Abstract
An efficient Rh(III)-catalyzed dehydrative C−H allylation of indoles with allyl alcohols via β-hydroxide elimination under oxidant-free conditions has been developed. This method features very mild reaction conditions, excellent regioselectivity and stereoselectivity, and compatibility with various functional groups. In addition, the directing group can be removed under mild reaction conditions, which further underscores the synthetic utility of this method.

An efficient Rh(III)-catalyzed direct dehydrative C−H allylation of indoles with allyl alcohols under oxidant-free conditions has been developed, which favors an uncommon β-hydroxide elimination pathway rather than a β-hydride elimination pathway.
Introduction
Due to the versatility of olefin transformations, the allylation of (hetero)arenes is important in organic synthesis.1 Arguably, transition metal-catalyzed direct C−H allylation reactions with various allylation reagents represent a powerful strategy for atom- and step-economic syntheses.2 In recent years, direct C−H allylation reactions with preactivated allyl alcohol derivatives via β-oxygen elimination have been explored by utilizing various transition metals, such as Rh, Ru, Co, and Mn.3–5 Among them, Cp*Rh(III)-catalyzed allylation reactions with allyl acetates, allyl carbonates, and allyl halides were reported by Glorius, Loh, Li, Wang, and other groups (Scheme 1, eq 1).3 The use of unactivated allyl alcohols as the coupling partners was reported to result in ketones or aldehydes under rhodium catalysis (Scheme 1, eq 2),6 probably due to the preference for the β-hydride over β-hydroxide elimination. Kanai and Matsunaga demonstrated that allyl alcohols could be used directly for C−H allylation under Cp*Co(III) catalysis, which proceeded via the β-hydroxide elimination pathway (Scheme 1, eq 3).7 Sundararaju and co-workers compared the behavior of Cp*Co(III) for β-hydroxide elimination and Cp*Rh(III) for β-hydride elimination in one reaction system.6e Recently, Kapur and Ji reported Ru(II)-catalyzed allylation of indoles with allyl alcohols, respectively.4e,4g To the best of our knowledge, the Rh(III)-catalyzed C-H allylation with allyl alcohols via β-hydroxide elimination remains unexplored. Therefore, the development of the reaction that allows Cp*Rh(III) to undergo direct C-H allylation with allyl alcohols through an uncommon β-hydroxide elimination pathway is highly desirable. In our design and synthesis of β-catenin/T-cell factor (Tcf) protein-protein interaction (PPI) inhibitors, we need to search for new reactions to functionalize position 2 of the indole ring.8 Herein, we disclose the first example of an efficient Cp*Rh(III)-catalyzed C−H allylation of indoles with unactivated allyl alcohols at room temperature (Scheme 1, eq 4). This approach features good yields for most substrates, excellent regio- and stereoselectivity. Further, the reaction can easily be scaled up, and the directing group can be removed under mild conditions.
Scheme 1.
Transition metal-catalyzed C−H allylation reactions
Results and discussion
We initiated our investigation by choosing the coupling reaction of N-substituted indole (1) with 1-phenylprop-2-en-1-ol (2a) under Cp*Rh(III) catalysis as the model reaction to optimize the reaction conditions. First, various directing groups were screened; we found that the reaction did not proceed when using common directing groups 1a-1–1a-4 (Table 1, entries 1–4). By contrast, the reaction offered the desired product in 78% yield with excellent regio- and stereoselectivity when using ethoxycarbamoyl as the directing group (entry 5). Then, we investigated the effects of solvents, and the results showed that the yields of product were decreased when the reaction was conducted in methanol, dioxane, dichloromethane, and toluene (entries 6–9). Subsequently, the investigation of various bases indicated that KOAc, Na2CO3, and CsOAc led to lower efficiency (entries 10–12). The reaction did not afford the desired product without the catalyst [Cp*RhCl2]2 or the base NaOAc (entry 13 and 14). Different from rhodium catalysis (entry 5), the previously reported reaction using [RuCl2(p-cymene)]2 as the catalyst4g only offered 3a with the yield of 36% at room temperature (entry 15). For the reaction conditions that either did not work or gave low yields of he desired product in Table 1, we only observed unreacted starting material. No β-hydride elimination product was observed.
Table 1.
Optimization of Reaction Conditiona
 | ||||
|---|---|---|---|---|
| No. | Solvent | Base | 3a Yield (%)b | E/Z ratioc |
| 1d | DCE | NaOAc | 0 | - |
| 2e | DCE | NaOAc | 0 | - |
| 3f | DCE | NaOAc | trace | - |
| 4g | DCE | NaOAc | 0 | - |
| 5h | DCE | NaOAc | 83(78) | >25:1 |
| 6h | MeOH | NaOAc | 47 | >25:1 |
| 7h | dioxane | NaOAc | 42 | >25:1 |
| 8h | DCM | NaOAc | 63 | >25:1 |
| 9h | toluene | NaOAc | 56 | >25:1 |
| 10h | DCE | KOAc | 52 | - |
| 11h | DCE | Na2CO3 | 37 | - |
| 12h | DCE | CsOAc | 50 | - |
| 13h,i | DCE | NaOAc | 0 | - |
| 14h,j | DCE | - | trace | - |
| 15h,k | DCE | NaOAc | 36 | - |
 | ||||
Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), catalyst (5 mol %), base (0.6 equiv.) in 2 mL solvent at room temperature, Ar atmosphere.
NMR yields using CH2Br2 as an internal standard, and isolated yields in parentheses.
Determined by 1H-NMR analysis of the crude reaction mixtures.
1a-1 instead of 1a.
1a-2 instead of 1a.
1a-3 instead of 1a.
1a-4 instead of 1a.
1a was used, in which ethoxycarbamoyl is the directing group.
No [Cp*RhCl2]2.
No NaOAc.
[{RuCl2(p-cymene)}2] was used instead of [Cp*RhCl2]2.
With the optimized conditions in hand, the scope of indoles was investigated. In general, the reaction produced the corresponding allylation products in good yields with excellent regio- and stereoselectivity, regardless of electron-donating and electron-withdrawing substituents on the indole ring (Table 2). When halogen groups (such as fluorine, chlorine, and bromine) were attached to the C-4, C-5 or C-6 position of indoles, the reactions all afforded the products in good yields with excellent selectivity (3b-3f). Further, a methyl or methoxyl group at the C-5 position of indoles also allowed the reaction to generate the corresponding products smoothly (3g and 3h). To investigate the influence of the steric hindrance at the C-3 position of indoles, the methyl and phenyl groups were introduced, and the desired products were obtained in good yields, respectively (3i and 3j).
