Abstract
Physical frailty is an age-associated syndrome of decreased reserve leading to vulnerability to physiological stressors and associated with negative outcomes. The underlying structural brain abnormalities of physical frailty are unclear. We investigated the association between brain volume, cortical brain infarcts, and physical frailty. In this multicenter study, 214 nondemented participants were classified as frail (n = 32), prefrail (n = 107), or nonfrail (n = 75) based on the Fried frailty phenotype. The associations between frailty and brain volumes and cortical brain infarcts were investigated by linear or logistic regression analyses. Participants in the frail group showed a lower total brain volume (−19.67 mL [95% confidence interval −37.84 to −1.50]) and lower gray matter volume (−12.19 mL [95% confidence interval −23.84 to −0.54]) compared to nonfrail participants. Frailty was associated with cortical brain infarcts [frail 16% [n = 5], prefrail 11% [n = 12], and nonfrail 3% [n = 2]). Reduced total brain volume and gray matter volume and increased cortical brain infarcts seem therefore to be part of the structural substrate of the physical frailty phenotype.
Keywords: Physical frailty, Aging, Neurodegenerative brain changes, Neurovascular brain changes, Magnetic resonance imaging
1. Introduction
Frailty is defined as an age-associated biological syndrome of decreased reserve that leads to a vulnerability to physiological stressors (Clegg et al., 2013, Fried et al., 2001). Frail individuals have an increased risk of adverse events, such as hospitalization, falls, institutionalization, and complications after surgery including postoperative delirium (Brown et al., 2016, Fried et al., 2001). Frailty is most often described using the physical frailty phenotype (Buta et al., 2016, Fried et al., 2001). This phenotype is assessed with 5 frailty components: slowness, weakness, exhaustion, weight loss, and a low level of activity (Fried et al., 2001). A combination of 3 or more of these components classifies an individual as frail.
Previous studies showed that physical frailty is associated with an increased risk of cognitive decline and dementia (Avila-funes et al., 2012, Buchman et al., 2014, Solfrizzi et al., 2013). These findings may suggest that neurodegenerative or neurovascular changes are the structural substrate of the physical frailty phenotype. However, only few small studies have assessed the underlying structural brain magnetic resonance imaging (MRI) correlates of physical frailty. These studies have shown that signs of neurodegenerative or neurovascular changes, that is, lower global or regional brain volume, a higher number of cerebral microbleeds, and a higher burden of white matter hyperintensities of presumed vascular origin (WMH), were related to frailty in older individuals (Avila-funes et al., 2012, Avila-funes et al., 2017, Chen et al., 2015, Chung et al., 2016, Del Brutto et al., 2017, Jung et al., 2014, Newman et al., 2001, Siejka et al., 2017). These studies were however limited to community-dwelling individuals and included only a low number of frail individuals. Knowledge on the biological basis and development of physical frailty could lead to strategies to prevent dependence and eventually reduce the burden on an economic, societal, and individual level. To date, it is unknown if brain alterations are already present in prefrail individuals. Furthermore, the association between cortical brain infarcts and physical frailty has never been investigated.
The aim of the present study was to investigate differences in brain volumes, WMH, and cortical brain infarcts in physical frail, prefrail, and nonfrail older nondemented individuals who were scheduled for elective surgery. In addition, we studied the relation between these brain markers and individual frailty components.
2. Methods
2.1. Study design and participants
This investigation is part of the BioCog consortium study: an ongoing multicenter prospective cohort study performed in the Charité Universitätsmedizin Berlin and the University Medical Center Utrecht. The general aim of the BioCog study is to identify determinants of perioperative neurocognitive disorders (Winterer et al., 2018). For the BioCog study, participants were included who were (1) scheduled for major elective surgery of a minimum of 60 minutes, (2) at least 65 years of age, (3) able to undergo cognitive tests (no blindness, deafness, neurological or psychiatric diseases) and MRI scanning, and (4) had a Mini–Mental State Examination (MMSE) score of 24 or higher. The present study uses data from the first n = 400 participants of the BioCog consortium study. All participants signed an informed consent form, and all procedures were approved by the medical ethics committee of both centers under ethical approval number EA2/092/14 (Berlin) and 14-469 (Utrecht).
2.2. Procedure
All participants were invited before surgery for a visit that included questionnaires, a frailty assessment, and an MRI scan. Trained researchers collected data on age, gender, body mass index (BMI), diabetes, smoking, and history of cardiovascular events. All participants were assessed with the MMSE (Folstein et al., 1975) to determine preoperative cognitive status. An MMSE score of 24 or higher was considered as absence of severe dementia. The American Society of Anesthesiologists classification was assessed in a preoperative interview by an anesthesiologist (in training) (Dripps et al., 1961).
2.3. Frailty assessment
Frailty was assessed by trained researchers based on a modified version of the Fried frailty phenotype by Rockwood et al. and consisted of 5 frailty components: slowness, weakness, exhaustion, weight loss, and a low level of activity (Fried et al., 2001, Rockwood et al., 2007); see Supplementary Table A for a detailed description of these components. Participants who had a combination of 3 or more components were considered frail, participants who had a combination of 1 or 2 components were considered prefrail, and participants who had none of these components were considered nonfrail.
