Abstract
Rehabilitation of oral functions following surgery on the jaws is a goal that is often difficult to achieve. Removable dentures supported by remaining teeth or gum are often unstable and seldom satisfactory. On the other hand, endosseous (dental) implants offer a mechanism to provide stability to the dentures. This review, discusses factors related to the tumor, patient, treatment, and physicians which impact upon the feasibility and success of dental implants in patients with oral cancer.
Keywords: Oral rehab, Implants, oral cancer, mandible, rehabilitation, surgery
INTRODUCTION
The American Cancer Society estimates over 51,000 cases of cancer of the oral cavity and pharynx in 2018 in the United States. These comprise 3% of all cancers [1, 2]. It is estimated that there will be over 13,000 deaths from oral cancer this year leading to a mortality of 26%. The median survival time of patients dying from oral cancer is approximately 24 months [3]. In addition to threat to life, the aesthetic and functional consequences of oral cancer and its treatment are prohibitive and impact significantly on patient’s quality of life. These issues are challenging not only for the patients and their families but also for the healthcare providers. The ultimate goal of treatment of oral cancer is long term control of cancer and complete rehabilitation of all oral functions for an optimal quality of life. To achieve cancer control, surgery with or without post-operative radiotherapy or chemoradiotherapy remain the mainstay of treatment. The aesthetic and functional sequelae of these treatment approaches, particularly in advanced stage patients, impact upon the ability to speak, chew, swallow and create an esthetic impact on external appearance of the patient, leading to decline in the quality of life [4]. The ability to achieve total rehabilitation of all oral functions is, however dependent on tumor factors, patient factors, treatment related factors and physician factors. Tumor factors include the site and stage of disease while patient factors include age, life style habits, oral hygiene, status of dentition, status of the available bone and soft tissues following treatment in the oral cavity and overall prognosis of the patient. Treatment related factors include the impact of surgery and radiation with or without chemotherapy on all oral cavity structures, including the mandible, soft tissues and mucosa of the oral cavity as well as function of salivary glands. Physician related factors include the available expertise of the multidisciplinary team in providing cancer care and rehabilitative care of all oral functions. The purpose of this review is to define optimal oral rehabilitation and the factors that impact upon achieving this goal.
Optimal rehabilitation of the oral cancer patient
Complete rehabilitation of all oral functions following treatment for cancers in the oral cavity is a desirable but difficult goal to achieve in all patients. Early stage oral cancers are easily treated by simple surgery with minimal alteration in oral function. On the other hand, surgical treatment of advanced tumors alters the ability to produce clear speech, to retain normal mastication and normal swallowing. Although soft tissue reconstruction can replace volume loss of the tongue, the ability to articulate clear speech, chew normal foods and swallow all types of foods is difficult to achieve.
On the other hand, tumors that involve or are in the vicinity of the mandible or maxilla require resection of some part of the involved or adjacent bone [5]. These result in several deformities including aesthetic deformity, functional deformity due to loss of continuity of the mandibular arch and loss of dentition as well as loss of sensation to the teeth anterior to the site of mandible resection. Surgical reconstruction of the mandible often achieves the goal of restoration of acceptable external aesthetic appearance but does little for restoration of oral function. Historically loss of dentition due to any cause was restored by fabrication of a removable dental prosthesis. However, in the patient with oral cancer, rehabilitation with removable resection prosthesis is seldom satisfactory and rarely optimal due to the altered anatomy in the oral cavity [6]. Retention of the prosthesis can be improved with institution of endosseous (dental) implants [7]. Endosseous implants can be utilized in a variety of applications including fabrication of prosthetic teeth or as retaining elements for removable resection prosthesis. The ideal rehabilitation following treatment for advanced cancers include restoration of the external appearance of the patient, reconstruction of the mandibular arch and facial contour, retaining or restoring oral competency, restoring clarity of speech, restoring stable dentition to achieve the ability to chew all types of foods and preserving or restoring the ability to swallow. There is conflicting information in the literature, regarding success rates of implants, when placed immediately at the time of reconstruction, or secondarily, after complete healing of the reconstructed mandible with FFF. Fenlon et al, reported significant difficulties with immediate implants including implant failures, and complications [8]. On the other hand, a more recent report by Jackson et al, supports placement of immediate implants. In their study of 46 patients with primary and secondary implants, they did not report any difference in implant failure rate or complications in the two groups [9]. Thus, it would seem logical to consider primary implant placement in FFF to reduce, cost, multiple procedures, morbidity and early accomplishment of dental rehabilitation. However, there are several factors that impact upon selection of patients for implant based dental rehabilitation. These s are factors related to the tumor, the patient, the treatment given and finally the facilities available.