Table 2.
 |
Reaction conditions: 1 (0.2 mmol), 2a (0.3 mmol), [Cp*RhCl2]2 (5 mol %), NaOAc (0.6 equiv) in DCE (2 mL) at room temperature, Ar atmosphere, 2~6 h. Unless otherwise noted, the E/Z ratios of all products are > 25:1.
Isolated yields are reported.
Allyl methyl carbonate was used.
AgOAc (0.6 equiv) was used instead of NaOAc, 50 °C.
Next, we investigated the scope of allyl alcohols as coupling partners under standard reaction conditions. In general, the reaction gave the desired products in good yields with excellent regio- and stereoselectivity when a variety of allyl alcohols were employed as the coupling partners. Regardless of the benzene ring of allyl alcohols bearing electron-withdrawing groups (F, Cl, Br, and CF3) or electron-donating groups (Me and MeO-), the reaction provided the corresponding products smoothly (3k-3p). Further, the reaction was proceeded with good efficiency when using thiophenyl substituted allyl alcohols or naphthalenyl substituted allyl alcohols as the coupling partners (3q and 3r). In addition, the reaction afforded the desired products in good yields when alkyl substituted allyl alcohols were used (3s and 3t). However, the reaction did not give the corresponding product when using prop-2-en-1-ol as the coupling partner. Nonetheless, the desired product 3u could be obtained using allyl methyl carbonate. Besides, the reaction afforded the desired product 3v smoothly when 2-methylbut-3-en-2-ol was employed as the coupling partner and silver acetate as the base. The use of non-terminal allyl alcohols (2n-2p) did not offer the products.
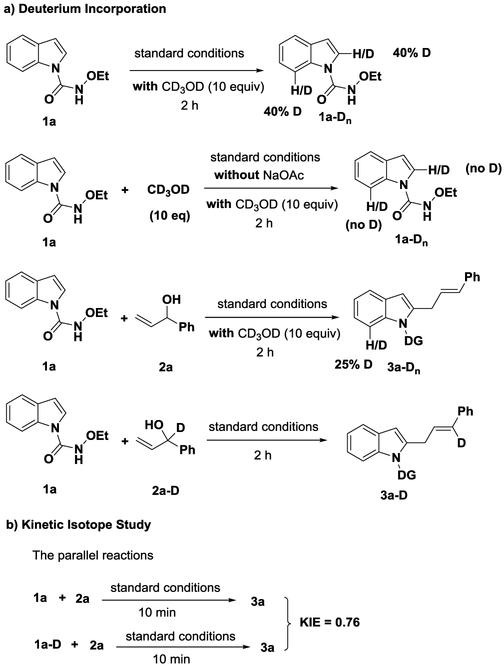
We then carried out a series of deuterium labeling experiments to probe the mechanism of the reaction. The reaction was performed with an excess amount of isotopically labeled CD3OD to determine the reversibility of the step of the C−H bond cleavage (Scheme 2a). A facile H/D scrambling was observed at the C-2 and C-7 positions of the indole ring, respectively (see the ESI for details). In contrast, the H/D exchange was not observed in the absence of the base NaOAc. Further, the reaction resulted in 25% deuteration at the C-7 position when performed with 2a. These results indicated that the step of C−H bond cleavage was reversible, and the reaction could follow a concerted metalation-deprotonation (CMD) mechanism.9 Further, we conducted the reaction with deuterium-labeled allyl alcohol 2a-D, and deuterium-labeled product 3a-D was obtained, indicating the C-D bond of 2a-D was intact in the reaction. The kinetic isotope effect (KIE) was measured in two parallel reactions using 1a and deuterium-labeled 1a-D under the standard conditions (Scheme 2b). A KIE value of 0.76 was obtained, implicating that the step of C−H bond activation was probably not involved in the rate-limiting step.10
Scheme 2.
Preliminary mechanism studies
A plausible reaction pathway was proposed in Scheme 3a based on the preliminary mechanistic experiments and those in the literatures.3,7 The first step is the generation of the active Rh(III) catalyst with the assistance of sodium acetate. The second step is the C-H bond activation to form the rhodacycle I. Subsequently, the insertion of allyl alcohol 2 leads to intermediate II. Finally, β-hydroxide elimination and protonation with the aid of AcOH provides the allylation product 3 and regenerates the active rhodium catalyst.
Scheme 3.
Proposed mechanism
The reason why the β-hydroxide elimination is more favored by this reaction might be determined by the stability of the transition state structures from intermediate II to intermediates III1 and III2, or by the stability of the transition state structures from intermediates III1 and III2 to IV1 and IV2, respectively, as shown in Figure 3b. The key transition state structure of the β-hydroxide elimination pathway might be more stable than that of the β-hydride elimination pathway. The detailed mechanism requires more mechanistic studies and/or DFT calculations.
To evaluate the efficiency and potential synthetic utility of this methodology, a gram-scale reaction and the removal of the directing group reaction were performed (Scheme 4a). The result showed that the gram-scale preparation of 3i with [Cp*RhCl2]2 gave good yield. By contrast, the reaction using [RuCl2(p-cymene)]2 was used as the catalyst at 45 °C only provided the product in a moderate yield. In combination with the different results of entries 5 and 15 of Table 1, and the stereoselectivities of 3t, and 3u of Table 2, these results indicated that the Rh(III)-catalyzed reaction offered higher yield, milder reaction condition and higher seteroselectivity than the Ru(II)-catalyzed reaction4g. Further, after many conditions were screened (described in the ESI), we found an approach that the directing group can be removed under mild reaction conditions by converting the N-ethoxycarbamoyl group to the Weinreb amide and then undergoing the reduction reaction.
Scheme 4.
Gram-scale reaction and the removal of the directing group
Conclusions
In this study, we have reported the first example of an efficient Rh(III)-catalyzed C−H allylation of indoles by utilizing unactivated allyl alcohols, which favors an interesting β-hydroxide elimination over β-hydride elimination. For most substrates, the reaction can afford allylated products in good yields. In addition, this method features very mild reaction conditions, excellent regio- and stereoselectivity, and compatibility with various functional groups.
Experimental
General procedure for the synthesis of compound 3
To a dry tube was equipped with N-ethoxy-indole-1-carboxamides 1 (0.2 mmol, 1 equiv), allylic alcohols 2 (0.3 mmol, 1.5 equiv), [Cp*RhCl2]2 (0.01 mmol, 0.05 equiv), NaOAc (0.12 mmol, 0.6 equiv) and 2 mL 1,2-dichloroethane. The resulting mixture was stirred at room temperature for 2~6 h under Ar atmosphere. Then, the solution was diluted with dichloromethane and washed by water. The combined organic layers were dried with Na2SO4, filtered, concentrated and purified by column chromatography on silica gel (hexanes/EtOAc = 6/1 ~ 4/1, v/v) to give desired products 3.
