.D. Dissertation, North Carolina State University, 2017. [Google Scholar]
- ICIS. Chemical Prices – D https://www.icis.com/resources/news/2000/07/03/117434/chemical-prices-d/ (accessed May 10, 2018).
- Bureau of Labor Statistics. Producer price index for crude petroleum and natural gas extraction—primary products https://beta.bls.gov/dataViewer/view/timeseries/PCU211111211111P (accessed Mar 21, 2018).
- FERTECON. Weekly review of the ammonia market https://agribusinessintelligence.informa.com/products-and-services/data-and-analysis/fertecon (accessed Mar 21, 2018).
- Airgas. Technical Bulletin - Aqua ammonia - Delivery & Storage Information http://airgasspecialtyproducts.com/wp-content/uploads/2016/02/Appendix_D_-_Delivery_and_Storage_Information.pdf (accessed Mar 21, 2018).
- EIA. Electric Power Monthly: With Data for August 2016; Washington, D.C., 2016.
- ICIS. Indicative Chemical Prices A–Z http://www.icis.com/chemicals/channel-info-chemicals-a-z/ (accessed Nov 28, 2016).
- Environmental Protection Agency. Drinking Water Costs & Federal Funding https://nepis.epa.gov/Exe/ZyPDF.cgi/300065YY.PDF?Dockey=300065YY.PDF (accessed Apr 3, 2017).
- U.S. Treasury. U. S. Economic Statistics - Monthly Data (2012–2015) https://www.treasury.gov/resource-center/data-chart-center/monitoring-the-economy/Documents/monthly ECONOMIC DATA TABLES.pdf (accessed Oct 28, 2016).
- Humbird D.; Davis R.; Tao L.; Kinchin C.; Hsu D.; Aden A.; Schoen P.; Lukas J.; Olthof B.; Worley M.. et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol. Technical Report NREL/TP-5100–47764; Golden, CO, 2011; p 147.
- Damodaran, A. Cost of Capital by Industry Sector http://people.stern.nyu.edu/adamodar/New_Home_Page/datafile/wacc.html (accessed Jan 30, 2017).
- NIST Standard Reference Database. Heat of vaporization - dimethylformamide https://webbook.nist.gov/cgi/cbook.cgi?ID=C68122&Mask=4, (accessed May 7, 2018).
- National Center for Biotechnology Information - PubChem Compound Database. Heat of vaporization—water https://pubchem.ncbi.nlm.nih.gov/compound/962 (accessed May 7, 2018).
- Balakshin M.; Capanema E.. Rethinking Biorefinery Lignins: Breaking Dogmas. In 14th European Workshop on Lignocellulosics and Pulp; Autrans, France, 2016; pp 63–66.
- NIST Standard Reference Database. Heat of vaporization - acetone https://webbook.nist.gov/cgi/cbook.cgi?ID=C67641&Mask=4, (accessed May 7, 2018).
- Fortunati E.; Yang W.; Luzi F.; Kenny J.; Torre L.; Puglia D. Lignocellulosic Nanostructures as Reinforcement in Extruded and Solvent Casted Polymeric Nanocomposites: An Overview. Eur. Polym. J. 2016, 80, 295–316. 10.1016/j.eurpolymj.2016.04.013. [DOI] [Google Scholar]
- Gîlcă I.-A.; Popa V. I. Study on Biocidal Properties of Some Nanoparticles Based on Epoxy Lignin. Cellul. Chem. Technol. 2013, 47 (3–4), 239–245. [Google Scholar]
- Yang W.; Owczarek J. S.; Fortunati E.; Kozanecki M.; Mazzaglia A.; Balestra G. M.; Kenny J. M.; Torre L.; Puglia D. Antioxidant and Antibacterial Lignin Nanoparticles in Polyvinyl Alcohol/Chitosan Films for Active Packaging. Ind. Crops Prod. 2016, 94, 800–811. 10.1016/j.indcrop.2016.09.061. [DOI] [Google Scholar]
- Qian Y.; Deng Y.; Qiu X.; Li H.; Yang D. Formation of Uniform Colloidal Spheres Based on Lignosulfonate, a Renewable Biomass Resource Recovered from Pulping Spent Liquor. Green Chem. 2014, 16, 2156–2163. 10.1039/c3gc42131g. [DOI] [Google Scholar]
- Tortora M.; Cavalieri F.; Mosesso P.; Ciaffardini F.; Melone F.; Crestini C. Ultrasound Driven Assembly of Lignin into Microcapsules for Storage and Delivery of Hydrophobic Molecules. Biomacromolecules 2014, 15 (5), 1634–1643. 10.1021/bm500015j. [DOI] [PubMed] [Google Scholar]