Tumor Factors
Early staged primary tumors (T1 and T2) can be managed easily with simple surgical resection without the need for any major reconstructive effort or adjuvant therapy. On the other hand, advanced cancers require major ablative surgery, reconstructive surgery and adjuvant therapy with radiation or chemoradiotherapy. Advanced tumors involving the mandible or adjacent to the mandible require either marginal or segmental mandibulectomy with loss of teeth and teeth bearing bone. These patients also need adjuvant radiation therapy with or without chemotherapy which will alter the status of intraoral mucosa and soft tissues as well as salivary function. Thus, in advanced staged patients, pre-operative treatment planning inclusive of the maxillofacial prosthodontics team, reconstructive surgery team and the ablative surgeons, should take place to devise a plan of resection, reconstruction and rehabilitation in concert to achieve the desired goal. In spite of all the coordinated extensive treatments offered, the long-term prognosis for patients with advanced stage tumors is guarded. The five-year disease specific survival is stage dependent as shown in Figure 1 from a recent study from our institution [10]. It is well known that the median survival time for patients with Stage IV oral cancer is approximately 2 years of which more than 6 months are consumed in implementation and completion of the required treatment for the primary cancer. Thus, the feasibility of meaningful long term oral rehabilitation to maximize patient quality of life may be achievable in only a minority of patients unless efforts are made to provide intraoral rehabilitation expeditiously.
Figure 1.
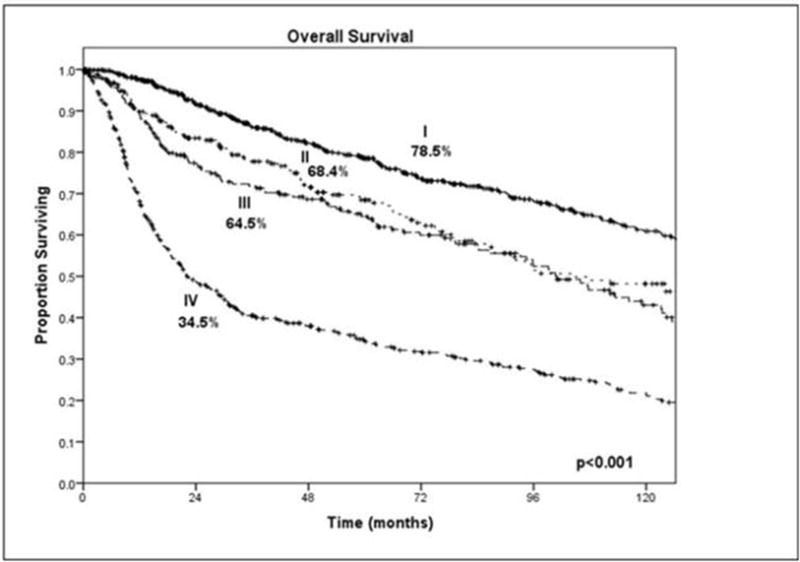
Clinical stage and overall survival. Courtesy of Memorial Sloan Kettering Cancer Center database, New York, NY
Patient Factors
Elderly and totally edentulous patients with a severely atrophic mandible are not candidates for any meaningful dental rehabilitation following ablative surgery for advanced oral tumors. An example of atrophic mandible (pipe stem mandible) is shown in Figure 2. These patients often lack sufficient residual bone to consider endosseous implants for fixed dentures and do not have adequate alveolar ridge or sulci to maintain conventional removable dentures. As a result, these patients will remain with a suboptimal oral function and thus a suboptimal quality of life. Many of these patients are able to consume only pureed foods through the mouth. In addition to this, elderly patients with other comorbidities are not good candidates for major ablative and reconstructive inclusive of surgery to achieve the desired goal of optimal oral rehabilitation. Life style including consumption of oral tobacco and alcohol, as well as poor dental hygiene also creates a milieu in the oral cavity not suitable for any meaningful dental rehabilitation [3].
Figure 2.
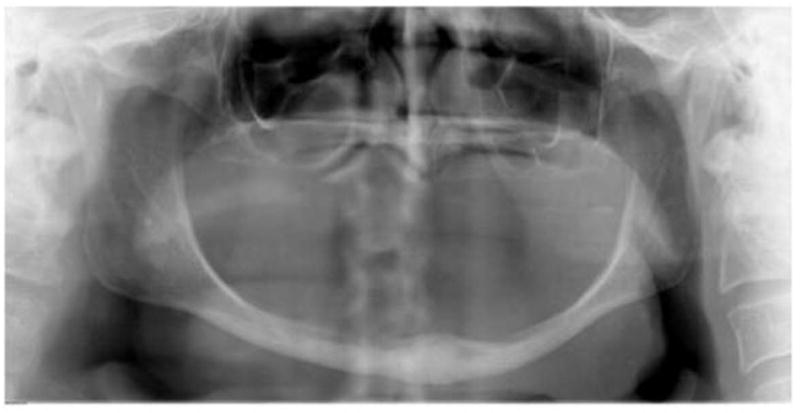
Panoramic radiograph of atrophic mandible in an edentulous patient
Treatment Factors
The treatment of advanced oral cancer requires a multidisciplinary approach with ablative surgery, often with reconstructive surgery followed by radiotherapy and chemotherapy in a variety of combinations. These treatment modalities heavily impact upon the aesthetic appearance of the patient as well as oral functions of clarity of speech, mastication and swallowing. Retention or restoration of oral competence, and dentition are also important factors in restoring oral function. Each of the treatment modalities mentioned, require detailed discussion as to their impact on oral function and the feasibility of achieving optimal oral rehabilitation.