The gram-scale preparation of 3i:
With Cp*RhIII catalyst: To a dry round bottom flask was equipped with N-ethoxy-indole-1-carboxamides 1i in Scheme 4 (1.0 g, 4.58 mmol, 1 equiv), allylic alcohols 2a (922 mg, 6.87 mmol, 1.5 equiv), [Cp*RhCl2]2 (85.0 mg, 0.13 mmol, 0.03 equiv), NaOAc (225.5 mg, 2.75 mmol, 0.6 equiv), and 40 mL 1,2-dichloroethane (DCE). The resulting mixture was stirred at room temperature for 6 h under Ar atmosphere. Then, the solution was diluted with dichloromethane and washed by water. The combined organic layers were dried with Na2SO4, filtered, concentrated, and purified by column chromatography on silica gel (hexanes/EtOAc = 6/1 ~ 4/1, v/v) to give the desired product 3i (1.13 g, 74% yield).
With RuII catalyst: To a dry round bottom flask was equipped with N-ethoxy-indole-1-carboxamides 1i (1.0 g, 4.58 mmol, 1 equiv), allylic alcohols 2a (922 mg, 6.87 mmol, 1.5 equiv), [RuCl2(p-cymene)]2 (140.3 mg, 0.23 mmol, 0.05 equiv), NaOAc (375.9 mg, 4.58 mmol, 1 equiv) and 40 mL 1,2-dichloroethane. The resulting mixture was stirred at 45 °C for 6 h under Ar atmosphere. Then, the solution was diluted with dichloromethane and washed by water. The combined organic layers were dried with Na2SO4, filtered, concentrated, and purified by column chromatography on silica gel (hexanes/EtOAc = 6/1 ~ 4/1, v/v) to give the desired product 3i (597.0 mg, 39% yield).
Removal of the directing group:
To a solution of 3i (0.52 g, 1.55 mmol, 1 equiv) in dry DMF (20 mL) was added NaH (74.6 mg, 60%, dispersion in mineral oil, 1.2 equiv) portionwise at 0 °C. The resulting mixture was stirred for 30 min at room temperature, and then MeI (441.0 mg, 2 equiv) was added into the solution. The reaction was stirred for 6 h at room temperature. Then, the reaction was quenched with saturated NH4Cl solution. The solution was diluted with EtOAc and washed by brine. The combined organic layers were dried over Na2SO4, filtered, concentrated, and purified by column chromatography on silica gel (hexanes/EtOAc = 15/1 ~ 10/1, v/v) to give the desired product 3ia (498.0 mg, 92% yield).
To a solution of 3ia (50.0 mg, 0.14 mmol, 1 equiv) in anhydrous THF (5 mL) was added LiAlH4 (27.2 mg, 5 equiv) portionwise at room temperature. The resulting mixture was stirred for 30 min, and then the reaction was heated to 60 °C for 12 h under Ar atmosphere. The reaction was quenched with saturated NH4Cl solution. The solution was diluted with EtOAc and washed by brine. The combined organic layers were dried over Na2SO4, filtered, concentrated and purified by column chromatography on silica gel (hexanes/EtOAc = 15/1 ~ 10/1, v/v) to give the product 4a (21.0 mg, 59% yield).
2-cinnamyl-N-ethoxy-1H-indole-1-carboxamide (3a).
3a was obtained as colorless oil in 78% yield, 50 mg. 1H NMR (500 MHz, CDCl3) δ 8.32 (s, 1H), 7.73 – 7.70 (m, 1H), 7.54 – 7.48 (m, 1H), 7.38 – 7.34 (m, 2H), 7.32 – 7.28 (m, 2H), 7.25 – 7.16 (m, 3H), 6.51 (d, J = 15.9 Hz, 1H), 6.46 (d, J = 0.8 Hz, 1H), 6.39 (dt, J = 15.9, 6.5 Hz, 1H), 4.09 (q, J = 7.0 Hz, 2H), 3.88 (d, J = 6.5 Hz, 2H), 1.31 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.6, 139.0, 137.1, 135.9, 132.6, 129.3, 128.7, 127.6, 126.5, 126.4, 123.3, 122.5, 120.7, 112.5, 107.1, 72.9, 32.0, 13.6 ppm. HRMS (ESI) m/z: calculated for C20H19N2O2− [M − H]−: 319.1452, found: 319.1453.
2-cinnamyl-N-ethoxy-5-fluoro-1H-indole-1-carboxamide (3b).
3b was obtained as colorless oil in 74% yield, 50 mg. 1H NMR (500 MHz, CDCl3) δ 8.29 (s, 1H), 7.66 (dd, J = 9.0, 4.3 Hz, 1H), 7.38 – 7.34 (m, 2H), 7.33 – 7.28 (m, 2H), 7.25 – 7.21 (m, 1H), 7.15 (dd, J = 8.8, 2.5 Hz, 1H), 6.95 (td, J = 9.1, 2.6 Hz, 1H), 6.51 (d, J = 15.9 Hz, 1H), 6.42 (d, J = 0.7 Hz, 1H), 6.37 (dt, J = 15.9, 6.5 Hz, 1H), 4.08 (q, J = 7.0 Hz, 2H), 3.86 (d, J = 6.4 Hz, 2H), 1.31 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 159.1 (d, J = 238.6 Hz), 152.5, 140.5, 136.9, 133.0, 132.5, 130.0 (d, J = 10.1 Hz), 128.7, 127.8, 126.4, 126.2, 113.6 (d, J = 9.3 Hz), 111.2 (d, J = 25.5 Hz), 107.1 (d, J = 4.0 Hz), 106.0 (d, J = 23.7 Hz), 73.0, 32.1, 13.6 ppm. 19F NMR (471 MHz, CDCl3) δ −121.40 ppm. HRMS (ESI) m/z: calculated for C20H18N2O2F− [M − H]−: 337.1358, found: 337.1363.
5-chloro-2-cinnamyl-N-ethoxy-1H-indole-1-carboxamide (3c).