2.4. MRI scans
Participants were scanned on a Siemens Magnetom TrioTim MRI scanner (Berlin) or a Philips Achieva 3T MRI scanner (Utrecht). The MRI scanning protocol was standardized and consisted of a 3-dimensional (3D) T1-weighted sequence (voxel size =1.0 × 1.0 × 1.0 mm3; Berlin: 3D T1 magnetization-prepared rapid acquisition gradient echo sequence, repetition time [TR]/echo time [TE] = 2500/4.77 ms; Utrecht: TR/TE = 7.9/4.5 ms) and a fluid-attenuated inversion recovery (FLAIR) sequence (Berlin: TR/TE/inversion time = 4800/388/1800 ms; voxel size = 0.49 × 0.49 × 1.00 mm3; Utrecht: TR/TE/inversion time = 4800/125/1650 ms; voxel size = 1.11 × 1.11 × 0.56 mm3).
2.5. MRI processing steps and analysis
A robust approach to brain segmentation of multicenter data was used (Heinen et al., 2016, Mendrik et al., 2015). 3D FLAIR images were registered to the 3D T1-weighted images by using statistical parametric mapping software (SPM12, Wellcome Institute of Neurology, University College London, UK, http://www.fil.ion.ucl.ac.uk/spm/doc/), running on Matlab R2013a (Mathworks, Natick, MA, USA). WMH segmentations were performed on the FLAIR scans by the lesion prediction algorithm (Schmidt, 2017) as implemented in the Lesion Segmentation Toolbox version 2.0.15 (www.statistical-modeling.de/lst.html) for SPM12. All resulting WMH segmentations were visually checked for segmentation errors by trained researchers (I.M.J.K., and E.A.) and in doubt by a radiologist (J.B.) with 10 years of experience in brain segmentation. WMH segmentations were thresholded on a 0.5 probability, and WMH volumes were calculated using the Lesion Segmentation Toolbox. Lesion filling was performed on the 3D T1-weighted images by using the WMH segmentations. The resulting “lesion filled” 3D T1-weighted images were subsequently segmented in the CAT12 toolbox for SPM12 (Gaser and Dahnke, Jena University Hospital, Departments of Psychiatry and Neurology, http://www.neuro.uni-jena.de/cat/index.html#About). This resulted in segmentations of gray matter, white matter, and cerebrospinal fluid. Intracranial volume (ICV), total brain volume, gray matter volume, white matter volume, and cerebrospinal fluid volume were calculated by the SPM12 option “tissue volumes.” All scans were checked by a neuroradiologist (J.B.) for presence of cortical brain infarcts and major artifacts that might hinder accurate segmentations. Subsequently, all brain tissue segmentations were visually checked for segmentation errors (e.g., registration errors, wrong classification of tissue) by a trained researcher (I.M.J.K.). All cases that contained errors were discussed in a consensus meeting with an expert neuroradiologist (J.B.). All final decisions on exclusion of MRI data were made in this consensus meeting. All scans that contained cortical brain infarcts over 1.5 cm were excluded from the WMH and brain volume analysis because of segmentation errors. We have used the threshold of 1.5 cm based on the standards for reporting vascular changes on neuroimaging (STRIVE) criteria for large subcortical infarcts (Wardlaw et al., 2013). Brain surfaces were estimated by a fully automated method that estimates cortical thickness and the reconstruction of the central surface in 1 step (Gaser and Dahnke, 2012). To allow intersubject analysis, a spherical map was plotted and images were smoothed by a 15-mm Gaussian kernel.
2.6. Statistical analysis
Demographic variables were compared between the 3 groups (frail, prefrail, and nonfrail) by a one-way ANOVA or chi-square test depending on the type of variable. For analysis of brain volumes, participants with cortical brain infarcts over 1.5 cm were excluded. To study differences in brain volumes (total brain volume, gray matter volume, white matter volume, and WMH volume) between frail, prefrail, and nonfrail participants, linear regression analyses were performed adjusted for age, gender, ICV, and study center. Analyses of WMH volume were additionally corrected for vascular risk factors (hypertension, hypercholesterolemia, smoking, BMI, history of cardiovascular events, and diabetes). For the assessment of the difference in presence of cortical brain infarcts over 1.5 cm between frail, prefrail, and nonfrail participants, logistic regression analyses were performed with the presence of a cortical brain infarct as the dependent variable, adjusted for age, gender, and study center. Analyses of brain volume differences and WMH differences per frailty component were performed by linear regression analyses corrected for age, gender, ICV, and study center. Presence of cortical brain infarcts was analyzed by a logistic regression analysis corrected for age, gender, and study center. All regression analyses of global brain volumes, WMH, and cortical brain infarcts were performed by using IBM SPSS version 21.
For analysis of cortical thickness differences between frail, prefrail, and nonfrail participants, a linear regression model was implemented in CAT12 and SPM12, with age, gender, and center as covariates. All cortical thickness analyses were family-wise error corrected and thresholded at p < 0.05. A cluster was considered significant at a minimal amount of 86 vertices, based on the expected number of voxels per cluster.