- Xiong F.; Han Y.; Wang S.; Li G.; Qin T.; Chen Y.; Chu F. Preparation and Formation Mechanism of Renewable Lignin Hollow Nanospheres with a Single Hole by Self-Assembly. ACS Sustainable Chem. Eng. 2017, 5 (3), 2273–2281. 10.1021/acssuschemeng.6b02585. [DOI] [Google Scholar]
- Li H.; Deng Y.; Wu H.; Ren Y.; Qiu X.; Zheng D.; Li C. Self-Assembly of Kraft Lignin into Nanospheres in Dioxane-Water Mixtures. Holzforschung 2016, 70 (8), 725–731. 10.1515/hf-2015-0238. [DOI] [Google Scholar]
- Li H.; Deng Y.; Liang J.; Dai Y.; Liu B.; Ren Y.; Qiu X.; Li C. Direct Preparation of Hollow Nanospheres with Kraft Lignin: A Facile Strategy for Effective Utilization of Biomass Waste. BioResources 2016, 11 (2), 3073–3083. 10.15376/biores.11.2.3073-3083. [DOI] [Google Scholar]
- Byrappa K.; Ohara S.; Adschiri T. Nanoparticles Synthesis Using Supercritical Fluid Technology - towards Biomedical Applications. Adv. Drug Delivery Rev. 2008, 60 (3), 299–327. 10.1016/j.addr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Loscertales I. G.; Barrero A.; Guerrero I.; Cortijo R.; Marquez M.; Ganan-Calvo A. M. Micro/Nano Encapsulation via Electrified Coaxial Liquid Jets. Science (Washington, DC, U. S.) 2002, 295 (5560), 1695–1698. 10.1126/science.1067595. [DOI] [PubMed] [Google Scholar]
- Díaz J. E.; Barrero A.; Márquez M.; Loscertales I. G. Controlled Encapsulation of Hydrophobic Liquids in Hydrophilic Polymer Nanofibers by Co-Electrospinning. Adv. Funct. Mater. 2006, 16, 2110–2116. 10.1002/adfm.200600204. [DOI] [Google Scholar]
- Richter A. P.; Bharti B.; Armstrong H. B.; Brown J. S.; Plemmons D.; Paunov V. N.; Stoyanov S. D.; Velev O. D. Synthesis and Characterization of Biodegradable Lignin Nanoparticles with Tunable Surface Properties. Langmuir 2016, 32 (25), 6468–6477. 10.1021/acs.langmuir.6b01088. [DOI] [PubMed] [Google Scholar]
- Browning W. C. Lignosulfonate Stabilized Emulsions in Oil Well Drilling Fluids. JPT, J. Pet. Technol. 1955, 7 (6), 9–15. 10.2118/393-G. [DOI] [Google Scholar]
- Wei Z.; Yang Y.; Yang R.; Wang C. Alkaline Lignin Extracted from Furfural Residues for PH-Responsive Pickering Emulsions and Their Recyclable Polymerization. Green Chem. 2012, 14 (11), 3230–3236. 10.1039/c2gc36278c. [DOI] [Google Scholar]
- Ruiz-Rosas R.; Bedia J.; Lallave M.; Loscertales I. G.; Barrero A.; Rodríguez-Mirasol J.; Cordero T. The Production of Submicron Diameter Carbon Fibers by the Electrospinning of Lignin. Carbon 2010, 48 (3), 696–705. 10.1016/j.carbon.2009.10.014. [DOI] [Google Scholar]
- Chen F.; Liu W.; Seyed Shahabadi S. I.; Xu J.; Lu X. Sheet-Like Lignin Particles as Multifunctional Fillers in Polypropylene. ACS Sustainable Chem. Eng. 2016, 4 (9), 4997–5004. 10.1021/acssuschemeng.6b01369. [DOI] [Google Scholar]
- Jiang C.; He H.; Jiang H.; Ma L.; Jia D. M. Nano-Lignin Filled Natural Rubber Composites: Preparation and Characterization. eXPRESS Polym. Lett. 2013, 7 (5), 480–493. 10.3144/expresspolymlett.2013.44. [DOI] [Google Scholar]
- Del Saz-Orozco B.; Oliet M.; Alonso M. V.; Rojo E.; Rodríguez F. Formulation Optimization of Unreinforced and Lignin Nanoparticle-Reinforced Phenolic Foams Using an Analysis of Variance Approach. Compos. Sci. Technol. 2012, 72 (6), 667–674. 10.1016/j.compscitech.2012.01.013. [DOI] [Google Scholar]
- Mahmoud K. A.; Zourob M. Fe3O4/Au Nanoparticles/Lignin Modified Microspheres as Effectual Surface Enhanced Raman Scattering (SERS) Substrates for Highly Selective and Sensitive Detection of 2,4,6-Trinitrotoluene (TNT). Analyst 2013, 138 (9), 2712–2719. 10.1039/c3an00261f. [DOI] [PubMed] [Google Scholar]
- Centre for the Promotion of Imports—Netherlands. Exporting gum arabic to Europe https://www.cbi.eu/market-information/natural-food-additives/gum-arabic/ (accessed May 10, 2018).