3c was obtained as colorless oil in 77% yield, 55 mg. 1H NMR (500 MHz, CDCl3) δ 8.29 (s, 1H), 7.64 (d, J = 8.8 Hz, 1H), 7.46 (d, J = 1.9 Hz, 1H), 7.37 – 7.33 (m, 2H), 7.33 – 7.28 (m, 2H), 7.25 – 7.21 (m, 1H), 7.18 (dd, J = 8.8, 2.1 Hz, 1H), 6.50 (d, J = 15.9 Hz, 1H), 6.40 (d, J = 0.8 Hz, 1H), 6.39 – 6.33 (m, 1H), 4.08 (q, J = 7.0 Hz, 2H), 3.86 (d, J = 6.5 Hz, 2H), 1.30 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.3, 140.2, 136.9, 134.4, 133.0, 130.4, 128.8, 128.1, 127.8, 126.4, 126.1, 123.5, 120.1, 113.7, 106.6, 73.0, 32.0, 13.6 ppm. HRMS (ESI) m/z: calculated for C20H18N2O2Cl− [M − H]−: 353.1062, found: 353.1066.
5-bromo-2-cinnamyl-N-ethoxy-1H-indole-1-carboxamide (3d).
3d was obtained as colorless oil in 82% yield, 66 mg. 1H NMR (500 MHz, CDCl3) δ 8.42 (s, 1H), 7.60 (d, J = 1.9 Hz, 1H), 7.56 (d, J = 8.8 Hz, 1H), 7.36 – 7.33 (m, 2H), 7.32 – 7.27 (m, 3H), 7.25 – 7.20 (m, 1H), 6.48 (d, J = 15.9 Hz, 1H), 6.39 – 6.31 (m, 2H), 4.05 (q, J = 7.0 Hz, 2H), 3.82 (d, J = 6.5 Hz, 2H), 1.29 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.2, 140.1, 136.9, 134.8, 133.0, 130.9, 128.8, 127.8, 126.4, 126.1, 126.0, 123.2, 115.7, 114.1, 106.4, 73.1, 31.9, 13.6 ppm. HRMS (ESI) m/z: calculated for C20H18N2O2Br− [M − H]−: 397.0557, found: 397.0546.
4-chloro-2-cinnamyl-N-ethoxy-1H-indole-1-carboxamide (3e).
3e was obtained as colorless oil in 79% yield, 56 mg. 1H NMR (500 MHz, CDCl3) δ 8.53 (s, 1H), 7.57 (d, J = 8.1 Hz, 1H), 7.38 – 7.33 (m, 2H), 7.30 (t, J = 7.6 Hz, 2H), 7.27 – 7.21 (m, 1H), 7.16 (dd, J = 7.7, 0.7 Hz, 1H), 7.12 (t, J = 7.9 Hz, 1H), 6.55 (s, 1H), 6.50 (d, J = 15.9 Hz, 1H), 6.36 (dt, J = 15.9, 6.5 Hz, 1H), 4.05 (q, J = 7.0 Hz, 2H), 3.82 (d, J = 6.5 Hz, 2H), 1.29 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.1, 139.6, 136.9, 136.6, 133.0, 128.7, 127.9, 127.7, 126.4, 125.9, 125.9, 123.9, 122.1, 111.0, 105.0, 73.0, 31.9, 13.6 ppm. HRMS (ESI) m/z: calculated for C20H18N2O2Cl− [M − H]−: 353.1062, found: 353.1064.
2-cinnamyl-N-ethoxy-6-fluoro-1H-indole-1-carboxamide (3f).
3f was obtained as colorless oil in 68% yield, 46 mg. 1H NMR (500 MHz, CDCl3) δ 8.32 (s, 1H), 7.47 (dd, J = 10.2, 2.3 Hz, 1H), 7.40 (dd, J = 8.6, 5.4 Hz, 1H), 7.37 – 7.33 (m, 2H), 7.33 – 7.28 (m, 2H), 7.25 – 7.19 (m, 1H), 6.97 – 6.93 (m, 1H), 6.50 (d, J = 15.9 Hz, 1H), 6.42 (d, J = 0.8 Hz, 1H), 6.37 (dt, J = 15.9, 6.4 Hz, 1H), 4.08 (q, J = 7.0 Hz, 2H), 3.84 (d, J = 6.4 Hz, 2H), 1.31 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 160.3 (d, J = 239.5 Hz), 152.4, 139.0, 138.9, 136.9, 136.1 (d, J = 12.1 Hz), 132.9, 128.7, 127.7, 126.4, 125.4 (d, J = 1.1 Hz), 121.2 (d, J = 9.8 Hz), 110.8 (d, J = 24.0 Hz), 106.9, 100.2 (d, J = 28.4 Hz), 73.0, 32.0, 13.6 ppm. 19F NMR (471 MHz, CDCl3) δ −118.38 ppm. HRMS (ESI) m/z: calculated for C20H18FN2O2− [M − H]−: 337.1358, found: 337.1359.
2-cinnamyl-N-ethoxy-5-methyl-1H-indole-1-carboxamide (3g).
3g was obtained as colorless oil in 71% yield, 48 mg. 1H NMR (500 MHz, CDCl3)) δ 8.28 (s, 1H), 7.59 (d, J = 8.4 Hz, 1H), 7.40 – 7.34 (m, 2H), 7.32 – 7.27 (m, 3H), 7.24 – 7.19 (m, 1H), 7.04 (dd, J = 8.5, 1.3 Hz, 1H), 6.50 (d, J = 15.9 Hz, 1H), 6.43 – 6.34 (m, 2H), 4.07 (q, J = 7.0 Hz, 2H), 3.86 (d, J = 6.4 Hz, 2H), 2.42 (s, 3H), 1.31 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.8, 139.2, 137.2, 134.1, 132.5, 131.9, 129.6, 128.7, 127.6, 126.7, 126.3, 124.7, 120.6, 112.3, 106.9, 72.9, 32.1, 21.3, 13.6 ppm. HRMS (ESI) m/z: calculated for C21H21N2O2− [M − H]−: 333.1609, found: 333.1601.
2-cinnamyl-N-ethoxy-5-methoxy-1H-indole-1-carboxamide (3h).
3h was obtained as colorless oil in 78% yield, 55 mg. 1H NMR (500 MHz, CDCl3) δ 8.26 (s, 1H), 7.62 (d, J = 9.0 Hz, 1H), 7.39 – 7.33 (m, 2H), 7.33 – 7.27 (m, 2H), 7.24 – 7.17 (m, 1H), 6.96 (d, J = 2.5 Hz, 1H), 6.85 (dd, J = 9.0, 2.5 Hz, 1H), 6.50 (d, J = 15.9 Hz, 1H), 6.44 – 6.35 (m, 2H), 4.08 (q, J = 7.0 Hz, 2H), 3.86 (d, J = 6.4 Hz, 2H), 3.83 (s, 3H), 1.31 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 155.8, 152.8, 139.7, 137.1, 132.6, 130.6, 130.2, 128.7, 127.6, 126.6, 126.3, 113.5, 112.3, 107.2, 103.1, 72.9, 55.8, 32.2, 13.7 ppm. HRMS (ESI) m/z: calculated for C21H21N2O3− [M − H]−: 349.1558, found: 349.1560.