3. Results
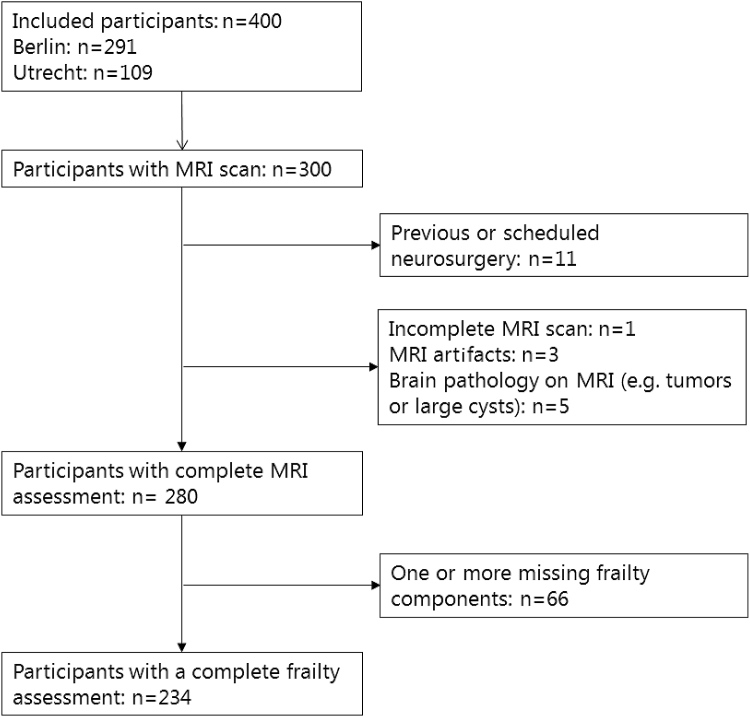
Of the initial 400 included participants in the BioCog study, 300 completed the preoperative MRI scanning protocol. Of these participants, 20 had to be excluded because of a brain tumor, previous trauma, MRI artifacts, or an incomplete MRI scanning protocol (see Fig. 1). Furthermore, 66 participants had one or more missing frailty components and were thus excluded. This resulted in inclusion of 214 participants (mean age 72.4 years [SD: 4.9 years]; 37% female) in the present study (see Fig. 1). In total, 32 participants (15%) were classified as frail (3 or more frail components), 107 participants (50%) were classified as prefrail (1 or 2 frail components), and 75 participants (35%) were classified as nonfrail (no frail components). Frail participants were older [F(2,211)=3.98, p < 0.05; planned contrasts revealed that frail and prefrail participants were significantly older than nonfrail participants {t(211)=2.42, p < 0.05} and that frail participants were significantly older than prefrail participants {t(211)=2.21, p < 0.05}], more often female [χ2(2)=7.90, p < 0.05], and had higher preoperative American Society of Anesthesiologists scores [χ2(4)=12.39, p < 0.05] compared to prefrail and nonfrail participants (see Table 1 for demographics). The frailty groups showed no differences in vascular risk factors. Patients with cortical brain infarcts over 1.5 cm (n = 19) were excluded from the brain volume analysis due to segmentation errors. The demographics of the brain volume analysis group after exclusion of participants with cortical brain infarcts were comparable to the original group (for demographics of the brain volume analysis group, see Supplementary Table B).
Fig. 1.
Flowchart representing the included participants. MRI, magnetic resonance imaging.
Table 1.
Demographics
| Total (N = 214) | Frail (n = 32) | Prefrail (n = 107) | Nonfrail (n = 75) | p-value | |
|---|---|---|---|---|---|
| Age | 72.4 ± 4.9 | 74.7 ± 5.4 | 72.3 ± 5.0 | 71.6 ± 4.5 | 0.020 |
| Female gender | 80 (37%) | 19 (59%) | 37 (35%) | 24 (32%) | 0.019 |
| MMSE | 29 (28, 30) | 28 (27, 29) | 29 (28, 30) | 29 (28, 30) | 0.021 |
| Center | 0.11 | ||||
| Utrecht | 74 (35%) | 13 (41%) | 42 (39%) | 19 (25%) | |
| Berlin | 140 (65%) | 19 (59%) | 65 (61%) | 56 (75%) | |
| ASA score (n = 211)∗ | 0.015 | ||||
| I | 12 (6%) | 0 (0%) | 4 (4%) | 8 (11%) | |
| II | 134 (64%) | 18 (58%) | 64 (60%) | 53 (71%) | |
| III | 65 (31%) | 13 (42%) | 38 (36%) | 14 (19%) | |
| Vascular risk factorsa | |||||
| Diabetes | 24 (11%) | 4 (13%) | 12 (12%) | 8 (12%) | 0.22 |
| BMI | 27 (24, 29) | 29 (26, 33) | 26 (24, 29) | 27 (24, 28) | 0.42 |
| Hypertension | 128 (60%) | 22 (69%) | 66 (62%) | 40 (53%) | 0.11 |
| Hyperlipidemia | 54 (25%) | 10 (31%) | 30 (28%) | 14 (19%) | 0.09 |
| Current smoker | 27 (13%) | 2 (6%) | 15 (14%) | 10 (13%) | 0.46 |
| Self-reported previous cardiovascular events | 6 (3%) | 2 (6%) | 2 (2%) | 2 (3%) | 0.04 |
| Frailty components | |||||
| Slowness | 52 (24%) | 25 (78%) | 27 (25%) | 0 (0%) | |
| Weakness | 63 (29%) | 22 (69%) | 41 (38%) | 0 (0%) | |
| Weight loss | 14 (7%) | 4 (13%) | 10 (8%) | 0 (0%) | |
| Exhaustion | 48 (22%) | 23 (72%) | 25 (23%) | 0 (0%) | |
| Mobility | 82 (38%) | 30 (94%) | 52 (49%) | 0 (0%) | |
Data represent n (percentage), mean ± SD, or the median (interquartile range). A one-way ANOVA comparison of 3 groups was performed on continuous data. A chi-square comparison of 3 groups was performed for categorical data.
