Abstract
The fruit fly, Drosophila melanogaster, is a popular model organism for studying neurological processes and diseases due to the availability of sophisticated genetic tools. While endogenous neurotransmitter release has been characterized in Drosophila larvae, here, we measured endogenous dopamine release in isolated adult Drosophila brains for the first time. Dopamine was measured with fast-scan cyclic voltammetry (FSCV), and acetylcholine or nicotine were used as the stimulus, as both interact with nicotinic acetylcholine receptors (nAChRs) to evoke endogenous dopamine release. Stimulations with 10 pmol acetylcholine elicited 0.26 ± 0.05 μM dopamine while 70 fmol nicotine stimulations evoked 0.29 ± 0.03 μM in the central complex. Nicotine-stimulated dopamine release lasted much longer than acetylcholine-stimulated release. Dopamine release is reduced in the presence of nAChR antagonist α-bungarotoxin and the sodium channel blocker tetrodotoxin, indicating release is mediated by nAChRs and exocytosis. The identity of dopamine was confirmed by using 3-iodotyrosine, a dopamine synthesis inhibitor, and by confirming that release was not changed in octopamine synthesis mutant flies, Tdc2RO54. Additionally, the half decay time (t50) in fumin (67 ± 15 s), dopamine transporter mutant flies, was larger than in wild type flies (16 ± 3.7 s) further proving that acetylcholine stimulation evokes dopamine release. This study demonstrates that stimulation of nAChRs can be used to elicit endogenous dopamine release in adult fly brains, which will be a useful technique for future studies probing dopamine changes during aging or in neurodegenerative diseases.
For TOC Only

Introduction
The fruit fly, Drosophila melanogaster, is a versatile model system for the investigation of fundamental neurological processes, especially related to disease, because it can be easily genetically manipulated.1–3 Similar to mammals, fruit flies utilize neurotransmitters, such as dopamine, serotonin, acetylcholine, and glutamate to regulate a wide range of complex behaviors such as aggression, flight navigation, learning, and memory.4,5 In contrast to mammals, fruit flies employ octopamine, which performs analogous functions to mammalian norepinephrine.6 The fruit fly genome has been completely sequenced and approximately 75% of human diseases genes have a counterpart in the Drosophila genome.7 Drosophila is a valuable model system to study human neurodegenerative disease, such as Parkinson’s and Alzheimer’s disease, and has been used to elucidate the molecular and genetic mechanisms of disease progression. Nevertheless, the study of changes in neurochemicals in fly disease models is still not well understood. There is a lack of techniques to measure endogenous neurotransmission in adult flies, which is necessary to follow the age-related progression of neurodegenerative diseases.
Most of the neuroscience studies in Drosophila are performed with advanced imaging techniques, which allow researchers to identify and understand neuronal morphology and neuronal activities.8,9 However, to understand real-time changes in neurochemistry, electrochemical detection is typically used. Fast-scan cyclic voltammetry (FSCV) at a carbon-fiber microelectrode (CFME) has been used in larvae to measure stimulated dopamine, serotonin, or octopamine release.10–13 In adult flies, FSCV revealed rapid clearance of exogenously applied dopamine.14 Many of these studies relied on optogenetic stimulations, where an exogenous light-sensitive channel, such as CsChrimson or channelphodopsin2, is expressed in specific cells using the Gal4/UAS system. Optogenetics is a good tool for precise control of neuronal firing, but it has been difficult to implement in adult fly models and requires genetic manipulation of the flies. Methods to evoke neurotransmitters without the need to express an exogenous channel would be useful because they are easier to employ in genetically-modified fly models of disease.
In vertebrates, acetylcholine regulates dopamine release by directly activating nicotinic acetylcholine receptors (nAChRs)15 and is associated with depression16 and neurodegenerative diseases, such as Parkinson’s disease.17 Similarly, in insects, acetylcholine is an abundant excitatory neurotransmitter that modulates neural activity.18 Nicotine, a natural insecticide, is biosynthesized in the tobacco plant and activates nAChRs as well. Recently, we demonstrated that acetylcholine and nicotine can be used as natural stimuli to evoke endogenous dopamine release in larvae without further genetic manipulations.19 In addition, the Campusano group has measured nicotine-evoked octopamine release in adults with chronoamperometry.20 However, measurements of acetylcholine or nicotine evoked endogenous dopamine release have not been made in adult flies.
In this work, we evoked endogenous dopamine release in the central complex of isolated adult brains without genetically manipulating flies, using acetylcholine and nicotine as natural stimuli. The release of dopamine was confirmed by using a dopamine synthesis inhibitor 3-iodo-tyrosine (3-IT). The measured analyte was not octopamine, as there was no change in acetylcholine-stimulated release in octopamine synthesis mutant flies. Treatment with tetrodotoxin, a sodium channel blocker, and α-bungarotoxin, a nAChR antagonist, decreased stimulated release proving that acetylcholine stimulated dopamine release is exocytotic and mediated by nAChRs. Furthermore, evoked dopamine release and uptake were studied in fumin (fmn) flies, a dopamine transporter (DAT) mutant flies without functional DAT activity.21 Nicotine also evokes dopamine release, but the release lasts much longer than acetylcholine-stimulated release. This method will enable measurements of dopamine release in different life stages of any fly line, including genetically altered disease models.
Experimental Methods
Electrochemical measurements.
