Abstract
The relapsing fever spirochete, Borrelia miyamotoi, is increasingly recognized as a cause of human illness (hard tick-borne relapsing fever) in the United States. We previously demonstrated that single nymphs of the blacklegged tick, Ixodes scapularis, can transmit B. miyamotoi to experimental hosts. However, two recent epidemiological studies from the Northeastern United States indicate that human cases of hard tick-borne relapsing fever peak during late summer, after the spring peak for nymphal tick activity but coincident with the peak seasonal activity period of larval ticks in the Northeast. These epidemiological findings, together with evidence that B. miyamotoi can be passed from infected I. scapularis females to their offspring, suggest that bites by transovarially-infected larval ticks can be an important source of human infection. To demonstrate experimentally that transovarially-infected larval I. scapularis ticks can transmit B. miyamotoi, outbred Mus musculus CD1 mice were exposed to 1 or 2 potentially infected larvae. Individual fed larvae and mouse blood taken 10 d after larvae attached were tested for presence of B. miyamotoi DNA, and mice also were examined for seroreactivity to B. miyamotoi 8 wk after tick feeding. We documented B. miyamotoi DNA in blood from 13 (57%) of 23 mice exposed to a single transovarially-infected larva and in 5 (83%) of 6 mice exposed to two infected larvae feeding simultaneously. All 18 positive mice also demonstrated seroreactivity to B. miyamotoi. Of the 11 remaining mice without detectable B. miyamotoi DNA in their blood 10 d after infected larvae attached, 7 (64%) had evidence of spirochete exposure by serology 8 wk later. Because public health messaging for risk of exposure to Lyme disease spirochetes focuses on nymphal and female I. scapularis ticks, our finding that transovarially-infected larvae effectively transmit B. miyamotoi should lead to refined tick-bite prevention messages.
Keywords: Borrelia miyamotoi, Ixodes scapularis, Hard tick-borne relapsing fever, Larvae, Transmission
1. Introduction
The relapsing fever spirochete, Borrelia miyamotoi, is increasingly recognized as a cause of human illness (hard tick-borne relapsing fever) in the United States (Krause et al., 2013, 2014; Molloy et al., 2015; Fiorito et al., 2017). The primary vector of B. miyamotoi to humans in the eastern part of the United States is the blacklegged tick, Ixodes scapularis, which also transmits the Lyme disease spirochetes, Borrelia burgdorferi sensu stricto (s.s.) and Borrelia mayonii (Krause et al., 2015; Breuner et al., 2017; Eisen, 2018). For nymphal and adult I. scapularis ticks, local infection prevalence with the relapsing fever spirochete, B. miyamotoi, is commonly 10-fold lower than for the Lyme disease spirochete, B. burgdorferi s.s. (Tsao et al., 2004; Krause et al., 2015; Nelder et al., 2016). However, in contrast to B. burgdorferi s.s., female ticks infected with B. miyamotoi can pass spirochetes transovarially to their offspring, resulting in host-seeking infected larval ticks (Scoles et al., 2001; Rollend et al., 2013; Breuner et al., 2017).
Two recent epidemiological studies from the Northeastern United States indicate that human cases of hard tick-borne relapsing fever peak during late summer (Molloy et al., 2015; Fiorito et al., 2017). This peak in cases coincides with the peak seasonal activity period of larval ticks in the Northeast, but occurs later in the year than the peak for nymphal tick activity and earlier than the fall peak activity period for adult ticks (Sonenshine, 1993; Stafford, 2007). Support for involvement of larval I. scapularis ticks in the natural transmission of B. miyamotoi in the Northeast was provided by an earlier study showing rising infection prevalence of white-footed mice (Peromyscus leucopus) with this spirochete during the summer period of peak larval activity (Barbour et al., 2009)
One previous study from Europe demonstrated experimentally that transovarially-infected larval Ixodes ricinus ticks are capable of transmitting B. miyamotoi (van Duijvendijk et al., 2016). However, all instances where transmission to an individual experimental host was demonstrated resulted from mass feeding by mixed infected and non-infected larvae. This scenario most likely better represents natural enzootic transmission than transmission to a human host bitten by a single or a few larvae. We previously demonstrated that single infected nymphal I. scapularis ticks effectively transmit B. miyamotoi to experimental hosts (Breuner et al., 2017). The objective of this study was to similarly demonstrate that single transovarially-infected larval I. scapularis ticks can transmit B. miyamotoi to experimental hosts.
2. Materials and methods
2.1. Source of B. miyamotoi-infected larval I. scapularis ticks
The larval I. scapularis ticks used in this transmission experiment were first generation transovarially B. miyamotoi-infected larvae originating from two female ticks (MN17-30 and MN17-52) collected by drag sampling at the Camp Ripley Training Center, Morrison County, Minnesota, in May 2017. The field-collected females were fed on a New Zealand white rabbit (Charles River Laboratories, Wilmington, MA, USA) in the laboratory and allowed to lay eggs as described previously (Piesman et al., 1991). Infection of the spent females and their larval offspring with B. miyamotoi was confirmed by TaqMan polymerase chain reaction (PCR) as described in Section 2.3. The two B. miyamotoi-infected females (MN17-30 and MN17-52) produced larval batches with high prevalence of infection with B. miyamotoi: 91% (29/32) of tested resulting larvae were infected for female MN17-52 and 62% (15/24) for female MN17-30. Additional larvae originating from these two females were used in the transmission experiment described in Section 2.2. Larvae originating from a third infected female (MN17-43) were not used due to their low infection prevalence (12%; 3/26).