Surgery
Surgical treatment of oral cancer may produce significant changes in the intra oral anatomy with loss of mucosal surface, soft tissue volume, effacement of the vestibular sulci and bone loss if any part of the mandible or maxilla is resected. Simple surgical excision of early stage oral cancer produces minimal soft tissue loss and results in very little functional disability. On the other hand, major glossectomies or major resection of floor of the mouth or buccal mucosa impacts upon the intra oral anatomy enough to make the retention of a dental prosthesis unstable. Thus, for mucosal or soft tissue volume loss appropriate reconstructive measures should be undertaken to bring in soft tissue volume and lining with a free flap. This will allow restoration of the mobility of the tongue and add soft tissue volume to permit mastication and swallowing. However, this does not address the issue of denture stability. In this setting consideration should be given to endosseous dental implants for stability and a permanent denture.
On the other hand, patients requiring resection of maxilla or mandible have altogether different considerations. Mandible resection may be in the form of a marginal mandibulectomy or a segmental mandibulectomy. Marginal mandibulectomy in the premolar and molar regions of the mandible causes effacement of the alveolar crest and loss of mandibular sulci making a removable dental prosthesis unstable. These patients are unable to have a satisfactory denture for mastication and swallowing. The feasibility of dental implants in this situation is also unlikely because of the lack of sufficient bone height above the mandibular canal to permit endosseous implants. An orthopantomogram of the mandible in a patient following marginal mandibulectomy in the lateral segment is shown in Figure 3, demonstrating inadequate height of the residual alveolar crest above the mandibular canal. A minimum of 1.5 mm distance between in the inferior alveolar canal and the implant is required to prevent any temporary or permanent neuropathy of the inferior alveolar nerve [11]. Thus, adequate residual bone is unlikely to be available in the posterior part of the mandible or the posterior part of the maxillary alveolus due to the proximity of the maxillary sinuses. These anatomical deficits in bone volume can be addressed through alveolar ridge or sinus augmentation or nerve translocation procedures depending upon the patient’s comorbidities and other medical considerations for intervention [12]. However, oral cancer patients have often received previous radiation therapy or are at high risk of local recurrence and hence are not considered good candidates for alveolar augmentation procedures [13].
Figure 3.
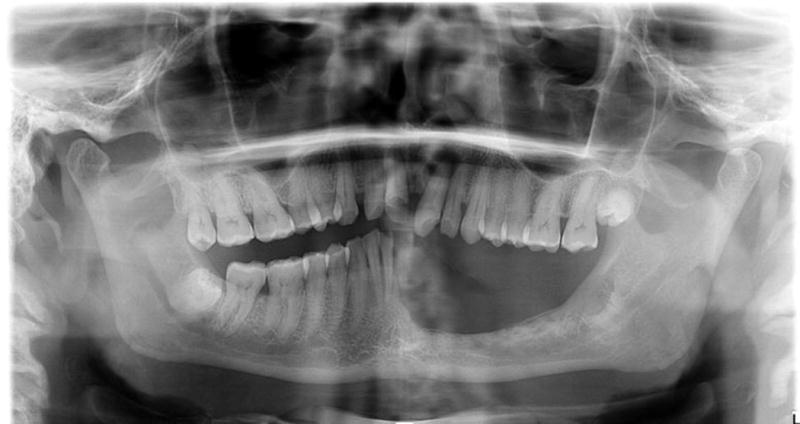
Panoramic radiograph of patient with left posterior marginal mandibulectomy, showing inadequate vertical height above the mandibular canal
On the other hand, marginal mandibulectomy in the anterior segment of the mandible between the mental foramina leaves sufficient vertical height permitting endosseous implants for a permanent fixed denture [Figure 4]. Therefore, if, eventual complete dental rehabilitation is the ultimate goal, in patients with posteriorly located lesions of the mandible, marginal mandibulectomy should not be considered and a segmental mandibulectomy with fibula free flap reconstruction should be planned for tumors located in the molar and premolar regions as endosseous implant placement can then be considered. Endosseous implants require adequate bone stock to completely accommodate their placement within the alveolar bony complex. The diameter (generally 3 to 5 mm) and length (generally 5 to 15 mm) of the implant should be selected with these considerations in mind. Fibula free flaps provide sufficient bone volume for dental implants [14]. On the other hand, radius or iliac osteocutaneous flaps often do not have sufficient bone volume for consideration of implants [15]. Endosseous implant placement surgery that is not completed during the primary reconstructive procedure should be deferred until after complete healing of the osteotomies in the bone flap as to not fracture the neo maxilla or neo mandible. But there are other considerations for secondary implants such as surgical cost and patient morbidity, with multiple surgical procedures. Considering the various factors that impact upon the status of the reconstructed mandible with a fibula free flap, such as postoperative radiotherapy, bone atrophy and the need for multiple procedures, only a minority of patients are suitable, and eventually do undergo secondary dental implants for permanent prosthesis [16]. Patients who have undergone maxillectomy may be candidates for craniofacial or maxillofacial implants to assist in retaining a maxillofacial prosthetic device. However, these implants are often retained in alternative bones of the craniofacial region such as the zygoma [17, 18].