A detailed description of electrode fabrication methods can be found in SI. The dopamine waveform (−0.4 V to 1.3 V and back to –0.4V) was applied to the carbon-fiber microelectrode (7 μm diameter) every 100 ms at a scan rate of 400 V/s. Prior to the experiment, electrodes were calibrated with 1μM dopamine by using flow cell injection analysis, and this calibration factor was used to convert currents into concentrations. For picospritzing, an empty glass capillary was pulled, the tip was trimmed, and it was filled with 5 mM acetylcholine or 20 μM nicotine for stimulation. A Picospritzer III (Parker Hannfin, Fairfield, NJ) was used to pressure eject acetylcholine or nicotine into the brain tissue. Capillaries were calibrated by picospritzing a droplet in oil and the diameter of the pressure ejected droplet was measured using DS-Qi2 monochrome CMOS camera and NIS-Elements BR imaging software (Nikon Instruments Inc. Melville, NY). The amount of acetylcholine applied was controlled by changing ejected volumes, relying on different pulse durations, and applied pressure was maintained at 10 psi. For 10 pmol stimulation, 2 nL (~78 μm droplet radius, approximately 250 ms pulse, depending on pipette calibration) of 5 mM acetylcholine was applied. The fly brain is 80 nL in volume, so 2 nL is only 1/40 of the volume of the brain.22
Drosophila and Brain Tissue Preparation
The Canton-S (stock 64349) and UAS-GFP (stock 4776) were obtained from the Bloomington Stock Center (Indiana University, Bloomington, IN, USA). Th-Gal4, w1118, fumin, and Tdc2RO54 were provided by Dr. Jay Hirsh at the University of Virginia, Charlottesville, VA, USA. Drosophila stocks were maintained as described previously.13 Four to 10 day old adult Drosophila were anesthetized by rapid chilling; an empty Petri dish was placed in ice, and then flies were put on the chilled petri dish for 5 min. The anesthetized fly was placed in a chilled PBS dissecting buffer (4 °C) with 11.1 mM glucose and 5.3 mM trehalose. Then the fly was decapitated and the brain removed using fine tweezers under a dissecting stereoscope. The harvested brain was transferred to a Petri dish containing room temperature dissecting PBS. The brain tissue was immobilized by simply placing it at the bottom of the Petri dish, where it adheres. Brains were situated dorsal side up to provide easy access for the electrode and capillary injector. All the experiments were conducted under a SMZ800N stereoscope (Nikon Instruments Inc, NY) and brains were allowed to equilibrate 15 min prior to the experiment. The electrode and capillary injector were placed using a micromanipulator (Narishige International USA, Inc, Amityville, NY). The electrode was placed at the center of protecerebrum between antennal lobes, and inserted approximately 80 to 100 μm from the brain tissue surface. Then the capillary injector was placed approximately 10 to 15 μm laterally from the tip of the electrode, at the same depth.
Statistical Analysis
All statistics were performed using Graphpad Prism 7.02 (GraphPad Software, Inc., La Jolla, CA). Error bar represent mean ± standard error of the mean.
Results and Discussion
Endogenous Dopamine Release Evoked by Acetylcholine.
The objective of this study was to develop a method for endogenous stimulation of dopamine in isolated adult fly brains. Adult fly brains consist of the protocerebrum, the central brain involved with major fundamental processes, and two optic lobes, associated with the visual system, attached on either side of protocerebrum (Fig. 1A). The central complex is located in the central part of the protocerebrum and consists of a fan-shaped and ellipsoid body which are associated with complex behavior processes including learning, locomotion, and courtship.23 The central complex contains clusters of dopaminergic neurons, and was the target in this study for measurement of stimulated dopamine release.24
Figure 1.
(A) Schematic diagram of adult Drosophila Brain. This diagram labels the major regions of the adult brain. (B) Fluorescence microscopy image of adult fly brain (TH-Gal4:UAS-GFP) with GFP expressed in dopaminergic neurons in the protocerebrum. (C) Microscopy image of adult brain with a carbon-fiber microelectrode and capillary injector placement. Antennal lobes are marked with black dashed lines. All brain images are anterior side up.
Four to 10 day old adult flies were used for this study. To prove the tissue is viable in buffer for 2 hours, longer than a typical electrochemical experiment, we used calcein imaging, a live cell fluorescent marker.25 Fig. S1 shows that there is no difference in average fluorescent signal for calcein treated tissue 2 hours after dissection compared to that directly after dissection (0 min: 2290 ± 190 a.u.; 2 hrs: 3080 ± 340 a.u.; n=4, p = 0.1103, One-way ANOVA) and both values are higher than fluorescent intensity in fixed brain tissue (540 ± 54 a.u., n= 4, p< 0.01, One-way ANOVA). Thus, the harvested brain can be kept viable incubated in buffer for the time course of the experiment.
The isolated brain was placed anterior side up for easy access to the central complex (Fig. 1C). Initially, to place a microelectrode and a micropipette in the central complex, dopaminergic neurons were visualized using fluorescence imaging in TH-Gal4/UAS-GFP flies, expressing GFP in dopaminergic neurons (Fig. 1B). Microelectrodes were positioned in the medial dorsal part of the protocerebrum right above the antennal lobes (circled in dashed line in Fig. 1C) and inserted approximately 80 to 100 μm deep from the tissue surface. The micropipette, loaded with 5 mM acetylcholine, was inserted to the same depth and positioned approximately 10 to 15 μm away from the tip of the microelectrode. Once the investigators were good at placing the electrodes, the GFP was not necessary and most experiments were performed in Canton-S flies, a wild-type line that does not express GFP. The amount of acetylcholine pressure ejected was controlled by the pressure and duration of the injection, and volumes injected were calibrated for each pipette.