In preoperative ASA scores, 2 values were missing; therefore, a percentage of n = 212 participants was calculated.Key: ASA, American Society of Anesthesiologists; BMI, body mass index; MMSE, Mini–Mental State Examination; SD, standard deviation.
In vascular risk factors, 3 values were missing; therefore, a percentage of n = 211 participants was calculated. Previous cardiovascular events include previous stroke and cortical brain infarcts.
3.1. Brain volumes and frailty
Table 2 shows the analyses on the association between total brain volume, gray matter volume, white matter volume, WMH volume, and frailty (see Supplementary Table B). Participants in the frail group showed a lower total brain volume (−19.67 mL [95% confidence interval {CI} −37.84, −1.50]) and lower gray matter volume (−12.19 mL [95% CI −23.84, −0.54]) compared to participants in the nonfrail group. Participants in the frail group further showed a lower total brain volume (−22.40 mL [95% CI −40.38, −4.41]) and lower gray matter volume (−12.26 mL [95% CI −23.09, −1.43]) compared to participants in the prefrail group. No significant differences were found between the prefrail and nonfrail group in total brain volume (1.16 mL [95 % CI −10.78, 13.11]), gray matter volume (−0.43 (95% CI −8.11, 7.25)), white matter volume (1.59 [95% CI −7.09, 10.27]), and WMH volume (0.32 [95% CI −0.21, 0.84]) (see Table 2). A larger WMH volume was found in the frail group (9.53 ± 13.13 mL) compared to the nonfrail group (3.36 ± 3.43 mL), but this difference was not statistically significant (0.45 [95% CI −0.25, 0.93]) (see Table 2). Secondary analyses with additional adjustment for vascular risk factors (hypertension, hypercholesterolemia, smoking, BMI, history of cardiovascular events, and diabetes) showed an unchanged, nonsignificant association between WMH volume and physical frailty (β = 0.52 [95% CI −0.01, 1.04], p = 0.05; see Table 2). Analysis of cortical thickness showed a lower global cortical thickness in frail participants compared to prefrail and nonfrail participants. However, no association between regional cortical thickness and physical frailty was found as no clusters remained significant after family-wise error correction for multiple comparisons and cluster size.
Table 2.
Brain volumes and regression analyses of frail, prefrail, and nonfrail participants
| Total brain volume | Gray matter volume | White matter volume | WMH volume | WMH volume model 2 | |
|---|---|---|---|---|---|
| Brain volumes | |||||
| Frail | 1024.78 ± 100.72 | 556.64 ± 55.66 | 468.15 ± 55.56 | 9.53 ± 13.13 | |
| Prefrail | 1047.10 ± 103.58 | 567.88 ± 51.89 | 479.22 ± 59.55 | 5.81 ± 7.25 | |
| Nonfrail | 1060.47 ± 110.22 | 572.45 ± 57.92 | 488.01 ± 58.20 | 3.36 ± 3.43 | |
| Regression analyses | |||||
| Frail versus nonfrail | −19.67 (−37.84, −1.50)a | −12.19 (−23.84, −0.54)a | −7.48 (−20.72, 5.75) | 0.45 (−0.25, 0.93)b | 0.52 (−0.01, 1.04) |
| Frail versus prefrail | −22.40 (−40.38, −4.41)a | −12.26 (−23.09, −1.43)a | −10.14 (−24.05, 3.77) | 0.32 (−0.21, 0.84) | 0.42 (−0.12, 0.95) |
| Prefrail versus nonfrail | 1.16 (−10.78, 13.11) | −0.43 (−8.11, 7.25) | 1.59 (−7.09, 10.27) | 0.11 (−0.23, 0.44) | 0.15 (−0.21, 0.50) |
Brain volumes are in mL and are represented as mean ± SD. Regression analysis adjusted for age, gender, intracranial volume, and study center. Regression beta coefficients are presented with a 95% confidence interval. WMH volumes were multiplied by 100 and natural log–transformed before performing regression analyses. WMH volumes model 2 are regression beta coefficients that were additionally corrected for cardiovascular risk factors (hypertension, hypercholesterolemia, BMI, diabetes, previous cardiovascular events, and smoking).