Figure 4.
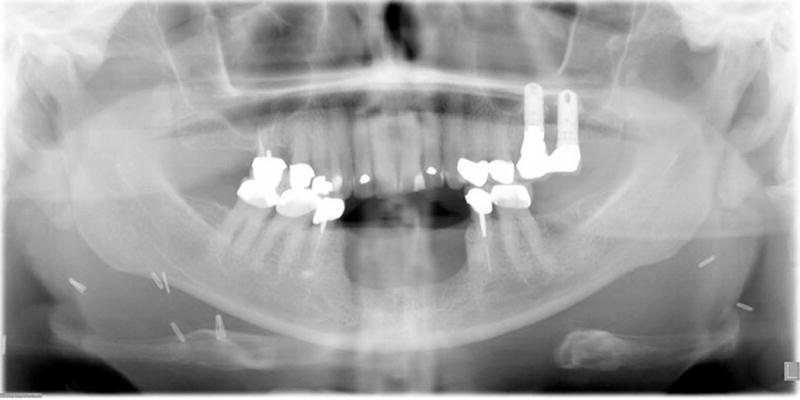
Panoramic radiograph of patient with anterior marginal mandibulectomy, showing adequate vertical height in the anterior segment after surgery
Radiotherapy
The target for delivery of radiation, type of radiation and dose of radiation are important factors when considering a patient who would be otherwise, suitable for optimal dental rehabilitation. Ionizing radiation causes endothelial damage to endosseous blood vessels in the irradiated bone and adjacent tissues. This leads to irreversible bone damage, leading to tissue hypoxia and a significant risk of osteoradionecrosis (ORN) following trauma or infection. Additionally, the patient’s natural process of tissue repair is also compromised [13, 16]. Therefore, the risk of performing surgery or invasive dental procedures in an irradiated bone raises the possibility of initiating osteoradionecrosis [19]. An example of a patient with an inflammatory process at the dental roots is shown in Figure 5a. Following extraction of the involved tooth, ORN has occurred at that site [Figure 5b]. The potential for osteoradionecrosis is dependent on the degree, progression and irreversibility of ischemic changes in the bone related to the dose and fractionation of radiation. The reported incidence of osteoradionecrosis of the mandible in the literature varies from 1% to 37.5%. Quite often management of radionecrosis requires surgical intervention. Clearly this depends on the extent of the necrotic bone. [20, 21, 22].
Figure 5.
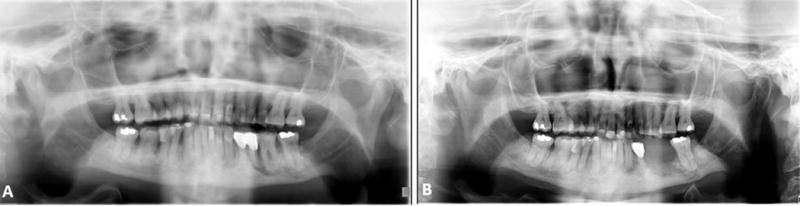
(a) Panoramic radiograph of patient with previously irradiated mandible showing inflammatory process at dental roots; (b) Panoramic radiograph following extraction of tooth and initiation of osteoradionecrosis.
The effects of radiotherapy are limited to the target portals and the delivered dose as well as fractionation. It is therefore imperative to review the treatment plan and dosimetry to assess the risk of ORN. Of particular interest is review of the PORT films to evaluate the areas at risk. Radiation doses more than 40 Gy increase the risk dental decay and caries, while dose in excess of 60 Gy is significantly associated with the risk of ORN [23, 24, and 25].