Acetylcholine stimulation was initially performed in adult flies with 10 pmol of acetylcholine pressure ejected into Canton-S wild type fly brains. A FSCV waveform of – 0.4 V to 1.3 V and back at 400 V/s was applied to the CFME at 10 Hz to measure stimulated dopamine release. In Fig. 2A, the false color plot shows all the data, with voltage on the y-axis, time on the x-axis, and current in false color. A cyclic voltammogram (insert), extracted at the vertical dashed line of the false color plot (bottom), shows oxidation and reduction peaks at 0.6 V and –0.2 V, respectively, the expected electrochemical signature of dopamine. Concentration vs. time traces (top) collected at the horizontal dashed line on the false color plot show dopamine rises immediately after acetylcholine stimulation and then is cleared in about 10 s. As a control, PBS was pressure ejected into the same brain, and it evoked no current responses or electrochemical signature of dopamine (Fig. 2B). These results confirm that exogenously applied acetylcholine stimulates dopamine release in adult Drosophila brain.
Figure 2.
Representative FSCV data of acetylcholine-stimulated dopamine release in adult Canton-S brains. (A) 10 pmol of acetylcholine was pressure injected (black arrow) to evoke dopamine release. False color plot (bottom), concentration vs. time traces (top), which sampled at the horizontal black dashed line, and cyclic voltammogram (insert), which sampled at the vertical black dashed line, indicate that dopamine was released upon the acetylcholine stimulation. (B) PBS was pressure ejected (black arrow) into the same area where the microelectrode was positioned. False color plot (bottom), concentration vs. time traces (top), and cyclic voltammogram (insert) verify that there is no characteristic of dopamine release upon PBS stimulation.
Optimization of Stimulation Parameters for Dopamine
To optimize stimulation parameters, first, the effect of different amounts of acetylcholine on dopamine release was studied by varying the pressure ejected volume. Figure 3A shows concentration vs. time traces of evoked dopamine release observed after 1, 3, 5, 10, 15, 20, or 40 pmol of acetylcholine stimulation. Averaged data show that the peak concentration of dopamine release increased linearly up to 10 pmol (Fig. 3B, n=8, R2= 0.95) and then plateaued after 15 pmol, likely because the entire readily releasable pool of dopamine was released. Also, dopamine clearance is slow for larger amounts of acetylcholine applied, and traces do not return to baseline. This effect could be due to dopamine adsorbing on the CFME surface, which sometimes causes a background shift and signals that do not return back to the baseline.26 It could also signify that uptake is slower with the larger amounts released, as dopamine transporters are fully saturated. For most studies, we used 10 pmol acetylcholine to maximize evoked dopamine, but to study changes in uptake, smaller amounts of acetylcholine are useful since the released dopamine was fully cleared.
Figure 3.
Effect of acetylcholine stimulating parameters on dopamine release. (A) Concentration vs. time traces of different amount of acetylcholine applied to evoke dopamine release. As the amount of pressure ejected acetylcholine increases, the signal takes longer to return to the baseline. (B) Average data of dopamine peak current vs different amounts of injected acetylcholine. The peak concentration of dopamine release increased linearly up to 10 pmol (n=8, R2 = 0.95) and then plateaued. (C) Stability of acetylcholine stimulated dopamine release. 10 pmol acetylcholine was applied at 5 or 10 min intervals. The peak oxidation current of dopamine release was normalized to the first stimulation for each experiment. For 10 min intervals, release did not significantly change (n=7, p = 0.458, two-way ANOVA, Sidak’s post-test), but for 5 min intervals, dopamine release significantly decreased after 7th stimulation (n=7, p < 0.005, Sidak’s post-test).
Evoked dopamine release needs to be stable throughout the experiment to differentiate changes during pharmacological studies. 10 pmol acetylcholine stimulations were repeated at 5 or 10 min intervals and currents normalized to the first stimulation. In Fig. 3C, a two-way ANOVA shows a significant effect of both the stimulation interval (F (1, 84) = 9.994, p = 0.0022) and the number of stimulations (F (6, 84) = 2.885, p = 0.0132). Post-tests indicate that dopamine release was significantly attenuated after 7th stimulation with 5 min intervals (n=7, p = 0.0028, Sidak’s post-test) but there were no significant changes with 10 min intervals (n=7, p> 0.05 for all Sidak’s post-tests). The decrease in signal with 5 min may be due to not enough time for the releasable dopamine pool replenishment or to time needed for cells to reset after the injection, but 10 min intervals were used in the following studies to obtain stable signals.
Acetylcholine evoked on average 0.26 ± 0.05 μM dopamine (n = 20) in isolated adult brains, about half that previously reported in the larval ventral nerve cord (VNC) (0.43 ± 0.04 μM).19 While the adult brain is bigger than the larval brain and has more dopaminergic neurons (130 compared to 70–90 in the larval CNS),24 the number of neurons that innervate the specific brain regions may be different and it is difficult to compare the density of dopaminergic terminals. Therefore, acetylcholine-stimulated dopamine release in adult brains might be lower if the particular region is smaller or if the region is less innervated with dopaminergic terminals.