Key: BMI, body mass index; SD, standard deviation; WMH, white matter hyperintensities of presumed vascular origin.
p-value < 0.05.
p-value = 0.06.
3.2. Cortical brain infarcts and frailty
In total, 19 participants (9%) had cortical brain infarcts over 1.5 cm (see Table 3). The infarct prevalence was associated with frailty status: frail (16%; n = 5), prefrail (11%; n = 12), and nonfrail (3%; n = 2). One of the participants with a cortical brain infarct had reported a previous cardiovascular event (a transient ischemic attack); this participant was classified as frail. All other participants therefore had silent cortical infarcts. None of these participants has reported a previous myocardial infarction. Although participants in the frail group had more cortical brain infarcts compared to those in the nonfrail group, this did not reach statistical significance (OR = 4.14 [95% CI 0.63, 27.40]; see Table 3). Participants in the prefrail group showed a higher odds ratio of having a cortical brain infarct compared to participants in the nonfrail group (OR = 4.66 [95% CI 1.00, 21.73]).
Table 3.
The presence of cortical brain infarcts in relation to physical frailty
| Cortical brain infarcts and physical frailty | |
|---|---|
| Presence of cortical brain infarcts | |
| Frail | 5 (16%) |
| Prefrail | 12 (11%) |
| Nonfrail | 2 (3%) |
| Logistic regression analyses | |
| Frail versus nonfrail | 4.14 (0.63, 27.40) |
| Frail versus prefrail | 1.48 (0.45, 4.84) |
| Prefrail versus nonfrail | 4.66 (1.00, 21.73)a |
Data on presence of cortical brain infarcts over 1.5 cm are presented as n (percentage). Logistic regression analysis adjusted for age, gender, and study center. Corrected odds ratios are presented with a 95% confidence interval.
p-value = 0.05.
3.3. Brain volumes, cortical brain infarcts, and individual frailty components
Analyses on individual frailty components across groups and brain volumes, and cortical brain infarcts are shown in Table 4. In these analyses, all groups (frail, prefrail, and nonfrail) were combined and assessed per component. Most frailty components were associated with brain volumes and occurrence of cortical brain infarcts, but these associations did not reach statistical significance (see Table 4).
Table 4.
Brain volume changes and presence of cortical brain infarcts per frailty component for the total group
| Total brain volume (N = 195) | Gray matter volume (n = 195) | White matter volume (n = 195) | WMH volume (n = 195) | Cortical brain infarcts (n = 234)a | |
|---|---|---|---|---|---|
| Slowness | β = −12.60 (−25.91, 0.70) | β = −5.63 (−14.02, 2.75) | β = −6.97 (−16.77, 2.83) | β = 0.23 (−0.14, 0.60) | OR = 2.28 (0.84, 6.23) |
| Weakness | β = −8.08 (−20.75, 4.59) | β = −4.39 (−12.34, 3.57) | β = −3.69 (−13.01, 5.63) | β = 0.01 (−0.34, 0.36) | OR = 1.31 (0.48, 3.59) |
| Weight loss | β = −18.18 (−39.84, 3.47) | β = −9.12 (−22.74, 4.50) | β = −9.06 (−25.01, 6.88) | β = 0.24 (−0.36, 0.84) | -b |
| Exhaustion | β = −2.94 (−16.56, 10.68) | β = −2.93 (−11.47, 5.61) | β = −0.01 (−10.01, 9.99) | β = 0.36 (−0.02, 0.73) | OR = 2.02 (0.73, 5.56) |
| Mobility | β = −4.27 (−15.80, 7.25) | β = −5.23 (−12.43, 1.97) | β = 0.96 (−7.51, 9.42) | β = −0.01 (−0.33, 0.31) | OR = 1.78 (0.68, 4.65) |
Linear regression analysis on the association between individual frailty components and total brain volume, gray matter volume, white matter volume, and WMH volume per frailty component adjusted for age, gender, intracranial volume, and study center. Regression beta coefficients are presented with a 95% confidence interval.
Key: WMH, white matter hyperintensities of presumed vascular origin.
Logistic regression analysis on cortical brain infarcts adjusted for age, gender, and study center. Adjusted odds ratios are presented with a 95% confidence interval.
There were no participants who fulfilled the criterion for weight loss and had a cortical brain infarct over 1.5 cm.
4. Discussion
We investigated differences in brain volumes and cortical brain infarcts between frail, prefrail, and nonfrail older individuals in a large group of older individuals. We showed that frail individuals had more neurodegenerative and neurovascular abnormalities compared to prefrail and nonfrail individuals. Frail individuals had a significantly lower total brain volume and lower gray matter volume and showed a trend for more cortical brain infarcts and a higher WMH volume compared to nonfrail individuals. Frail individuals showed a lower global cortical thickness compared to prefrail and nonfrail individuals; however, no regional clusters of a lower cortical thickness were found. Individual frailty components showed a relation with a lower global gray matter volume, but this did not reach statistical significance. Furthermore, prefrail individuals had more cortical brain infarcts compared to nonfrail individuals.