It is imperative that a careful review of the port films is undertaken while planning implant based dental rehabilitation. These images will demonstrate the radiated areas and the high-risk areas for ORN. This is critical in implant placement planning. In addition to the port films, review of the isodose curves on the treatment plan is important to assess how much radiation was delivered to adjacent tissue outside of the target area from the scatter effect. Such a review would be also important in implant planning. In general areas of the mandible beyond the radiation ports are considered safe for dental interventions. Contemporary techniques of radiation delivery such as intensity modulated radiotherapy (IMRT) or and use of proton beam therapy (PBRT) deliver precise dose to the target area and minimize excessive dose delivery to adjacent normal tissues [26, 27]. The assessment of the dosimetry to the adjacent tooth bearing bone is thus complicated and requires the assistance of a radiation oncologist and radiation physicist to specifically contour the area of interest to determine the dose delivered. Contouring data from our institution for patients treated with IMRT have shown that the dosimetric distribution to the tooth bearing region of the mandible is directly related to the tumor site and location of the tooth. An example of a patient with carcinoma of the base of the tongue treated with chemoradiotherapy is shown in Fig. 6a, which shows dose distribution in various parts of the mandible. Clearly the molar regions received the maximum dose, putting this region at risk for osteoradionecrosis. Development of sepsis in the last molar tooth initiates the process of radionecrosis. (Fig.6b). Once ORN sets in, the potential to reverse the process is nearly impossible. A pathological fracture, thru the area of necrosis is inevitable.
Figure 6.
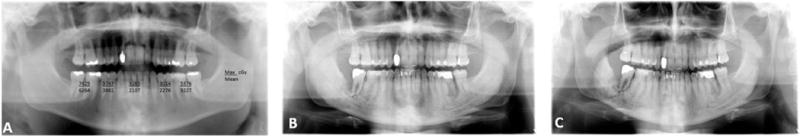
(a) Panoramic radiograph of the mandible of a patient treated with chemoradiotherapy for carcinoma of the base of the tongue. The mean and maximum dose delivery in various regions of the mandible is shown; (b) Initiation of osteoradionecrosis following extraction of the second molar tooth; (c) Pathological fracture thru the area of osteoradionecrosis.
(Fig.6c). Oropharynx (base of tongue and tonsil) tumors and tumors of the lower gingiva and floor of mouth in the oral cavity lead to delivery of highest radiation doses to the contralateral molar region. In this regard, proton beam therapy has the advantage of greater capability of sparing radiation delivery to adjacent normal tissues, since its penumbra is much sharper and smaller than photons. Thus, some radiation exposure is unavoidable to adjacent tissues [23]. In contrast, brachytherapy or administration of radioactive iodine are reasonably safe for implant placement since there is no high dose exposure to the adjacent mandible, depending on the site of the primary tumor. Thus, it is crucial that in planning implant placement, the multidisciplinary team be consulted, all radiation factors carefully reviewed and a risk assessment for ORN is considered. Needless to say, patients deemed at high risk are best rehabilitated by other means, avoiding implant placement and the potential development of ORN.
Chemotherapy and Immunotherapy
Cytotoxic chemotherapy is an important factor to consider in any oral surgery since it produces bone marrow suppression resulting in leukopenia by day 10 after the start of chemotherapy; thrombocytopenia after 10 to 14 days and anemia in a longer time frame [28]. These changes in peripheral blood may increase the risk of spontaneous or traumatically induced bleeding, at the site of the tumor or any dental intervention [29]. Therefore, all patients on chemotherapy should be screened for bleeding risk prior to consideration of any dentoalveolar surgery. Coagulation profile, including platelet count provides adequate information on platelet quantity and function [14]. Thus, all elective dental surgery should be deferred in patients receiving chemotherapy if the platelet count is < 100,000 /mm3 or if the patient has leucopenia of <1,000/mm3 [30].
Immunotherapy and biological therapy or biotherapy, are used in the prevention or treatment of disease. These agents stimulate the immune response. The experience with immunotherapeutic agents in head and neck cancer is relatively new, and there is not enough information in the literature at this time to assess the impact of immunotherapeutic agents on dental health. This is an area for future research as it relates to risk assessment for optimal dental rehabilitation, particularly with reference to endosseous implant placement.
Systemic therapy with antiresorptive medications
Patients with osteoporosis, and those with bone metastases, often receive antiresorptive medications such as bisphosphonates, to reduce the risk of fracture. However, they are at risk of antiresorptive agent-induced osteonecrosis of the jaw (ARONJ). Thus, any rehabilitative dental intervention such as implant surgery is largely contraindicated in this patient population. The incidence of ARONJ in patients taking oral antiresorptive agents such as Alendronate or Etidronate, for the management of osteoporosis is low (approximately 1: 100,000), in contrast to those receiving intravenous bisphosphonates (BPs) for the treatment of metastatic bone diseases [31, 32]. Highly potent agents such as Pamidronate and Zoledronate are used for bone remodelling [33]. They are used as intravenous infusions at regular intervals in patients with bone metastases. Their potency exceeds by a factor of 5 to 20 compared to oral agents [34, 35]. The half-life of these drugs ranges from 1-10 years, and therefore the extent and intensity of dental care remains the same in those patients who are currently on these drugs as those who have received these drugs in the past. The risk of ARONJ increases with the length of period for which the patient is on bisphosphonate therapy [36]. When a dental implant is placed in the mandible, a series of metabolic changes take place, eventually forming new bone which binds to the implant. If the bone adjacent to the implant contains medium to high levels of BPs, such bone turnover and remodeling is compromised leading to a high probability of osteonecrosis. Once that sets in, there is very little that can be done to avert or prevent progressive bone necrosis.