- Sudan’s manna from heaven and strategic weapon http://www.sudantribune.com/Sudan-s-manna-from-heaven-and,27807 (accessed May 10, 2018).
- Zauba Technologies & Data Services. Zauba platform—importat and export www.zauba.com (accessed May 10, 2018).
- Santhanam N.; Balu M.; Sreevatsan S.. Production and Uses of Key Castor Oil Oleochemicals; White paper; Chennai, India, 2015. [Google Scholar]
- Alibaba webpage www.alibaba.com (accessed May 10, 2018).
- ICIS News. US flexible polyurethane foam prices set to rise in May https://www.icis.com/resources/news/2010/04/13/9350500/us-flexible-polyurethane-foam-prices-set-to-rise-in-may/ (accessed May 10, 2018).
- PR Newswire. Global Polyols and Polyurethane Markets are Expected to Reach USD 22.6 Billion and USD 66.4 Billion Respectively by 2018: Transparency Market Research http://www.prnewswire.com/news-releases/global-polyols-and-polyurethane-markets-are-expected-to-reach-usd-226-billion-and-usd-664-billion-respectively-by-2018-transparency-market-research-207681271.html (accessed May 10, 2018).
- Lookchem webpage www.lookchem.com (accessed May 10, 2018).
- National Toxicology Program. Chemical Information Review Document for Phenolic Benzotriazoles; Research Triangle Park, NC, 2011. [Google Scholar]
- Tan L. Asia buyers may switch to non-China TiO2 after price surge https://www.icis.com/press-releases/focus-story-tio2-price-surge/ (accessed May 10, 2018).
- Market Research Store. Global Titanium Dioxide Market Set for Rapid Growth, To Reach Around USD 17.00 Billion by 2020 http://www.marketresearchstore.com/news/global-titanium-dioxide-market-156 (accessed May 10, 2018).
- PR Newswire. Global Zinc Oxide Market Trends and Forecasts to 2020 https://www.prnewswire.com/news-releases/global-zinc-oxide-market-trends-and-forecasts-to-2020-300142173.html (accessed May 10, 2018).
- Smajda R.; Mionic M.; Duchamp M.; Andresen J. C.; Forró L.; Magrez A. Production of High Quality Carbon Nanotubes for Less than $1 per Gram. Phys. Status Solidi C 2010, 7 (3–4), 1236–1240. 10.1002/pssc.200982972. [DOI] [Google Scholar]
- Wolf E. L.Practical Productions of Graphene, Supply and Cost. In Applications of Graphene - An Overview; Wolf E. L., Ed.; Springer: New York, 2014; pp 19–38. [Google Scholar]
- Ita P.Carbon Black Global Outlook—The Year in Carbon Black 2017. In Carbon Black 2017—Perspectives in Asia Pacific; Chennai, India, 2017.
- Intratec. Phenol Price History & Forecast https://www.intratec.us/chemical-markets/phenol-price (accessed May 10, 2018).
- Stewart D. Lignin as a base material for materials applications: Chemistry, application and economics.. Ind Crops and Prod. 2008, 27, 202–207. [Google Scholar]
- Maleniak R. F. Aramid Fibers https://www.chem.uwec.edu/chem405_s01/malenirf/project.html 9/9 (accessed May 15, 2018).
- Mazumdar S.; Karthikeyan D.; Pichler D.; Benevento M.; Frassine R. State of the Composites Industry Report for 2017 http://compositesmanufacturingmagazine.com/2017/01/composites-industry-report-2017/2/ (accessed May 15, 2018).
- Grandview Research. Aramid Fiber Market Projected To Reach $ 6. 51 Billion By 2024 https://www.grandviewresearch.com/press-release/global-aramid-fiber-market (accessed May 15, 2018).