Using optimized stimulating parameters, acetylcholine-stimulated dopamine release was compared between female and male flies. Dopamine release in 4–10 day-old flies was not significantly different for females and males (Fig. S2, female: 0.18 ± 0.04 μM, n=10; male: 0.34 ± 0.10 03bcM, n=10, p = 0.1754, t-test). Previous measurements found higher dopamine tissue content in adult females,27 but the acetylcholine stimulated release data trend in the opposite direction, perhaps due to different amounts in the releasable pool. Since there were no significant sex differences, results from both female and male adult flies were combined for future studies.
Pharmacological Characterization of Acetylcholine Stimulated Dopamine Release
There are two types of acetylcholine receptors: nicotinic and muscarinic acetylcholine receptors (mAChRs). nAChRs are pentameric ligand gated ion channels that are activated by acetylcholine and nicotine to trigger action potentials for rapid synaptic neurotransmission.28 In mammals, nAChRs are presynaptically expressed to regulate dopamine and other neurotransmitter release from nigrostriatal terminals.29–31 In Drosophila, nAChRs are abundantly expressed throughout the central nervous system.32–34 To demonstrate that dopamine release was mediated by nAChRs, we used α-bungarotoxin (α-BTX), a neurotoxin antagonist that is known to bind specifically to nAChRs but not mAChRs.
Dopamine release was measured before and after the brains were incubated in 2 μM α-BTX for 20 min (Fig. 4A). To quantify the concentration of evoked dopamine, the oxidation current was converted to the peak concentration by using an in vitro calibration factor. Evoked dopamine decreased about 50% after α-BTX incubation compared to pre-incubation (Fig. 4B, pre-drug, 0.46 ± 0.08 μM; post-drug, 0.22 ± 0.06 μM; p < 0.05, n=6, t-test). The dopamine release was not completely eliminated, similar to results in larvae where acetylcholine-stimulated dopamine release was reduced by almost 50% after α-BTX incubation,19 perhaps because α-BTX did not fully diffuse into the brain tissue within 20 min. However, the more likely explanation for the only 50% drop is that some nAChRs are not sensitive to α-BTX. In Drosophila, ten different types of nAChR subunits have been identified (Dα1 – Dα7 and Dβ1 – Dβ3), and receptors are pentamers with that are either homomeric, with all the same subunit type, or heteromeric, with different receptor subtypes.35,36 Dα5- Dα7 subunits are known to be α-BTX sensitive,35,36 but the subunit make-up of the nAChRs being activated here is not known. In larvae, dihydro β-erythrodine (DHβE) application, which specifically blocks nAChRs consisting of α4β2 subunits, the most abundant neuronal subunits, reduced dopamine release by 94%.19 While nAChRs with different subunits may play a role, these data show that nAChRs mediate the acetylcholine-stimulated dopamine release.
Figure 4.
Pharmacological characterization of evoked dopamine release. (A) Concentration vs. time trace of evoked dopamine release collected before and after 2 μM α-bungarotoxin (α-BTX) bath application. (B) The effect of α-BTX, a nAChR antagonist, on dopamine release. The peak concentration of dopamine release after α-BTX incubation was significantly lower than pre-incubation (pre-drug, 0.46 ± 0.08 μM; post-drug, 0.22 ± 0.06 μM; p < 0.05, n=6, t-test). (C) Concentration vs. time traces of dopamine release before and after 0.1 μM tetrodotoxin (TTX) incubation. (D) The effect of TTX, a voltage sensitive sodium channel blocker, on dopamine release. Dopamine release was completely abolished 20 min after TTX treatment, demonstrating that released dopamine is due to activity dependent exocytosis (pre-drug, 0.34 ± 0.05 μM; post-drug, 0 μM; p < 0.001, n = 5, t-test).
Tetrodotoxin (TTX) is a neurotoxin that blocks voltage gated sodium channels, disrupting the generation of action potentials that lead to vesicular release.37 After 20 min of incubation in 0.1 μM TTX, there is no acetylcholine-evoked dopamine release (Fig. 4C–D), as it dropped from 0.34 ± 0.05 μM pre-drug to 0 μM after TTX incubation (p < 0.001, n = 5, t-test). The TTX traces do have a negative deflection that occurs right after stimulation, likely a background subtraction error is due to ion movement. This phenomenon could be a background capacitance shift that is caused by the movement of ions near the electrode, likely when nAChRs open and cause an influx of cations into the cell. Similar observation was reported in patch-clamp recording study where negative current was observed with nicotine stimulation in the presence of TTX.38 In mammals, acetylcholine modulates dopamine release either by directly activating nAChRs on presynaptic dopamine neurons or by indirectly modulating glutamate release.39 Here, we cannot rule out the indirect pathways, however, the TTX data suggests that acetylcholine is acting presynaptically.