Quantification of global and regional brain atrophy as an MRI marker for neurodegenerative diseases is widely performed in research on cognitive impairment and dementia (Mak et al., 2015, Verlinden et al., 2017). However, only few studies have performed these analyses in relation to frailty (Chen et al., 2015, Del Brutto et al., 2017). These studies included a low number of frail individuals and thus combined prefrail and frail individuals in 1 group, possibly reducing the contrast between groups (Chen et al., 2015). Furthermore, the method of determining frailty differed between studies. Some studies have used the physical frailty phenotype and others the Edmonton Frail Scale, which combines physical and cognitive frailty scores (Fried et al., 2001, Rolfson et al., 2006, Savva et al., 2013). This does not allow direct comparison of results between these studies. In line with the results of Chen et al., we showed an association between physical frailty and a lower gray matter volume (Chen et al., 2015). We additionally showed an association between physical frailty and a lower total brain volume and gray matter volume. Chen et al. previously showed that distinct patterns of regional (mainly cerebellar) gray matter volume changes were associated with individual frailty components and with physical frailty. Our results confirm their findings by showing an association of frailty and of all individual components with global gray matter volume changes. However, in contrast to previous findings, analysis of regional cortical thickness showed no significant regional differences in cortical thickness, which is possibly due to the heterogeneous nature of physical frailty.
WMHs are common in older individuals and are an MRI marker of cerebral small vessel disease (Wardlaw et al., 2013). WMHs are related to cognitive decline and physical deterioration such as gait problems (De Laat et al., 2011, Inzitari et al., 2009, Van Dijk et al., 2008). To date, only few studies have investigated the association between WMH and physical frailty (Avila-funes et al., 2017, Chen et al., 2015, Chung et al., 2016, Del Brutto et al., 2017, Siejka et al., 2017), of which only 2 studies have assessed WMH quantitatively (Avila-funes et al., 2017, Chen et al., 2015). The association between physical frailty and WMH is unclear, as 3 previous studies found no association between WMH and frailty (Chen et al., 2015, Chung et al., 2016, Del Brutto et al., 2017), and only one study did find an association (Avila-funes et al., 2017, Siejka et al., 2017). We found a trend for an association between increased WMHs in the frail versus the nonfrail group. Because of the large variability in WMH volumes between individuals, future studies with larger patient groups are needed to prove a potential association between WMH volume and physical frailty. Alternatively, other MRI methods that can investigate the structural integrity of the white matter, such as diffusion tensor imaging, may be performed to detect differences in white matter integrity between frail, prefrail, and nonfrail (Avila-funes et al., 2017).
Cortical brain infarcts are an MRI marker of large vessel disease and are associated with cognitive decline (Aggarwal et al., 2012). Only one study has specifically explored the association between brain infarcts and physical frailty (Newman et al., 2001). In this study, the presence of brain infarcts greater than 3 mm was associated with physical frailty; however, the authors did not distinguish different infarct types according to underlying pathophysiology (i.e., cortical, subcortical, or lacunar brain infarcts) (Newman et al., 2001). Another investigation that assessed physical and cognitive frailty in a combined way showed that brain infarcts occurred more frequently in frail compared to prefrail and nonfrail individuals (Del Brutto et al., 2016). Our investigation is the first to assess the association between a physical frailty state and the presence of cortical brain infarcts. Our results confirm the relation between physical frailty and cerebral infarcts, which was shown in previous studies (Del Brutto et al., 2017, Newman et al., 2001). In addition, we have shown an association between a physical prefrailty state and the presence of cortical brain infarcts and a nonsignificant increase in presence of cortical brain infarcts between prefrail and frail participants.
A limitation of our study may be between-center differences in MRI-based brain volumes. To minimize the effect of these possible between-center differences, MRI protocols were made comparable, and we used a brain segmentation pipeline that is relatively robust for between-center differences (Heinen et al., 2016). We also used “center” as a covariate in the analyses. This approach minimized the between-center differences in brain volumes. Another limitation may be that all participants were scheduled for major elective surgery. Therefore, we cannot rule out a possible association between the reason for elective surgery (e.g., total hip replacement) and components of physical frailty. Finally, our study is limited by its cross-sectional design. Therefore, we are unable to assess whether smaller brain volume measures were already present before development of the frailty phenotype. A strength of our study is the inclusion of a large group of participants, which enabled the separate analysis of frail, prefrail, and nonfrail individuals. This allowed us to study prefrail individuals separately, which is a group that has received almost no previous attention. Furthermore, the physical frailty phenotype is the most frequently used method to classify frailty in research and clinical practice (Buta et al., 2016). Therefore, use of this method enables comparison of our results with recent literature and gives us insight into the underlying mechanism of this clinical concept.
In conclusion, individuals with physical frailty showed lower global brain volumes and lower global gray matter volumes compared to prefrail and nonfrail individuals. Individuals with physical frailty and prefrailty also showed more cortical brain infarcts compared to nonfrail individuals. These brain changes could be the underlying substrate of the physical frailty phenotype.
Disclosure statement
The authors have no actual or potential conflicts of interest.