Physician Factors
Patients with oral cancer need multidisciplinary care to optimize their oncologic and functional outcomes. Therefore, a team of head and neck surgeons, reconstructive surgeons, radiation oncologists, medical oncologists, dental and prosthetic specialists, and rehabilitation specialists are necessary to being about the desired goal of optical outcome. For oral rehabilitation, dental surgeons with expertise in implantology, and speech and swallowing pathologists are crucial to optimize oral function and quality of life.
Current Status and Future possibilities
Long term cure of oral cancer is the fundamental goal of modern day multidisciplinary treatments of oral cancer. A second important goal however is complete rehabilitation of all oral functions, including external appearance, oral competency, clarity of speech and ability to chew and swallow all types of foods. Unfortunately, this goal is achieved in a minority of patients with advanced oral cancer. In the past when any part of the mandible is resected, oral rehabilitation was afforded with a removable denture. However, this has not been satisfactory in a substantial number of patients. Denture stability is a crucial factor to restore mastication, and clarity of speech. Dental implants can easily provide that. Unfortunately, dental implant placement is feasible after marginal mandibulectomy in only a few selected patients undergoing marginal mandibulectomy in the anterior segment of the mandible. The minimum height required for placement of implants in the lateral aspect of the mandible is 1.5 mms between the implant and the inferior alveolar canal. In a recent study of 52 patients at our institution the distance between the alveolar crest and the mandibular canal following marginal mandibulectomy ranged from 3.9 – 3.4 mms. This is clearly not adequate for implant placement because even the shortest implants (mini implants) are 6 mms long. On the other hand, the median height between the alveolar crest and the lower border of the mandible in the anterior segment after marginal mandibulectomy was 21.8 mms. But only 8 patients had marginal mandibulectomy in the anterior segment and were thus suitable for implant placement [37].
However, the risk of inducing osteoradionecrosis in the reconstructed mandible with a fibula free flap, due to previous radiotherapy is significantly high, and therefore a distinct minority of patients, ever get to that point [38]. From the 416 patients who had fibula free flap reconstruction following segmental mandibulectomy only 18 patients were felt suitable for secondary dental implants, in the last 24 years at our institution (1990-2014) [39]. All the patients who received post-operative radiotherapy were not felt suitable to receive dental implants due to the risk for osteoradionecrosis. Also excluded were patients who died within two years after surgery. With these exclusions, only 18 patients were felt suitable, and received secondary dental implants. From these, one patient dies within one year after implant placement. The implants were unsuccessful in two patients, both of whom had previous radiation. Further, the process of secondary dental implants is a long one, with multiple surgical procedures, hospitalizations, and financial burden. The median time to achieving satisfactory dental rehabilitation was 44 months, and the 11 % of the implants failed, requiring removal. On the other hand, primary implant placement during the time of tumor resection, either in native or reconstructed bone, offers an opportunity for implant supported restorations to the oral cancer patient. There are additional opportunities for research in this space as there is little data regarding this treatment approach which offers obvious benefits to patients’ quality of life.
SUMMARY
In summary, dental implants provide the best dental rehabilitation with a stabilized or immobilized intraoral prosthesis in patients who have lost teeth due to tumor or treatment related factors. However, there are several issues that impact upon the suitability of an individual patient for the feasibility of dental implant surgery. Aside from the technical knowledge required for endosseous implant surgery, practitioners should be aware of additional treatment factors that impact upon the feasibility, risks and sequela of dental implants in patients who have or are undergoing treatment of oral cancer. Early experience at MSKCC during the past two years with immediate implants in FFF reconstruction has been favorable, with minimal immediate complications. However, this is work in progress, and we need to have a larger experience with longer follow up, to draw definitive conclusions. This review covers the tumor, patient, treatment and Physician /Dentist related factors that need consideration before offering and performing implant surgery on suitable candidates. Consideration of these issues will identify eligible candidates for immediate or delayed dental implants, minimize post-operative complications, and maximize the prospect of optimal oral rehabilitation for the oral cancer patient.
Synopsis.
The purpose of this review is to discuss factors that impact upon optimal oral rehabilitation after surgery for cancer.
