ABSTRACT
Glioblastoma multiforme (GBM) is a frequent and aggressive glial tumor, containing a small population of therapy-resistant cells, glioma stem cells (GSCs). Current dogma suggests that tumors regrow from GSCs, and these cells contribute to therapy resistance, poor prognosis, and recurrence; highlighting the importance of GSCs in glioma pathophysiology and therapeutic targeting. Macroautophagy/autophagy-based cellular homeostasis can be changed from pro-survival to pro-cell death by modulating SDCBP/MDA-9/Syntenin (syndecan binding protein)-mediated signaling. In nonadherent conditions, GSCs display protective autophagy and anoikis-resistance, which correlates with expression of SDCBP/MDA-9/Syntenin. Conversely, SDCBP/MDA-9/Syntenin silencing induces autophagic death in GSCs, indicating that SDCBP/MDA-9/Syntenin regulates protective autophagy in GSCs under anoikis conditions. This process is mediated through phosphorylation of the anti-apoptotic protein BCL2 accompanied with suppression of high levels of autophagic proteins (ATG5, LAMP1, LC3B) through EGFR signaling. SDCBP/MDA-9/Syntenin-mediated regulation of BCL2 and EGFR phosphorylation is achieved through PTK2/FAK and PRKC/PKC signaling. When SDCBP/MDA-9/Syntenin is absent, this protective mechanism is deregulated, leading to highly elevated and sustained levels of autophagy and consequently decreased cell survival. Our recent paper reveals a novel functional link between SDCBP/MDA-9/Syntenin expression and protective autophagy in GSCs. These new insights into SDCBP/MDA-9/Syntenin-mediated regulation and maintenance of GSCs present leads for developing innovative combinatorial cancer therapies.
KEYWORDS: Anoikis resistance, autophagy, BCL2, cell death, EGFR, FAK, glioma stem cells, MDA-9/Syntenin, PKCα
SDCBP/MDA-9/Syntenin was cloned by the Fisher laboratory in 1993 and is receiving increasing attention for its central pathogenic role in multiple, diverse cancers. The current study provides important conceptual advances through in vitro and in vivo experiments on GSC survival under stressful anoikis-inducing conditions and how SDCBP/MDA-9/Syntenin regulates this process [1].
Functionally, SDCBP/MDA-9/Syntenin’s cancer-and metastasis-promoting activities depend to a significant extent on interactions with specific partner proteins. This research identified a pivotal and novel function of SDCBP/MDA-9/Syntenin in the regulation and maintenance of anoikis-resistant GSCs through protective autophagy. Autophagy may provide an alternative metabolic mechanism in these GSCs to obtain nutrients and energy under normally fatal stressful conditions, and our studies explain how SDCBP/MDA-9/Syntenin plays a seminal role in this protective mechanism. Anoikis-resistant GSCs express and depend on significantly higher levels of autophagy and SDCBP/MDA-9/Syntenin as compared to anoikis-sensitive non-stem glioma cells; and loss of sdcbp/mda-9/syntenin leads to sensitization of GSCs to anoikis in conjunction with toxic levels of autophagy. ATG5, LC3 and LAMP1 are key regulators of early, mid and late stages of autophagosome formation, respectively. EGFR regulates autophagy, whereas BCL2 (phosphorylated at S70) is an important inhibitor of apoptosis. We hypothesized that one or more of these signaling molecules must be responsible for maintenance of SDCBP/MDA-9/Syntenin-mediated protective autophagy. To test these possibilities, we studied the expression levels of these proteins in shcon and shsdcbp/mda-9 GSCs. Protein expression analysis of nonadherent shcon and shsdcbp/mda-9 GSCs indicated that p-EGFR and p-BCL2 expression along with expression of the EGFR and BCL2 phosphorylation regulator p-PRKCA/PKCα, are all significantly decreased in shsdcbp/mda-9 GSC neurospheres, in both in vitro and in vivo intracranial glioma xenografts.