2-cinnamyl-N-ethoxy-3-methyl-1H-indole-1-carboxamide (3i).
3i was obtained as colorless oil in 77% yield, 52 mg. 1H NMR (500 MHz, CDCl3) δ 8.34 (s, 1H), 7.78 – 7.75 (m, 1H), 7.53 – 7.48 (m, 1H), 7.33 – 7.18 (m, 8H), 6.40 – 6.37 (m, 2H), 4.05 (q, J = 7.0 Hz, 2H), 3.87 (d, J = 4.0 Hz, 2H), 2.27 (s, 3H), 1.28 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.7, 137.0, 135.3, 132.8, 131.7, 130.3, 128.6, 127.5, 127.1, 126.3, 123.6, 122.1, 118.8, 114.5, 112.7, 72.8, 29.0, 13.6, 8.8 ppm. HRMS (ESI) m/z: calculated for C21H21N2O2− [M − H]−: 333.1609, found: 333.1612.
2-cinnamyl-N-ethoxy-3-phenyl-1H-indole-1-carboxamide (3j).
3j was obtained as colorless oil in 80% yield, 64 mg. 1H NMR (500 MHz, CDCl3) δ 8.51 (s, 1H), 7.78 (dt, J = 8.4, 0.8 Hz, 1H), 7.56 – 7.54 (m, 1H), 7.49 – 7.46 (m, 4H), 7.41 – 7.34 (m, 1H), 7.31 – 7.24 (m, 5H), 7.23 – 7.17 (m, 2H), 6.41 – 6.37 (m, 2H), 4.03 (q, J = 7.0 Hz, 2H), 3.91 (d, J = 4.2 Hz, 2H), 1.24 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.3, 137.0, 135.6, 133.6, 133.6, 132.2, 129.9, 129.1, 128.8, 128.7, 127.8, 127.6, 127.4, 126.3, 124.0, 122.5, 121.2, 119.9, 112.5, 72.9, 29.4, 13.5 ppm. HRMS (ESI) m/z: calculated for C26H24N2O2Na+ [M + Na]+: 419.1735, found: 419.1727.
(E)-N-ethoxy-2-(3-(4-fluorophenyl)allyl)-1H-indole-1-carboxamide (3k).
The product was obtained as colorless oil in 70% yield, 48 mg. 1H NMR (500 MHz, CDCl3) δ 8.30 (s, 1H), 7.71 (d, J = 8.2 Hz, 1H), 7.54 – 7.48 (m, 1H), 7.38 – 7.29 (m, 2H), 7.25 – 7.15 (m, 2H), 7.03 – 6.94 (m, 2H), 6.48 – 6.43 (m, 2H), 6.31 (dt, J = 15.8, 6.5 Hz, 1H), 4.09 (q, J = 7.0 Hz, 2H), 3.87 (d, J = 6.5 Hz, 2H), 1.32 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 162.4 (d, J = 246.6 Hz), 152.6, 139.0, 135.8, 133.3 (d, J = 3.3 Hz), 131.4, 129.3, 127.8 (d, J = 7.9 Hz), 126.3, 126.3, 123.4, 122.5, 120.7, 115.6 (d, J = 21.5 Hz), 112.4, 107.1, 73.0, 31.9, 13.7 ppm. 19F NMR (471 MHz, CDCl3) δ −114.69 ppm. HRMS (ESI) m/z: calculated for C20H18N2O2F− [M − H]−: 337.1358, found: 337.1357.
(E)-2-(3-(4-chlorophenyl)allyl)-N-ethoxy-1H-indole-1-carboxamide (3l).
The product was obtained as colorless oil in 78% yield, 56 mg. 1H NMR (500 MHz, CDCl3) δ 8.31 (s, 1H), 7.71 – 7.68 (m, 1H), 7.53 – 7.48 (m, 1H), 7.29 – 7.15 (m, 6H), 6.47 – 6.43 (m, 2H), 6.36 (dt, J = 15.9, 6.4 Hz, 1H), 4.09 (q, J = 7.0 Hz, 2H), 3.86 (d, J = 6.3 Hz, 2H), 1.32 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.6, 138.9, 135.8, 135.7, 133.2, 131.3, 129.3, 128.8, 127.5, 127.3, 123.4, 122.5, 120.7, 112.4, 107.1, 72.9, 31.9, 13.7 ppm. HRMS (ESI) m/z: calculated for C20H18N2O2Cl− [M − H]−: 353.1062, found: 353.1061.
(E)-2-(3-(4-bromophenyl)allyl)-N-ethoxy-1H-indole-1-carboxamide (3m).
The product was obtained as colorless oil in 75% yield, 60 mg. 1H NMR (500 MHz, CDCl3) δ 8.27 (s, 1H), 7.70 (d, J = 8.2 Hz, 1H), 7.51 (d, J = 7.5 Hz, 1H), 7.44 – 7.37 (m, 2H), 7.26 – 7.16 (m, 4H), 6.47 – 6.34 (m, 3H), 4.09 (q, J = 7.0 Hz, 2H), 3.87 (d, J = 5.8 Hz, 2H), 1.32 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.6, 138.9, 136.1, 135.7, 131.8, 131.4, 129.3, 127.9, 127.5, 123.4, 122.5, 121.3, 120.8, 112.4, 107.1, 73.0, 32.0, 13.7 ppm. HRMS (ESI) m/z: calculated for C20H18N2O2Br− [M − H]−: 397.0557, found: 397.0562.
(E)-N-ethoxy-2-(3-(p-tolyl)allyl)-1H-indole-1-carboxamide (3n).
The product was obtained as colorless oil in 71% yield, 48 mg. 1H NMR (400 MHz, CDCl3)) δ 8.31 (s, 1H), 7.73 (d, J = 8.3 Hz, 1H), 7.55 – 7.46 (m, 1H), 7.27 – 7.16 (m, 5H), 7.13 – 7.08 (m, 2H), 6.50 – 6.44 (m, 2H), 6.34 (dt, J = 15.9, 6.4 Hz, 1H), 4.09 (q, J = 7.0 Hz, 2H), 3.86 (d, J = 6.4 Hz, 2H), 2.33 (s, 3H), 1.31 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.6, 139.1, 137.4, 136.0, 134.3, 132.6, 129.4, 129.3, 126.3, 125.5, 123.3, 122.4, 120.7, 112.6, 107.1, 72.9, 32.0, 21.3, 13.7 ppm. HRMS (ESI) m/z: calculated for C21H21N2O2− [M − H]−: 333.1603, found: 333.1608.