Acknowledgments
This study was supported, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748. The Straumann Maxillofacial Dental Implantology Research Fellowship is supported in part by the Straumann SUPER Grant award. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: A Cancer Journal for Clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures 2017. Atlanta, Ga: American Cancer Society; 2017. [Google Scholar]
- 4.Rogers SN. Quality of life perspectives in patients with oral cancer. Oral Oncol. 2010;46:445–447. doi: 10.1016/j.oraloncology.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Shah JP, Gil Z. Current concepts in management of oral cancer–surgery. Oral Oncol. 2009;45:394–401. doi: 10.1016/j.oraloncology.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis TA, Cantor R. The forgotten patient in maxillofacial prosthetics. J Prosthet Dent. 1974;31:662–680. doi: 10.1016/0022-3913(74)90122-x. [DOI] [PubMed] [Google Scholar]
- 7.Naert I, Alsaadi G, Quirynen M. Prosthetic aspects and patient satisfaction with two-implant-retained mandibular overdentures: a 10-year randomized clinical study. Int J Prosthodont. 2004;17:401–10. [PubMed] [Google Scholar]
- 8.Fenlon MR, Lyons A, Farrell S, Bavisha K, Banerjee A, Palmer RM. Factors affecting survival and usefulness of implants placed in vascularized free composite grafts used in post-head and neck cancer reconstruction. Clin Implant Dent Relat Res. 2012;14:266–72. doi: 10.1111/j.1708-8208.2009.00250.x. [DOI] [PubMed] [Google Scholar]
- 9.Jackson RS, Price DL, Arce K, Moore EJ. Evaluation of Clinical Outcomes of Osseointegrated Dental Implantation of Fibula Free Flaps for Mandibular Reconstruction. JAMA Facial Plast Surg. 2016 May 1;18(3):201–6. doi: 10.1001/jamafacial.2015.2271. [DOI] [PubMed] [Google Scholar]
- 10.Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24(3):491–508. doi: 10.1016/j.soc.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sammartino G, Prados-Frutos JC, Riccitiello F, Felice P, Cerone V, Gasparro R, Wang HL. The Relevance of the Use of Radiographic Planning in Order to Avoid Complications in Mandibular Implantology: A etrospective Study. Biomed Res Int. 2016;2016:8175284. doi: 10.1155/2016/8175284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abayev B, Juodzbalys G. Inferior Alveolar Nerve Lateralization and Transposition for Dental Implant Placement. Part II: a Systematic Review of Neurosensory Complications. J Oral Maxillofac Res. 2015 Mar 30;6(1):e3. doi: 10.5037/jomr.2014.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong HM. Oral Complications and Management Strategies for Patients Undergoing Cancer Therapy. The Scientific World Journal. 2014;2014:581795. doi: 10.1155/2014/581795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Disa JJ, Hidalgo DA, Cordeiro PG, Winters RM, Thaler H. Evaluation of bone heigh in osseous free flap mandible reconstruction: an indirect measure of bone mass. Plastic and Reconstructive Surgery. 1999;103(5):1371–1377. doi: 10.1097/00006534-199904050-00005. [DOI] [PubMed] [Google Scholar]
- 15.Frodel JL, Jr, Funk GF, Capper DT, Fridrich KL, Blumer JR, Haller JR, Hoffman HT. Osseointegrated implants: a comparative study of bone thickness in four vascularized bone flaps. Plast Reconstr Surg. 1993 Sep;92(3):449–55. [PubMed] [Google Scholar]
- 16.Reuther T, Schuster T, Mende U, Kübler AC. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients—a report of a thirty year retrospective review. International Journal of Oral and Maxillofacial Surgery. 2003;32(3):289–295. doi: 10.1054/ijom.2002.0332. [DOI] [PubMed] [Google Scholar]
- 17.Bedrossian E. Rehabilitation of the edentulous maxilla with the zygoma concept: A 7-year prospective study. Int J of Oral Maxillofac Implants. 2010;25:1213–21. [PubMed] [Google Scholar]
- 18.Aparicio C, Manresa C, Francisco K, Claros P, Alández J, González-Martín O, et al. Zygomatic implants: indications, techniques and outcomes, and the zygomatic success code. Periodontol. 2014;66:41–58. doi: 10.1111/prd.12038. [DOI] [PubMed] [Google Scholar]
- 19.Schepers RH, Slagter AP, Kaanders JH, et al. Effect of postoperative radiotherapy on the functional result of implants placed during ablative surgery for oral cancer. Int J Oral Maxillofac Surg. 2006;35:803–808. doi: 10.1016/j.ijom.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Cuesta-Gil M, Ochandiano Caicoya S, Riba-Garcia F, et al. Oral rehabilitation with osseointegrated implants in oncologic patients. J Oral Maxillofac Surg. 2009;67:2485–2496. doi: 10.1016/j.joms.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Pompa G, Saccucci M, Di Carlo G, et al. Survival of dental implants in patients with oral cancer treated by surgery and radiotherapy: a retrospective study. BMC Oral Health. 2015;15:5. doi: 10.1186/1472-6831-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sulaiman F, Huryn JM, Zlotolow IM. Dental extractions in the irradiated head and neck patient: a retrospective analysis of Memorial Sloan-Kettering Cancer Center protocols, criteria, and end results. J Oral Maxillofac Surg. 2003;61:1123–1131. doi: 10.1016/s0278-2391(03)00669-4. [DOI] [PubMed] [Google Scholar]