Validation of Acetylcholine Stimulated Dopamine Release
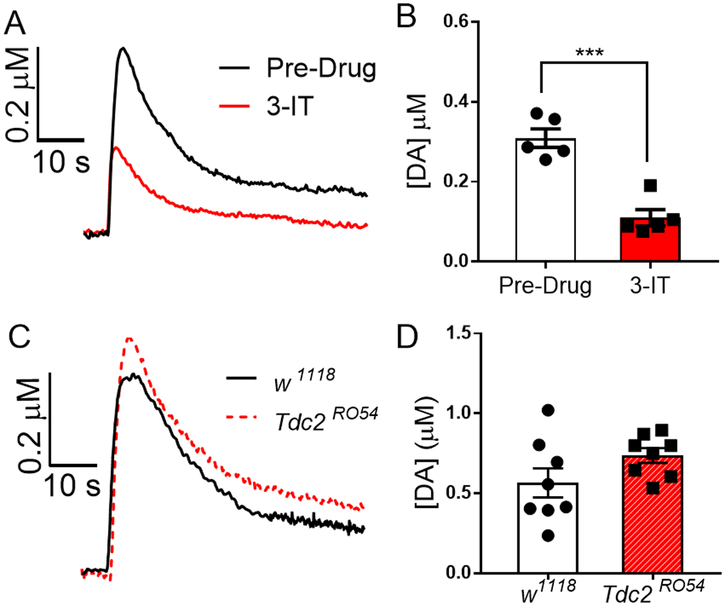
Although dopamine was identified as the released neurotransmitter via its cyclic voltammogram and possible interferents such as norepinephrine are not present in insects, we conducted further studies to prove the observed responses were due to dopamine release. First, we validated the released signal using 3-iodotyrosine (3-IT), an inhibitor of tyrosine hydroxylase, the rate-limiting enzyme of dopamine biosynthesis. Three acetylcholine stimulations were collected, then the isolated brain was incubated in 100 μM 3-IT for 20 min, and additional three stimulations were collected. The example data in Fig. 5A show that 3-IT application decreased dopamine release on average by 60% (Fig. 5B, pre-drug, 0.31 ± 0.02 μM; post-drug, 0.10 ± 0.02 μM; n=5, p<0.001, t-test). Control data of three measurements before and after 20 min PBS incubation show no significant differences in evoked dopamine release (Fig. S3). Therefore, 3-IT incubation decreases dopamine release in adults, consistent with reported observations in larvae where 3-IT incubation40 and feeding,19 lowered dopamine by 50 or 70%, respectively.
Figure 5.
Validation of stimulated dopamine release. (A-B). The effect of 100 μM bath application of 3-idotyrosine (3-IT), a dopamine synthesis inhibitor, on dopamine release. (A) Concentration vs. time trace of acetylcholine-stimulated dopamine release before and after 20 min 3-IT. (B) The peak concentration of the release after 3-IT was significantly lower (n=5, p<0.001, t-test) proving that the observed signal is due to dopamine release. (C-D). Measurements in octopamine synthesis mutant flies. (C) Concentration vs. time traces of acetylcholine-evoked dopamine release in Tdc2RO54 mutant flies, octopamine synthesis knock out flies, and in control flies, w1118. (D) The peak concentration of dopamine release in octopamine synthesis mutants is not significantly different than control (p= 0.11, t-test, n=8).
Evoked dopamine release was also studied in octopamine synthesis mutant flies because Campusano’s group had reported nicotine-stimulated octopamine release; thus we wanted to rule out that the release was due to octopamine.20 White-eyed w1118 were used as controls because that is the background strain for the mutant strain Tdc2RO54, octopamine synthesis mutant flies. Tdc2RO54 flies have a mutation in tyrosine decarboxylase (dTDC), the enzyme used to synthesize the octopamine precursor tyramine.6 If the observed signal was due to octopamine, the signal should be dramatically attenuated in Tdc2RO54 compared to w1118 but Fig. 5C shows that concentration vs. time traces are similar. The average dopamine peak concentration in Tdc2RO54 (0.74 ± 0.05 μM, n=8) is not significantly different than in w1118 (Fig. 5D, 0.57 ± 0.09 μM, n=8, p=0.11, t-test). Furthermore, release was tested in Canton-S flies with a waveform optimized for optogenetically-stimulated octopamine release (- 0.4V to 1.4 V at 100 V/s) in larval VNC.12 This waveform produces two characteristic oxidation peaks (the primary peak at 1.1V and the secondary peak at 0.56 V on anodic scan) for octopamine, but these peaks are not observed in Fig. S4B, indicating that release is not due to octopamine.
Campusanos’s group studied nicotine induced octopamine release in adult Drosophila brains using chronoamperometry.20 They positioned a Nafion-coated, 30 μm carbon-fiber electrode in the mid ventral side of fly brain where the ellipsoid body is located in the central complex. Then, large amounts of nicotine were applied at the brain surface and neurotransmitter efflux was measured in control and octopamine synthesis mutant flies, to prove it was octopamine. Thus, in different brain regions, nAChRs may activate other neurotransmitters besides dopamine. The advantages of our technique are that FSCV is better able to discriminate signals than chronoamperometry, our electrode is smaller and can localize in the central complex, and our stimulations were applied locally, rather than on the whole brain.
Characterization of Dopamine Clearance
The mechanisms of dopamine regulation, such as synthesis, exocytosis, and uptake, are conserved between human and flies.24 The dopamine transporter (DAT) regulates extracellular dopamine by uptake of released dopamine into presynaptic neurons; thus, it is essential for dopamine clearance. To validate the use of acetylcholine-stimulated release to study dopamine uptake in Drosophila, we compared acetylcholine-stimulated dopamine clearance in DAT mutant and control (Canton-S) flies. Canton-S, a strain control, and w1118 flies, the background strain for the genetic mutants, have similar amounts of dopamine release and clearance times with 10 pmol stimulations (Fig. S5).