(E)-N-ethoxy-2-(3-(4-methoxyphenyl)allyl)-1H-indole-1-carboxamide (3o).
The product was obtained as colorless oil in 74% yield, 52 mg. 1H NMR (500 MHz, CDCl3) δ 8.37 (s, 1H), 7.74 – 7.70 (m, 1H), 7.54 – 7.45 (m, 1H), 7.33 – 7.26 (m, 2H), 7.25 – 7.15 (m, 2H), 6.86 – 6.80 (m, 2H), 6.47 – 6.41 (m, 2H), 6.24 (dt, J = 15.8, 6.5 Hz, 1H), 4.08 (q, J = 7.0 Hz, 2H), 3.84 (d, J = 6.5 Hz, 2H), 3.79 (s, 3H), 1.31 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 159.3, 152.6, 139.1, 136.0, 132.1, 129.9, 129.3, 127.5, 124.3, 123.3, 122.4, 120.6, 114.1, 112.6, 107.0, 72.9, 55.4, 31.9, 13.6 ppm. HRMS (ESI) m/z: calculated for C21H21N2O3− [M − H]−: 349.1558, found: 349.1561.
(E)-N-ethoxy-2-(3-(4-(trifluoromethyl)phenyl)allyl)-1H-indole-1-carboxamide (3p).
The product was obtained as colorless oil in 72% yield, 56 mg. 1H NMR (500 MHz, CDCl3) δ 8.29 (s, 1H), 7.69 (dd, J = 8.2, 0.8 Hz, 1H), 7.56 – 7.50 (m, 3H), 7.45 – 7.42 (m, 2H), 7.25 – 7.16 (m, 2H), 6.55 – 6.44 (m, 3H), 4.10 (q, J = 7.0 Hz, 2H), 3.91 (d, J = 4.8 Hz, 2H), 1.33 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.6, 140.7, 138.8, 135.7, 131.2, 129.5, 129.3, 126.5, 125.7 (q, JC-F = 3.8 Hz), 123.5, 122.6, 120.8, 112.4, 107.2, 73.0, 32.0, 13.7 ppm. 19F NMR (471 MHz, CDCl3) δ −62.49 ppm. HRMS (ESI) m/z: calculated for C21H20F3N2O2+ [M + H]+: 389.1477, found: 389.1467.
(E)-N-ethoxy-2-(3-(thiophen-2-yl)allyl)-1H-indole-1-carboxamide (3q).
The product was obtained as colorless oil in 73% yield, 48 mg. 1H NMR (500 MHz, CDCl3) δ 8.33 (s, 1H), 7.72 – 7.68 (m, 1H), 7.54 – 7.48 (m, 1H), 7.24 – 7.15 (m, 2H), 7.12 (d, J = 5.1 Hz, 1H), 6.96 – 6.88 (m, 2H), 6.64 – 6.58 (m, 1H), 6.45 (d, J = 0.8 Hz, 1H), 6.21 (dt, J = 15.7, 6.6 Hz, 1H), 4.09 (q, J = 7.0 Hz, 2H), 3.85 (d, J = 6.6 Hz, 2H), 1.31 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.5, 142.2, 138.8, 135.9, 129.3, 127.5, 126.2, 125.8, 125.5, 124.1, 123.4, 122.5, 120.7, 112.4, 107.1, 73.0, 31.7, 13.6 ppm. HRMS (ESI) m/z: calculated for C18H17N2O2S− [M − H]−: 325.1016, found: 325.1013.
(E)-N-ethoxy-2-(3-(naphthalen-2-yl)allyl)-1H-indole-1-carboxamide (3r).
The product was obtained as colorless oil in 76% yield, 57 mg. 1H NMR (500 MHz, CDCl3) δ 8.33 (s, 1H), 7.80 – 7.67 (m, 5H), 7.59 (dd, J = 8.6, 1.7 Hz, 1H), 7.55 – 7.51 (m, 1H), 7.48 – 7.40 (m, 2H), 7.26 – 7.16 (m, 2H), 6.67 (d, J = 15.9 Hz, 1H), 6.57 – 6.47 (m, 2H), 4.08 (q, J = 7.0 Hz, 2H), 3.94 (d, J = 6.4 Hz, 2H), 1.29 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.6, 139.0, 135.9, 134.6, 133.7, 133.1, 132.7, 129.3, 128.3, 128.1, 127.8, 127.0, 126.4, 126.2, 125.9, 123.6, 123.4, 122.5, 120.7, 112.5, 107.1, 73.0, 32.1, 13.6 ppm. HRMS (ESI) m/z: calculated for C24H22N2O2− [M − H]−: 369.1620, found: 369.1611.
(E)-N-ethoxy-2-(pent-2-en-1-yl)-1H-indole-1-carboxamide (3s).
A mixture of stereoisomers (E/Z = 93:7) was obtained as colorless oil in 82% yield, 45 mg. Spectral data for the major isomer (E): 1H NMR (500 MHz, CDCl3) δ 8.37 (s, 1H), 7.81 – 7.76 (m, 1H), 7.51 – 7.47 (m, 1H), 7.24 – 7.15 (m, 2H), 6.39 (d, J = 0.8 Hz, 1H), 5.65 – 5.62 (m, 2H), 4.14 (q, J = 7.0 Hz, 2H), 3.68 – 3.60 (m, 2H), 2.11 – 2.03 (m, 2H), 1.36 (d, J = 7.0 Hz, 3H), 1.00 (t, J = 7.5 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.7, 139.2, 136.2, 135.7, 129.3, 125.7, 123.3, 122.4, 120.5, 112.9, 107.0, 72.9, 31.7, 25.7, 13.8, 13.7 ppm. HRMS (ESI) m/z: calculated for C16H21N2O2+ [M + H]+: 273.1603, found: 273.1600.
(E)-N-ethoxy-2-(hex-2-en-1-yl)-1H-indole-1-carboxamide (3t).