2.2. Challenge of experimental hosts with transovarially B. miyamotoi-infected I. scapularis larvae and assessment of hosts for spirochete infection and exposure
Following our previous study (Breuner et al., 2017), 1–3 mo old female CD-1 outbred Mus musculus mice (Charles River Laboratories) were used as the experimental hosts to confirm transmission of B. miyamotoi by feeding ticks. Depending on the likelihood of a larval tick being infected (see Section 2.1), mice were exposed to either a single larva (from the MN17-52 female) or two larvae (from the MN17-30 female). Larval feeding was accomplished within capsules attached to the shaved backs of mice and sealed at the top with mesh to prevent the ticks from escaping (Mbow et al., 1994; Soares et al., 2006; Breuner et al., 2017).
A first set of 20 mice were each challenged with a single larva from female tick MN17-52. These larvae fed poorly: only 8 attached and took some blood, and none ingested a complete blood meal. A second set of 29 mice were each challenged with 2 larvae from female tick MN17-30. At least one fed larva was recovered from 26 of these mice, and most larvae had fed to completion. All larvae recovered from the mice were scored for feeding status (no visible blood ingested; minimal blood meal; more than minimal but still only partial blood meal; or complete blood meal) using a stereo microscope. Ticks that ingested at least a minimal amount of blood were tested for presence of B. miyamotoi DNA by TaqMan PCR as described in Section 2.3. Of the 34 mice from which larval ticks took at least some blood, 5 mice were found to have been exposed only to non-infected larvae. Those exposed only to non-infected larvae included one mouse with a partially fed tick, one mouse with a fully fed tick, and one mouse with two fully fed ticks.
Baseline blood samples were collected from all 49 experimental mice prior to tick challenge. For the 34 mice with recovered ticks that took at least a minimal amount of blood, this was followed by (i) a second blood sample 10 d after larvae attached (to test for the presence of B. miyamotoi DNA in the mouse blood, as described in Section 2.3) and (ii) a third blood sample at 8 wk post-tick infestation (to test for mouse seroreactivity to B. miyamotoi; as described in Section 2.4). This timeline for taking mouse blood samples to document spirochete infection and exposure followed Breuner et al. (2017). For selected mice, an attempt was made to culture B. miyamotoi from blood taken 10 d after larvae attached, as described in Section 2.5.
2.3. Detection of B. miyamotoi DNA from ticks and mouse blood
Nucleic acids were isolated from ticks as follows. Fed larval ticks were homogenized in 350 μl of lysis buffer using a Mini-Beadbeater-96 (BioSpec Products, Inc., Bartlesville, OK, USA) as previously described (Graham et al., 2016, 2018). Spent female ticks were homogenized by hand in 500 μl of lysis buffer using a 1.5 ml microcentrifuge tube and a pestle. Thereafter, the tick lysates (300 μl for fed larvae and 30 μl for spent females) were processed using the KingFisher DNA extraction system (Thermo Fisher Scientific, Waltham, MA, USA) and the MagMAX™ Pathogen RNA/DNA Kit (Thermo Fisher Scientific).
Nucleic acids were isolated from mouse blood as follows. First, 200 μl of blood was mixed with 450 μl of lysis buffer (426 μl ATL; 20 μl proteinase K; 2 μl Reagent DX; and 2 μl Carrier RNA, 1 μg/μl) (Qiagen, Valencia, CA, USA). Two aliquots of 300 μl from each blood lysate was then processed using the KingFisher DNA extraction system and the MagMAX™ Pathogen RNA/DNA Kit. Each blood lysis sample was eluted in 90 μl. Finally, DNA elutes from the same original blood sample were combined after processing to generate one elute from each mouse blood sample.
The subsequent real-time TaqMan PCR reactions included primers and probes for the following DNA targets: a pan Borrelia 16S rDNA target (Kingry et al., 2018) and the adenylosuccinate lyase (purB) target for B. miyamotoi (Graham et al., 2016). PCR and DNA purification controls included the I. scapularis actin target (Hojgaard et al., 2014) for tick samples and the rodent GAPDH target (Applied Biosystems® TaqMan® Rodent GAPDH ControlReagents kit; ThermoFisher) for mouse blood samples.
PCR reactions were performed in 10 μl solutions with 5 μl iQ Multiplex Powermix (Bio-Rad, Hercules, CA, USA) and 4.8 μl DNA extract, with forward and reverse primers (0.2 μl) at a final concentration of 300 nM each, and probes at a final concentration of 200 nM each. The real-time TaqMan PCR cycling conditions consisted of: denature DNA at 95 °C for 3 min followed by 40 cycles of 95 °C for 10 s, 58 °C for 10 s, and 60 °C for 45 s on a C1000 Touch thermal cycler with a CFX96™ real time system (Bio-Rad). We analyzed samples using CFX Manager 3.1 software (Bio-Rad) with the quantitation cycle (Cq) determination mode set to regression. Based on Graham et al. (2018), only Cq values < 40 were considered indicative of the B. miyamotoi purB target being present in the tested sample.