Acetylcholine-evoked dopamine release was measured in fumin flies (fmn), lacking functional DAT activity.41,42 For 10 pmol acetylcholine, the concentration traces for dopamine release in fmn show slower uptake compared to Canton-S control flies (Fig. 6A), similar to the slow clearance rate of exogenously-applied dopamine in fmn larvae43 and adult flies.44 The uptake rate is determined using the half-decay time (t50), the time it takes to decrease from the maximum to half of the maximal concentration. As expected, the average value of t50 for dopamine in fmn flies (68 ± 15 s, n=6) is significantly larger than that in Canton-S flies (16 ± 3.7 s, n=6, p<0.01, t-test, Fig. 6B). However, the average dopamine peak concentration in fmn (0.39 ± 0.05 μM, n=6, n=8) is not different than in Canton-S with 10 pmol acetylcholine (0.33 ± 0.06 μM, n=6, p = 0.46, t-test, Fig. 6C). When 1 pmol acetylcholine was used for the stimulation, the concentration trace in fmn shows a slower uptake and higher dopamine release (Fig. 6D). Moreover, there are significant differences in both t50 (fmn, 10 ± 2.1 s, n=6; Canton-S, 3.7 ± 0.4 s, n=6, p<0.05, t-test, Fig.6E) and dopamine peak concentration (fmn, 0.20 ± 0.02 μM, n=6; Canton-S, 0.07 ± 0.02 μM, n=6, p<0.01, t-test, Fig. 6F). The discrepancy in the differences in peak concentration with different stimulations may be due to the amount available for release, as 10 pmol maximizes dopamine release. In rodents, adding DAT inhibitors, cocaine or amphetamine, increase dopamine concentration45,46 and in fly larvae, the DAT inhibitor nisoxetine also increased peak dopamine concentration.11 However, in DAT KO mice, in the presence of an uptake inhibitor, evoked dopamine release was not increased whereas the uptake was altered.47 Smaller amounts of acetylcholine may be more useful for ascertaining differences in concentration and uptake, but these studies overall demonstrate that acetylcholine-stimulated release can be measured in a mutant fly without the need for any other genetic modifications.
Figure 6.
Characterization of dopamine clearance in wild-type (Canton-S) and DAT mutant (fumin, fmn) flies using either 10 pmol or 1 pmol acetylcholine stimulations. (A) Concentration vs. time traces of 10 pmol acetylcholine-evoked dopamine release in Canton-S and fmn. (B) Half decay time (t50), a measure of dopamine clearance is significantly higher in fmn flies than that in Canton-S flies (n=6, p < 0.01, t-test). (C) Dopamine peak concentration in Canton-S and fmn is not significantly different with 10 pmol (n= 6, p = 0.07, t-test) (D). Concentration vs. time traces of 1 pmol acetylcholine evoked dopamine release in Canton-S and fmn. There are significant differences in (E) t50 (n=6, p<0.05, t-test) and (F) dopamine peak concentration (n=6, p<0.01, t-test) between Canton-S and fmn flies.
Nicotine-Stimulated Dopamine Release.
Acetylcholine is the natural agonist for nAChRs, but other agonists, such as nicotine, also activate the receptor. Nicotine is a natural insecticide synthesized in tobacco plants that acts on the reward system and is addictive. Nicotine, like acetylcholine, activates nAChRs to evoke dopamine release in mammals and in larval Drosophila.48 The amount of nicotine needed to evoke dopamine release was much smaller than that for acetylcholine, as fmol levels of nicotine were sufficient to activate nAChRs. Nicotine binds to nAChRs more tightly than acetylcholine and has a higher binding affinity (~nM) than acetylcholine (~μM).49,50
Figure 7A shows representative data of dopamine evoked by 70 fmol nicotine stimulation compared with dopamine evoked by 10 pmol acetylcholine. While the CV (Fig. 7B) has a reduction peak that is most consistent with dopamine release, nicotine is also reported to increase serotonin release in rats.51 Thus, to check if there was also serotonin release, we repeated experiments with a serotonin waveform that is selective for serotonin over dopamine.52 With nicotine stimulation, a small peak is observed at the potential for serotonin, although about half of the current may be due to nicotine itself producing a signal (nicotine does not produce any oxidation peak that looks like dopamine with the dopamine waveform, Fig. S6B). Thus, there may be a small contribution of serotonin, but dopamine is the primary molecule detected after nicotine release.
Figure 7.
Nicotine stimulated dopamine release (A) Representative concentration vs. time traces of nicotine (70 fmol) and acetylcholine (10 pmol)-stimulated dopamine release. Compared to acetylcholine-stimulated dopamine release, nicotine-stimulated release takes much longer to reach the maximal concentration and be cleared. (B) Cyclic voltammogram of nicotine-evoked release proves the response is due to dopamine release. (C) Representative data of different concentration traces of nicotine-stimulated dopamine release. (D) Effect of different amount of nicotine on dopamine release. The peak concentration increases linearly up to 25 fmol (R2 = 0.99, n=4) and plateaued after 50 fmol.
While the peak concentrations of dopamine evoked are similar for acetylcholine and nicotine, the traces look very different. Nicotine-evoked dopamine release increases slower and lasts longer than acetylcholine-stimulated dopamine release. In the example trace, acetylcholine-evoked dopamine reached maximum release 3.9 s after the stimulation compared to nicotine-evoked release, which reached the maximum release after 53 s. The nicotine-evoked trace is 240 s long and the dopamine level is still not back to baseline at that time. The same phenomenon was also observed in the larval VNC, where nicotine gave much longer lasting dopamine release than acetylcholine stimulations.19 For both larvae and adults, the time of stimulus application was similar for both stimuli (<1 s), so these differences are due to different interactions with the nAChRs and not due to a longer stimulation.