A mixture of stereoisomers (E/Z = 92:8) was obtained as colorless oil in 75% yield, 43 mg. Spectral data for the major isomer (E): 1H NMR (500 MHz, CDCl3) δ 8.43 (s, 1H), 7.79 – 7.76 (m, 1H), 7.52 – 7.46 (m, 1H), 7.25 – 7.13 (m, 2H), 6.38 (d, J = 0.8 Hz, 1H), 5.68 – 5.53 (m, 2H), 4.13 (q, J = 7.0 Hz, 2H), 3.66 – 3.63 (m, 2H), 2.05 – 2.00 (m, 2H), 1.41 – 1.34 (m, 5H), 0.89 (t, J = 7.4 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.5, 139.0, 136.0, 133.9, 129.1, 126.7, 123.1, 122.2, 120.3, 112.8, 106.8, 72.7, 34.6, 31.6, 22.4, 13.7, 13.6 ppm. HRMS (ESI) m/z: calculated for C17H22N2O2+ [M + H]+: 287.1760, found: 287.1756.
2-allyl-N-ethoxy-1H-indole-1-carboxamide (3u).
The product was obtained as colorless oil in 71% yield, 35 mg. 1H NMR (500 MHz, CDCl3) δ 8.36 (s, 1H), 7.72 (d, J = 8.2 Hz, 1H), 7.49 (d, J = 7.8 Hz, 1H), 7.23 – 7.14 (m, 2H), 6.40 (d, J = 0.7 Hz, 1H), 6.10 – 6.01 (m, 1H), 5.21 – 5.10 (m, 2H), 4.13 (q, J = 7.0 Hz, 2H), 3.74 – 3.69 (m, 2H), 1.36 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.6, 138.5, 136.0, 135.3, 129.3, 123.3, 122.4, 120.6, 117.5, 112.7, 107.1, 72.9, 32.8, 13.7 ppm. HRMS (ESI) m/z: calculated for C14H15N2O2− [M − H]−: 243.1139, found: 243.1133.
N-ethoxy-2-(3-methylbut-2-en-1-yl)-1H-indole-1-carboxamide (3v).
The product was obtained as colorless oil in 40% yield, 22 mg. 1H NMR (500 MHz, CDCl3) δ 8.23 (s, 1H), 7.73 – 7.65 (m, 1H), 7.45 – 7.36 (m, 1H), 7.17 – 7.00 (m, 2H), 6.30 (d, J = 0.9 Hz, 1H), 5.30 – 5.22 (m, 1H), 4.08 (q, J = 7.0 Hz, 2H), 3.60 – 3.57 (m, 2H), 1.78 – 1.61 (m, 6H), 1.30 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 152.9, 140.3, 136.1, 135.4, 129.4, 123.2, 122.4, 120.6, 120.5, 112.8, 106.4, 72.9, 27.8, 25.9, 18.1, 13.7 ppm. HRMS (ESI) m/z: calculated for C16H21N2O2+ [M + H]+: 273.1603, found: 273.1601.
2-cinnamyl-N-ethoxy-N,3-dimethyl-1H-indole-1-carboxamide (3ia).
The product was obtained as colorless oil in 92% yield, 498 mg. 1H NMR (500 MHz, CDCl3) δ 7.53 – 7.46 (m, 1H), 7.38 – 7.34 (m, 1H), 7.31 – 7.26 (m, 3H), 7.26 – 7.16 (m, 4H), 6.44 (d, J = 15.8 Hz, 1H), 6.25 (dt, J = 15.8, 6.5 Hz, 1H), 3.96 – 3.70 (m, 4H), 3.19 (s, 3H), 2.29 (s, 3H), 1.12 (t, J = 7.1 Hz, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 153.9, 137.3, 135.4, 134.1, 131.5, 130.0, 128.6, 127.4, 127.1, 126.2, 123.0, 121.3, 118.7, 113.2, 112.0, 69.7, 37.6, 28.6, 13.7, 8.8 ppm. HRMS (ESI) m/z: calculated for C22H25N2O2+ [M + H]+: 349.1916, found: 349.1909.
2-cinnamyl-3-methyl-1H-indole (4a).
The product was obtained as colorless oil in 59% yield, 21 mg. 1H NMR (500 MHz, CDCl3) δ 7.75 (s, 1H), 7.55 – 7.48 (m, 1H), 7.38 – 7.33 (m, 2H), 7.33 – 7.27 (m, 2H), 7.27 – 7.19 (m, 2H), 7.17 – 7.06 (m, 2H), 6.51 (d, J = 15.8 Hz, 1H), 6.33 (dt, J = 15.8, 6.6 Hz, 1H), 3.65 (dd, J = 6.6, 1.2 Hz, 2H), 2.28 (s, 3H) ppm. 13C NMR (126 MHz, CDCl3) δ 137.1, 135.5, 132.2, 132.1, 129.5, 128.8, 127.6, 126.7, 126.3, 121.4, 119.2, 118.4, 110.5, 107.7, 29.9, 8.6 ppm. HRMS (ESI) m/z: calculated for C18H18N+ [M + H]+: 248.1439, found: 248.1430.
Supplementary Material
Acknowledgements
This work was supported by the Susan G. Komen Career Catalyst Research Grant CCR16380693. The H. Lee Moffitt Cancer Center & Research Institute is a NCI-designated Comprehensive Cancer Center, supported under NIH grant P30-CA76292.
Footnotes
Electronic Supplementary Information (ESI) available. Data for new compounds and experimental procedures. See DOI: 10.1039/x0xx00000x
Conflicts of interest
The authors declare no competing financial interest.