To test our hypothesis, we studied further the effect of WT EGFR, constitutively active EGFRvIII and the EGFR activation inhibitor erlotinib (10 and 20 µM) on autophagy. We observed that EGFR activation maintained basal autophagy levels via regulation of ATG5, LC3, and LAMP1, and loss of EGFR activation leads to excessive levels of autophagy that ultimately proved lethal to the GSCs, i.e., toxic autophagy. Through studies involving restoration of function using BCL2, constitutively active PRKCA/PKCα and the PTK2/FAK inhibitor FAKi (10 µM), we further confirmed that SDCBP/MDA-9/Syntenin expression regulates EGFR activation and PRKCA/PKCα signaling-mediated antiapoptotic BCL2 protein phosphorylation at S70 through PTK2/FAK, in an interconnected and codependent manner. PRKCA/PKCα controls survival in GSCs, by regulating the antiapoptotic protein BCL2. This complex and interrelated process maintains protective autophagy in GSCs, thereby protecting against anoikis. When loss of SDCBP/MDA-9/Syntenin occurs, this delicate balance is disrupted causing autophagy levels to exceed the threshold level, thereby shifting autophagy from protective to toxic (Figure 1). In summary, this innovative study demonstrates a link between SDCBP/MDA-9/Syntenin, protective autophagy and anoikis-resistance, identifying a potential vulnerability of glioblastoma multiforme that may be exploited to define enhanced therapeutic strategies for future clinical intervention.
Figure 1.
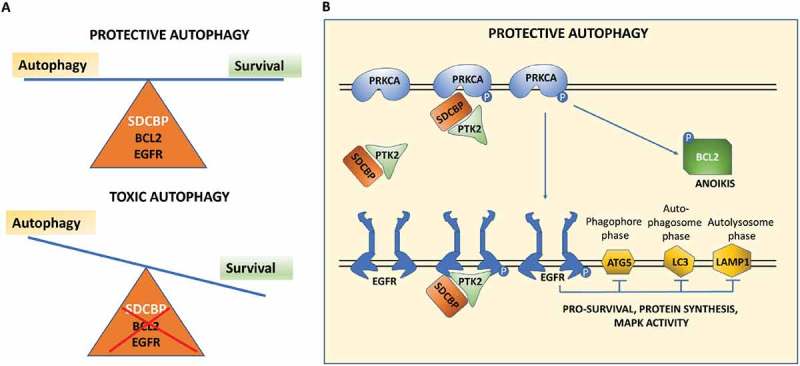
Representative model of SDCBP/MDA-9/Syntenin-mediated protective autophagy in anoikis-resistant GSCs. (A) SDCBP/MDA-9/Syntenin maintains protective autophagy in anoikis-resistant GSCs, whereas its loss deregulates this balance. (B) Schema of the multiple interconnected pathways that SDCBP/MDA-9/Syntenin regulates to maintain protective autophagy and anoikis-resistance in GSCs.
Funding Statement
This work was supported by the National Cancer Institute [P30 CA016059]; National Foundation for Cancer Research; Virginia Commonwealth University [Genetics Enhancement fund]; Virginia Commonwealth University [VCU Institute of Molecular Medicine].
Acknowledgments
Support was provided by the National Foundation for Cancer Research (P.B.F. and W.K.C.), the Virginia Commonwealth University Institute of Molecular Medicine (P.B.F.), and the Genetics Enhancement Fund (to P.B.F., S.K.D., and L.E.). Microscopy was performed at the Virginia Commonwealth University Department of Anatomy & Neurobiology Microscopy Facility, supported in part by funding from NIH-National Institute of Neurological Disorders and Stroke Center Core Grant 5 P30 NS047463 and, in part, by funding from NIH-National Cancer Institute (NCI) Cancer Center Support Grant P30 CA016059. The Virginia Commonwealth University Massey Cancer Center (MCC) Flow Cytometry Shared Resource, supported in part with funding from NIH-NCI Cancer Center Support Grant P30 CA016059 (to P.B.F. and D.S.), generated services and products in support of the research project. Histology services and products in support of the research project were generated by the MCC Mouse Model Shared Resource, supported in part with funding from NIH-NCI Cancer Center Support Grant P30 CA016059 (to J.J.W., P.B.F., and D.S.). P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research in the MCC. Services in support of the research project provided by the VCU Massey Cancer Center Tissue and Data Acquisition and Analysis Core, were supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
Disclosure statement
P.B.F. and W.K.C. are cofounders of InVaMet Therapeutics, Inc. P.B.F., W.K.C., Virginia Commonwealth University, and the Sanford-Burnham-Prebys Medical Discovery Institute own stock in InVaMet Therapeutics, Inc.
References
- [1].Talukdar S, Pradhan AK, Bhoopathi P, et al. MDA-9/Syntenin regulates protective autophagy in anoikis-resistant glioma stem cells. Proc Natl Acad Sci U S A. 2018;115(22):5768–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]