Acknowledgements
The research leading to these results has received funding from the European Union funded seventh framework research program (FP7 2007–2013) under grant agreement no. 602461/HEALTH-F2-2014-60246, BioCog (Biomarker Development for Postoperative Cognitive Impairment in the Elderly), www.biocog.eu. The research of Jeroen Hendrikse has received funding from the European Research Council under the European Union's Horizon 2020 Programme (H2020)/ERC grant agreement no. 637024 (HEARTOFSTROKE).
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.neurobiolaging.2018.06.032.
Appendix A. Supplementary data
References
- Aggarwal N.T., Schneider J.A., Wilson R.S., Beck T.L., Evans D.A., Carli C.D. Characteristics of MR infarcts associated with dementia and cognitive function in the elderly. Neuroepidemiology. 2012;38:41–47. doi: 10.1159/000334438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-funes A., Carcaillon L., Helmer C., Carrie I., Ritchie K., Rouaud O. Is frailty a prodromal stage of vascular Dementia ? Results from the three-city study. J. Am. Geriatr. Soc. 2012;60:1708–1712. doi: 10.1111/j.1532-5415.2012.04142.x. [DOI] [PubMed] [Google Scholar]
- Avila-funes J.A., Pelletier A., Meillon C., Periot O., Trevin I., Gonzalez-colaço M., Dartigues J., Pérès K., Allard M., Dilharreguy B., Nacional I., Médicas D.C. Vascular cerebral damage in frail older Adults ;: the AMImage study editor. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72:971–977. doi: 10.1093/gerona/glw347. [DOI] [PubMed] [Google Scholar]
- Brown C.H., Max L., Laflam A., Kirk L., Gross A., Arora R., Neufeld K., Hogue C.W., Walston J., Pustavoitau A. The association between preoperative frailty and postoperative delirium after cardiac surgery. Anesth. Analg. 2016;123:430–435. doi: 10.1213/ANE.0000000000001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A.S., Yu L., Wilson R.S., Boyle P.A., Schneider J.A., Bennett D.A. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014;69:1536–1544. doi: 10.1093/gerona/glu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buta B.J., Walston J.D., Godino J.G., Park M., Kalyani R.R., Xue Q.-L., Bandeen-Roche K., Varadhan R. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res. Rev. 2016;26:53–61. doi: 10.1016/j.arr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.-T., Chou K.-H., Liu L.-K., Lee P.-L., Lee W.-J., Chen L.-K., Wang P.-N., Lin C.-P. Reduced cerebellar gray matter is a neural signature of physical frailty. Hum. Brain Mapp. 2015;36:3666–3676. doi: 10.1002/hbm.22870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C.P., Chou K.H., Chen W.T., Liu L.K., Lee W.J., Chen L.K., Lin C.P., Wang P.N. Cerebral microbleeds are associated with physical frailty: a community-based study. Neurobiol. Aging. 2016;44:143–150. doi: 10.1016/j.neurobiolaging.2016.04.025. [DOI] [PubMed] [Google Scholar]
- Clegg A., Young J., Iliffe S., Rikkert M.O., Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laat K.F., Tuladhar A.M., Van Norden A.G.W., Norris D.G., Zwiers M.P., De Leeuw F.E. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134:73–83. doi: 10.1093/brain/awq343. [DOI] [PubMed] [Google Scholar]
- Del Brutto O.H., Mera R.M., Cagino K., Fanning K.D., Milla-Martinez M.F., Nieves J.L., Zambrano M., Sedler M.J. Neuroimaging signatures of frailty: a population-based study in community-dwelling older adults (the Atahualpa Project) Geriatr. Gerontol. Int. 2017;17:270–276. doi: 10.1111/ggi.12708. [DOI] [PubMed] [Google Scholar]
- Dripps R., Lamont A., Eckenhoff J. The role of anesthesia in surgical mortality. JAMA. 1961;178:261–266. doi: 10.1001/jama.1961.03040420001001. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., McBurnie M.A. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Gaser C., Dahnke R. CAT - a Computational Anatomy Toolbox for the Analysis of Structural MRI Data. 2012;32:7743. HBM Conf 2012. [Google Scholar]
- Heinen R., Bouvy W.H., Mendrik A.M., Viergever M.A., Biessels G.J., de Bresser J. Robustness of automated methods for brain volume measurements across different MRI field strengths. PLoS One. 2016;11:1–16. doi: 10.1371/journal.pone.0165719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzitari D., Pracucci G., Poggesi A., Carlucci G., Barkhof F., Chabriat H., Erkinjuntti T., Fazekas F., Ferro J.M., Hennerici M., Langhorne P., O’Brien J., Scheltens P., Visser M.C., Wahlund L.-O., Waldemar G., Wallin A., Pantoni L. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ. 2009;339:b2477. doi: 10.1136/bmj.b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H., Kim S.-W., Yoon S.-J., Choi J.-Y., Kim K., Kim C.-H. Associations between frailty, retinal microvascular changes, and cerebral white matter abnormalities in Korean older adults - letter to the editor. Am. J. Geriatr. Psychiatry. 2014;62:2210–2212. doi: 10.1111/jgs.13114. [DOI] [PubMed] [Google Scholar]