- 23.Thorn JJ, Hansen HS, Specht L, Bastholt L. Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation. J Oral Maxillofac Surg. 2000;58:1088–1093. doi: 10.1053/joms.2000.9562. discussion 93-5. [DOI] [PubMed] [Google Scholar]
- 24.Owosho AA, Yom SK, Han Z, et al. Comparison of mean radiation dose and dosimetric distribution to tooth-bearing regions of the mandible associated with proton beam radiation therapy and intensity modulated radiation therapy for ipsilateral head and neck tumor. Oral surgery, oral medicine, oral pathology and oral radiology. 2016;122(5):566–571. doi: 10.1016/j.oooo.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez DR, Estilo CL, Wolden SL, et al. Correlation of osteoradionecrosis and dental events with dosimetric parameters in intensity-modulated radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:e207–e213. doi: 10.1016/j.ijrobp.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Romesser PB, Cahlon O, Scher E, et al. Proton beam radiation therapy results in significantly reduced toxicity compared to intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2016;118(2):286–292. doi: 10.1016/j.radonc.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers MS, Toth BB, Martin JW, Fleming TJ, Lemon JC. Oral and dental management of the cancer patient: prevention and treatment of complications. Supportive Care in Cancer. 1995;3(3):168–175. doi: 10.1007/BF00368886. [DOI] [PubMed] [Google Scholar]
- 28.Lockhart PB, Sonis ST. Relationship of oral complications to peripheral blood leukocyte and platelet counts in patients receiving cancer chemotherapy. Oral Surgery Oral Medicine and Oral Pathology. 1979;48(1):21–28. doi: 10.1016/0030-4220(79)90230-5. [DOI] [PubMed] [Google Scholar]
- 29.Fillmore WJ, Leavitt BD, Arce K. Dental extraction in the thrombocytopenic patient is safe and complications are easily managed. J Oral Maxillofac Surg. 2013;71(10):1647–1652. doi: 10.1016/j.joms.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Ruggiero Salvatore L, et al. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. Journal of Oral and Maxillofacial Surgery. 72(10):1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita J, McCauley LK. Antiresorptives and osteonecrosis of the jaw. J Evid Based Dent Pract. 2012;12:233–47. doi: 10.1016/S1532-3382(12)70046-5. [DOI] [PubMed] [Google Scholar]
- 32.Migliorati CA. Intravenous bisphosphonate therapy may lead to osteonecrosis of the jaw in multiple myeloma, breast, and prostate cancer patients. J Evid Based Dent Pract. 2008;8(2):93–94. doi: 10.1016/j.jebdp.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Marx R. Oral & intravenous bisphosphonate-induced osteonecrosis of the jaws (history, prevention, and treatment) Quintessence Publishing Co, Inc.; Hanover Park: 2007. [Google Scholar]
- 34.Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, Koka S, et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: Executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011;142:1243–1251. doi: 10.14219/jada.archive.2011.0108. [DOI] [PubMed] [Google Scholar]
- 35.Estilo CL, Van Poznak CH, Wiliams T, et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist. 2008;13(8):911–920. doi: 10.1634/theoncologist.2008-0091. [DOI] [PubMed] [Google Scholar]
- 36.Flichy Fernández AJ, Balaguer-Martínez J, Peñarrocha-Diago M. Bisphosphonates and dental implants: current problems. Med Oral Patol Oral Cir Bucal. 2009;14:E355–60. [PubMed] [Google Scholar]
- 37.Petrovic I, Shah JP, Huryn JM, Rosen EB. Considerations for Intraoral Rehabilitation after Marginal Mandibulectomy. doi: 10.11607/ijp.6181. unpublished data. [DOI] [PubMed] [Google Scholar]
- 38.Celik N, Wei FC, Chen HC, et al. Osteoradionecrosis of the mandible after oromandibular cancer surgery. Plast Reconstr Surg. 2002;109:1875–1881. doi: 10.1097/00006534-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Petrovic I, Matros E, Huryn JM, Shah JP, Rosen EB. Assessment of the current treatment algorithm for secondary dental implant placement in mandibles reconstructed with a fibula free flap. unpublished data /in press. [Google Scholar]