Various amount of nicotine were applied to evoke dopamine release. As higher amounts of nicotine were applied, it takes longer to reach the maximum release and the clearance is also takes longer (Fig. 7C). The peak concentration of dopamine release increases linearly up to 25 fmol nicotine (n=4, R2 = 0.99) then plateaus, similar to the shape of the acetylcholine graph (compare Fig. 7D with Fig. 3B). A similar result was reported in an electrophysiological study where dopaminergic neuron firing rate escalated as higher nicotine concentration was perfused over a rat brain slice, and then reached a plateau.53
Nicotine is considered a natural insecticide that kills insects by overstimulating dopamine release, and our work confirms a large amount of dopamine release with a small nicotine stimulation. There are several reasons that nicotine may give a longer lasticotine is ng signal than acetylcholine. First, in mammals, Yin et al. demonstrated that nicotine increased firing rates of dopamine neurons in the midbrain ventral tegmental area (VTA) for 30 s after stimulation, followed by a gradual return to the baseline. Thus, dopamine neuronal firing might last longer with nicotine. Second, in mammals, nicotine-induced dopamine release is due to exocytosis and nicotine can alter the size of readily releasable pool of synaptic vesicles by increasing the rate of vesicle mobilization.54 Third, nicotine might be metabolized or cleared slower from the tissue, compared to the natural neurotransmitter acetylcholine, which is rapidly cleared, and thus have prolonged interaction with nAChRs.55 Future work could investigate which mechanism is responsible for the higher release by nicotine in vivo. Nicotine is an important natural insecticide and there are synthetic neonicotinoid insecticides that mimic its binding, so insight into the mechanism of nicotine action in the fly brain is critical to provide understanding of nicotine binding sites, upregulation of nAChRs, and insect resistance to neonicotinoids.
Conclusions
In this study, we measured endogenously stimulated dopamine release in the central complex of adult Drosophila brain for the first time using acetylcholine or nicotine as stimuli. Nicotine-evoked release lasts much longer than acetylcholine-evoked release. One major advantage of this stimulation method is that it is natural and does not require any exogenous optogenetic channels to be expressed. Thus, this stimulation can be performed in various different genetic strains, as evinced with the experiments using the fumin flies that lack functional DATs. Evoked dopamine release was sensitive to α-bungarotoxin and tetrodotoxin, indicating it is mediated by nAChRs and is exocytotic. Acetylcholine and nicotine evoked stimulation will facilitate new measurements of endogenous dopamine adult flies, where it can be used to study age-related changes in dopamine neurotransmission and how dopamine change in neurodegenerative disease models.
Supplementary Material
Acknowledgments
Authors would like to thank Dr. Jay Hirsh at the University of Virginia for donating Drosophila stocks.
Funding
This work is funded by NIHR01MH085159 to BJV.
Footnotes
The authors declare no competing financial interest.
Supporting Information. These materials are available free of charge on the ACS publication website at http://pubs.acs.org.
Methods for electrochemical measurements and Calcein-AM imaging and chemical information; Average fluorescent intensity of brains treated with calcein; Acetylcholine evoked dopamine release comparison between female and male flies; FSCV measurements using octopamine waveform; Control experiments; Evoked dopamine release and clearance time comparison between different wildtypes; Nicotine stimulation using serotonin and dopamine waveform
References
- (1).Kaun KR; Devineni AV; Heberlein U Human Genetics 2012, 131, 959–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Jeibmann A; Paulus W International Journal of Molecular Sciences 2009, 10, 407–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Sokolowski MB Nature Reviews Genetics 2001, 2, 879–890. [DOI] [PubMed] [Google Scholar]
- (4).Shin M; Copeland JM; Venton BJ ACS Chemical Neuroscience 2018, DOI: 10.1021/acschemneuro.1027b00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Chen S; Lee AY; Bowens NM; Huber R; Kravitz EA Proceedings of the National Academy of Sciences 2002, 99, 5664–5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hardie SL; Zhang JX; Hirsh J Developmental Neurobiology 2007, 67, 1396–1405. [DOI] [PubMed] [Google Scholar]
- (7).Bier E Nature Reviews Genetics 2005, 6, 9–23. [DOI] [PubMed] [Google Scholar]
- (8).Karim MR; Moore AW Journal of Visualized Experiments: JoVE 2011, 57, 3111, DOI: 10.3791/3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Lemon WC; Pulver SR; Höckendorf B; McDole K; Branson K; Freeman J; Keller PJ Nature Communications 2015, 6, 7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Borue X; Cooper S; Hirsh J; Condron B; Venton BJ Journal of Neuroscience Methods 2009, 179, 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Vickrey TL; Condron B; Venton BJ Analytical Chemistry 2009, 81, 9306–9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Pyakurel P; Privman Champaloux E; Venton BJ ACS Chemical Neuroscience 2016, 7, 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Privman E; Venton BJ Journal of Neurochemistry 2015, 135, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Makos MA; Han K-A; Heien ML; Ewing AG ACS Chemical Neuroscience 2009, 1, 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Albuquerque EX; Pereira EF; Alkondon M; Rogers SW Physiological reviews 2009, 89, 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Shytle R; Silver A; Lukas R; Newman M; Sheehan D; Sanberg P Molecular Psychiatry 2002, 7, 525–535. [DOI] [PubMed] [Google Scholar]