- Mak E., Su L., Williams G.B., Watson R., Firbank M., Blamire A.M., O’Brien J.T. Longitudinal assessment of global and regional atrophy rates in Alzheimer’s disease and dementia with Lewy bodies. Neuroimage. Clin. 2015;7:456–462. doi: 10.1016/j.nicl.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrik A.M., Vincken K.L., Kuijf H.J., Breeuwer M., Bouvy W.H., De Bresser J., Alansary A., De Bruijne M., Carass A., El-Baz A., Jog A., Katyal R., Khan A.R., Van Der Lijn F., Mahmood Q., Mukherjee R., Van Opbroek A., Paneri S., Pereira S., Persson M., Rajchl M., Sarikaya D., Smedby Ö., Silva C.A., Vrooman H.A., Vyas S., Wang C., Zhao L., Biessels G.J., Viergever M.A. MRBrainS challenge: online evaluation framework for brain image segmentation in 3T MRI scans. Comput. Intell. Neurosci. 2015;2015:1–16. doi: 10.1155/2015/813696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman a B., Gottdiener J.S., Mcburnie M.A., Hirsch C.H., Kop W.J., Tracy R., Walston J.D., Fried L.P. Associations of subclinical cardiovascular disease with frailty. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- Rockwood K., Andrew M., Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- Rolfson D.B., Majumdar S.R., Tsuyuki R.T., Tahir A., Rockwood K. Validity and reliability of the Edmonton frail scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva G.M., Donoghue O.A., Horgan F., O’Regan C., Cronin H., Kenny R.A. Using timed up-and-go to identify frail members of the older population. Journals Gerontol. Ser. A Biol. Sci. Med. Sci. 2013;68:441–446. doi: 10.1093/gerona/gls190. [DOI] [PubMed] [Google Scholar]
- Schmidt P. PhD thesis, Ludwig-Maximilians-Universität München; 2017. Chapter 6.1: Bayesion inference for structured additive regression models for large-scale problems with applications to medical imaging. Available at: http://nbn-resolving. de/urn:nbn:de:bvb:19-203731. Accessed January 2017. [Google Scholar]
- Siejka T.P., Srikanth V.K., Hubbard R.E., Moran C., Beare R., Wood A., Phan T., Callisaya M.L. Frailty and cerebral small vessel disease: a cross-sectional analysis of the tasmanian study of cognition and gait (TASCOG) J. Gerontol. A Biol. Sci. Med. Sci. 2017;73:255–260. doi: 10.1093/gerona/glx145. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V., Scafato E., Frisardi V., Seripa D., Logroscino G., Maggi S., Imbimbo B.P., Galluzzo L., Baldereschi M., Gandin C., Di A., Inzitari D. Frailty syndrome and the risk of vascular dementia : the Italian longitudinal study on Aging. Alzheimer’s Dement. 2013;9:113–122. doi: 10.1016/j.jalz.2011.09.223. [DOI] [PubMed] [Google Scholar]
- Van Dijk E.J., Prins N.D., Vrooman H.A., Hofman A., Koudstaal P.J., Breteler M.M.B. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam scan study. Stroke. 2008;39:2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- Verlinden V.J.A., van der Geest J.N., Hofman A., Niessen W.J., van der Lugt A., Vernooij M.W., Ikram M.A. Brain MRI-markers associate differentially with cognitive versus functional decline leading to dementia. J. Am. Geriatr. Soc. 2017;65:1258–1266. doi: 10.1111/jgs.14775. [DOI] [PubMed] [Google Scholar]
- Wardlaw J.M., Smith E.E., Biessels G.J., Cordonnier C., Fazekas F., Frayne R., Lindley R.I., O’Brien J.T., Barkhof F., Benavente O.R., Black S.E., Brayne C., Breteler M., Chabriat H., DeCarli C., de Leeuw F.E., Doubal F., Duering M., Fox N.C., Greenberg S., Hachinski V., Kilimann I., Mok V., Oostenbrugge R., Van Pantoni L., Speck O., Stephan B.C.M., Teipel S., Viswanathan A., Werring D., Chen C., Smith C., van Buchem M., Norrving B., Gorelick P.B., Dichgans M. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G., Androsova G., Bender O., Boraschi D., Borchers F., Dschietzig T.B., Feinkohl I., Fletcher P., Gallinat J., Hadzidiakos D., Haynes J.D., Heppner F., Hetzer S., Hendrikse J., Ittermann B., Kant I.M.J., Kraft A., Krannich A., Krause R., Kühn S., Lachmann G., Montfort S.J.T., Van Müller A., Nürnberg P., Ofosu K., Pietsch M., Pischon T., Preller J., Renzulli E., Scheurer K., Schneider R., Slooter A.J.C., Spies C., Stamatakis E., Volk H.D., Weber S., Wolf A., Yürek F., Zacharias N. Personalized risk prediction of postoperative cognitive impairment – rationale for the EU-funded BioCog project. Eur. Psychiatry. 2018;50:34–39. doi: 10.1016/j.eurpsy.2017.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.