References
- 1 (a).Farmer JL, Hunter HN and Organ MG, J. Am. Chem. Soc, 2012, 134, 17470; [DOI] [PubMed] [Google Scholar]; (b) Ni G, Zhang Q-J, Zheng Z-F, Chen R-Y and Yu D-Q, J. Nat. Prod, 2009, 72, 966; [DOI] [PubMed] [Google Scholar]; (c) Trost BM, Thiel OR and Tsui H-C, J. Am. Chem. Soc, 2002, 124, 11616. [DOI] [PubMed] [Google Scholar]
- 2.For a recent review on C-H allylation, see: Mishra NK, Sharma S, Park J, Han S and Kim IS, ACS Catal, 2017, 7, 2821. [Google Scholar]
- 3.For Cp*Rh(III)-catalyzed C-H allylation, see: (a) Wang H, Schröder N and Glorius F, Angew. Chem, Int. Ed., 2013, 52, 5386; [DOI] [PubMed] [Google Scholar]; (b) Feng C, Feng D and Loh T-P, Org. Lett, 2013, 15, 3670; [DOI] [PubMed] [Google Scholar]; (c) Feng C, Feng D and Loh T-P, Chem. Commun, 2015, 51, 3425; [DOI] [PubMed] [Google Scholar]; (d) Zhang S-S, Wu J-Q, Lao Y-X, Liu X-G, Liu Y, Lv W-X, Tan D-H, Zeng Y-F and Wang H, Org. Lett, 2014, 16, 6412; [DOI] [PubMed] [Google Scholar]; (e) Yu S and Li X, Org. Lett, 2014, 16, 1200; [DOI] [PubMed] [Google Scholar]; (f) Park J, Mishra NK, Sharma S, Han S, Shin Y, Jeong T, Oh JS, Kwak JH, Jung YH and Kim IS, J. Org. Chem, 2015, 80, 1818; [DOI] [PubMed] [Google Scholar]; (g) Wu JQ, Qiu ZP, Zhang SS, Liu JG, Lao YX, Gu LQ, Huang ZS, Li J and Wang H, Chem. Commun, 2015, 51, 77; [DOI] [PubMed] [Google Scholar]; (h) Debbarma S, Bera SS and Maji MS, J. Org. Chem, 2016, 81, 11716; [DOI] [PubMed] [Google Scholar]; (i) Dai H, Yu C, Wang Z, Yan H and Lu C, Org. Lett, 2016, 18, 3410; [DOI] [PubMed] [Google Scholar]; (j) Mei S-T, Wang N-J, Ouyang Q and Wei Y, Chem. Commun, 2015, 51, 2980; [DOI] [PubMed] [Google Scholar]; (k) Zhang S-S, Wu J-Q, Liu X and Wang H, ACS Catal, 2015, 5, 210; [Google Scholar]; (l)Qi Z, Kong L and Li X, Org. Lett, 2016, 18, 4392. [DOI] [PubMed] [Google Scholar]
- 4.For Ru(II)-catalyzed C-H allylation, see: (a) Oi S, Tanaka Y and Inoue Y, Organometallics 2006, 25, 4773; [Google Scholar]; (b) Kim M, Sharma S, Mishra NK, Han S, Park J, Kim M, Shin Y, Kwak JH, Han SH and Kim IS, Chem. Commun, 2014, 50, 11303; [DOI] [PubMed] [Google Scholar]; (c) Manikandan R, Madasamy P and Jeganmohan M, Chem. - Eur. J, 2015, 21, 13934; [DOI] [PubMed] [Google Scholar]; (d) Manikandan R and Jeganmohan M, Org. Biomol. Chem, 2016, 14, 7691; [DOI] [PubMed] [Google Scholar]; (e) Kumar GS and Kapur M, Org. Lett, 2016, 18, 1112; [DOI] [PubMed] [Google Scholar]; (f) Xia YQ and Dong L, Org. Lett, 2017, 19, 2258; [DOI] [PubMed] [Google Scholar]; (g) Wu X, and Ji H, Org. Lett, 2018, 20, 2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For Cp*Co(III) or Mn(I)-catalyzed C-H allylation, see: (a) Yu D-G, Gensch T, de Azambuja F, Vasquez-Cespedes S and Glorius F, J. Am. Chem. Soc, 2014, 136, 17722; [DOI] [PubMed] [Google Scholar]; (b) Gensch T, Vasquez-Cespedes S, Yu D-G and Glorius F, Org. Lett, 2015, 17, 3714; [DOI] [PubMed] [Google Scholar]; (c) Moselage M, Sauermann J, Koeller J, Liu W, Gelman D and Ackermann L, Synlett 2015, 26, 1596; [Google Scholar]; (d) Suzuki Y, Sun B, Sakata K, Yoshino T, Matsunaga S and Kanai M, Angew. Chem., Int. Ed, 2015, 54, 9944; [DOI] [PubMed] [Google Scholar]; (e) Bunno Y, Murakami N, Suzuki Y, Kanai M, Yoshino T and Matsunaga S, Org. Lett, 2016, 18, 2216; [DOI] [PubMed] [Google Scholar]; (f) Sk MR, Bera SS, and Maji MS, Org. Lett, 2018, 20, 134; [DOI] [PubMed] [Google Scholar]; (g) Liu W, Richter SC, Zhang Y and Ackermann L, Angew. Chem., Int. Ed, 2016, 55, 7747; [DOI] [PubMed] [Google Scholar]; (h) Meyer TH, Liu W, Feldt M, Wuttke A, Mata RA and Ackermann L, Chem. Eur. J, 2017, 23, 5443; [DOI] [PubMed] [Google Scholar]; (i) Lu Q, Klauck FJR and Glorius F, Chem. Sci, 2017, 8, 3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6 (a).Shi Z, Boultadakis-Arapinis M and Glorius F, Chem. Commun, 2013, 49, 6489; [DOI] [PubMed] [Google Scholar]; (b) Huang L, Wang Q, Qi J, Wu X, Huang K and Jiang H, Chem. Sci, 2013, 4, 2665; [Google Scholar]; (c) Han SH, Choi M, Jeong T, Sharma S, Mishra NK, Park J, Oh JS, Kim WJ, Lee JS and Kim IS, J. Org. Chem, 2015, 80, 11092; [DOI] [PubMed] [Google Scholar]; (d) Manoharan R and Jeganmohan M, Chem. Commun, 2015, 51, 2929; [DOI] [PubMed] [Google Scholar]; (e) Kalsi D, Laskar RA, Barsu N, Premkumar JR and Sundararaju B, Org. Lett, 2016, 18, 4198; [DOI] [PubMed] [Google Scholar]; (f) Xia J, Huang Z, Zhou X, Yang X, Wang F and L X, Org. Lett, 2018, 20, 740; [DOI] [PubMed] [Google Scholar]; (g) Wang Y, Chen Y, Yang Y and Zhou B, Org. Chem. Front, 2018, DOI: 10.1039/c8qo00265g. [DOI] [Google Scholar]
- 7 (a).Suzuki Y, Sun B, Sakata K, Yoshino T, Matsunaga S and Kanai M, Angew. Chem., Int. Ed, 2015, 54, 9944; [DOI] [PubMed] [Google Scholar]; (b) Bunno Y, Murakami N, Suzuki Y, Kanai M, Yoshino T and Matsunaga S, Org. Lett, 2016, 18, 2216. [DOI] [PubMed] [Google Scholar]
- 8.Huang Z, Zhang M, Burton SD, Katsakhyan LN and Ji H, ACS Chem. Biol, 2014, 9, 193. [DOI] [PubMed] [Google Scholar]
- 9 (a).Gorelsky SI, Lapointe D and Fagnou K, J. Am. Chem. Soc, 2008, 130, 10848; [DOI] [PubMed] [Google Scholar]; (b) Ma W, Mei R, Tenti G and Ackermann L, Chem. - Eur. J, 2014, 20, 15248. [DOI] [PubMed] [Google Scholar]
- 10.Simmons EM and Hartwig JF, Angew. Chem., Int. Ed, 2012, 51, 3066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.