- (17).Calabresi P; Picconi B; Parnetti L; Di Filippo M The Lancet Neurology 2006, 5, 974–983. [DOI] [PubMed] [Google Scholar]
- (18).Gundelfinger E; Schulz R In Neuronal nicotinic receptors; Springer, 2000, pp 497–521. [Google Scholar]
- (19).Pyakurel P; Shin M; Venton BJ Neurochemistry International 2018, 114, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Fuenzalida‐Uribe N; Meza RC; Hoffmann HA; Varas R; Campusano JM Journal of Neurochemistry 2013, 125, 281–290. [DOI] [PubMed] [Google Scholar]
- (21).Kume K; Kume S; Park SK; Hirsh J; Jackson FR Journal of Neuroscience 2005, 25, 7377–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Zheng Z; Lauritzen JS; Perlman E; Robinson CG; Nichols M; Milkie D; Torrens O; Price J; Fisher CB; Sharifi N BioRxiv 2017, 140905. [Google Scholar]
- (23).Chen A; Ng F; Lebestky T; Grygoruk A; Djapri C; Lawal HO; Zaveri HA; Mehanzel F; Najibi R; Seidman G Genetics 2013, 193, 159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Yamamoto S; Seto ES Experimental Animals 2014, 63, 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ross AE; Belanger MC; Woodroof JF; Pompano RR Analyst 2017, 142, 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Bath BD; Martin HB; Wightman RM; Anderson MR Langmuir 2001, 17, 7032–7039. [Google Scholar]
- (27).Denno ME; Privman E; Venton BJ ACS Chemical Neuroscience 2014, 6, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Karlin A In Handbook of Cell Signaling (Second Edition); Elsevier, 2009, pp 221–224. [Google Scholar]
- (29).Sher E; Chen Y; Sharples T; Broad L; Benedetti G; Zwart R; McPhie G; Pearson K; Baldwinson T; De GF Current Topics in Medicinal Chemistry 2004, 4, 283–297. [DOI] [PubMed] [Google Scholar]
- (30).Wonnacott S Trends in Neurosciences 1997, 20, 92–98. [DOI] [PubMed] [Google Scholar]
- (31).Wonnacott S; Kaiser S; Mogg A; Soliakov L; Jones IW European Journal of Pharmacology 2000, 393, 51–58. [DOI] [PubMed] [Google Scholar]
- (32).Grauso M; Reenan R; Culetto E; Sattelle D Genetics 2002, 160, 1519–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Collin C; Hauser F; de Valdivia EG; Li S; Reisenberger J; Carlsen EM; Khan Z; Hansen NØ; Puhm F; Søndergaard L Cellular and Molecular Life Sciences 2013, 70, 3231–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Silva B; Molina-Fernández C; Ugalde MB; Tognarelli EI; Angel C; Campusano JM Neural Plasticity 2015, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Wu P; Ma D; Pierzchala M; Wu J; Yang L-C; Mai X; Chang X; Schmidt-Glenewinkel T Journal of Biological Chemistry 2005, 280, 20987–20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Lansdell SJ; Collins T; Goodchild J; Millar NS BMC Neuroscience 2012, 13, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Goto T; Kishi Y; Takahashi S; Hirata Y Tetrahedron 1965, 21, 2059–2088. [DOI] [PubMed] [Google Scholar]
- (38).Coggan JS; Paysan J; Conroy WG; Berg DK Journal of Neuroscience 1997, 17, 5798–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Benarroch EE Neurology 2012, 79, 274–281. [DOI] [PubMed] [Google Scholar]
- (40).Xiao N; Venton BJ Journal of Neurochemistry 2015, 134, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Ueno T; Tomita J; Tanimoto H; Endo K; Ito K; Kume S; Kume K Nature Neuroscience 2012, 15, 1516–1523. [DOI] [PubMed] [Google Scholar]
- (42).Kume K Sleep and Biological Rhythms 2006, 4, 263–273. [Google Scholar]
- (43).Vickrey TL; Xiao N; Venton BJ ACS Chemical Neuroscience 2013, 4, 832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Makos MA; Kim Y-C; Han K-A; Heien ML; Ewing AG Analytical Chemistry 2009, 81, 1848–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Hoffman AF; Spivak CE; Lupica CR ACS Chemical Neuroscience 2016, 7, 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Daberkow D; Brown H; Bunner K; Kraniotis S; Doellman M; Ragozzino M; Garris P; Roitman M Journal of Neuroscience 2013, 33, 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Siciliano CA; Calipari ES; Ferris MJ; Jones SR Journal of Neuroscience 2014, 34, 5575–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Pidoplichko VI; DeBiasi M; Williams JT; Dani JA Nature 1997, 390, 401–404. [DOI] [PubMed] [Google Scholar]
- (49).Giorguieff-Chesselet M; Kemel M; Wandscheer D; Glowinski J Life Sciences 1979, 25, 1257–1261. [DOI] [PubMed] [Google Scholar]
- (50).Xiu X; Puskar NL; Shanata JA; Lester HA; Dougherty DA Nature 2009, 458, 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Takahashi H; Takada Y; Nagai N; Urano T; Takada A Synapse 1998, 28, 212–219. [DOI] [PubMed] [Google Scholar]
- (52).Jackson BP; Dietz SM; Wightman RM Analytical Chemistry 1995, 67, 1115–1120. [DOI] [PubMed] [Google Scholar]
- (53).Yin R; French ED Brain Research Bulletin 2000, 51, 507–514. [DOI] [PubMed] [Google Scholar]
- (54).Turner TJ Journal of Neuroscience 2004, 24, 11328–11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Self L; Guthrie F; Hodgson E Nature 1964, 204, 300–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.