2.4. Serological reactivity of mouse blood to B. miyamotoi
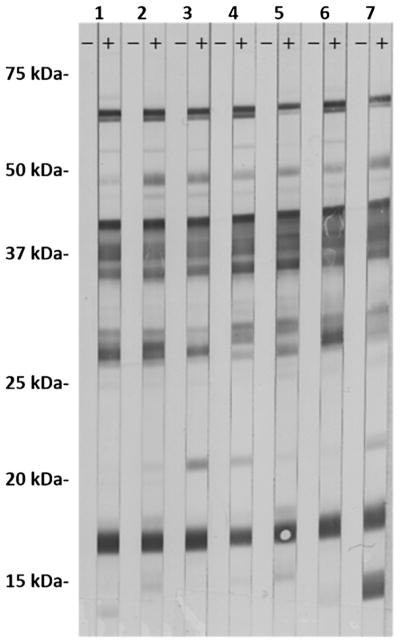
Antibody development to B. miyamotoi was assessed in mouse sera collected 8 wk after tick exposure for the 29 mice exposed to at least one infected larval tick, regardless of whether or not the blood of a given mouse tested positive for B. miyamotoi DNA 10 d after larvae attached. An additional 5 mice from which only non-infected fed ticks were recovered were included as negative controls. Serologic testing was as follows: whole cells from North American B. miyamotoi (strain CT13-2396; National Center for Biotechnology Information accession number: PRJNA310783) were cultivated in modified Barbour-Stoenner-Kelly (BSK) medium (in-house BSK-R medium) and harvested by centrifuging at 10,000 g for 10 min at 4 °C. The resulting cell pellet was frozen, thawed, and re-suspended in TE buffer, then sonicated, and finally diluted in TE buffer to a final protein concentration of 2.0 mg/ml. The sonicate was mixed with an equal volume of 2X Laemmli sample buffer with DTT (Bio-Rad), placed in a 95 °C heat block for 10 min, and run with 246 μl sonicate and sample buffer mixture on in-house made 12% Tris-HCL gels run in BioRad Protean IIxi Gel Electrophoresis Unit (Bio-Rad) at 25 mAmps for 150 min. Separated proteins were transferred to a 0.2 μm nitrocellulose membrane using BioRad Transblot SD Unit (Bio-Rad). Membranes were soaked overnight in 1% milk and TBS-t blocker, allowed to dry, and then cut into 3 mm strips for immunoblotting. Strips were hydrated in 1% milk and TBS-t wash buffer, then incubated on a rocking platform in wash buffer with 20 μl serum from experimental mouse hosts used in the transmission experiment at a concentration of 1:200 for 30 min. Strips were then washed and incubated for 15 min in wash buffer and phosphatase-labeled goat anti mouse IgG (H + L) conjugate (1 mg/ml; KPL, Gaithersburg, MD, USA) at a concentration of 1:10,000, followed by a final wash series. Finally, strips were developed for 4 min using BCIP/NBT phosphatase substrate (KPL). Mouse sera were considered positive when reactive bands were observed on the strips (Fig. 1).
Fig. 1.
Serological reactivity of paired pre-tick exposure sera (left strip) and 8 wk post-tick exposure sera (right strip) for 7 mice that were exposed to at least one B. miyamotoi-infected larval tick but tested negative for B. miyamotoi DNA in their blood 10 d after infected larvae attached.
2.5. Culture of B. miyamotoi from mouse blood
For a subset of mice, variable amounts (10–100 μl) of blood collected via cheek punch 10 d after larvae attached was introduced into in-house BSK-R medium with antibiotics (Cycloheximide, 20 μg/ml; Phosphomycin, 200 μg/ml; Rifampicin, 50 μg/ml; and Amphotericin B, 2.5 μg/ml). The amount of blood used varied among mice because these samples represented excess blood over the 200 μl set aside for detection of B. miyamotoi DNA. Cultures were incubated at 34 °C and aliquots examined under dark field microscopy at 400X magnification every 5–7 d over a 30 d period. Upon initial inoculation, blood was allowed to settle to the bottom of the culture tubes overnight. Media was then transferred to secondary culture tubes, leaving erythrocytes behind, and centrifuged for 8 min at 3300g in order to separate spirochetes from EDTA. The resultant pellets were then re-suspended in fresh media. Centrifugation and media replacement was repeated at 12 d post-inoculation.
2.6. Regulatory compliance
Animal use and experimental procedures were in accordance with an approved protocol on file with the Centers for Disease Control and Prevention, Division of Vector-Borne Diseases Animal Care and Use Committee.
3. Results and discussion
A total of 29 mice were exposed to at least minimal feeding by 1 or 2 transovarially B. miyamotoi-infected I. scapularis larvae. Outcomes for B. miyamotoi DNA detected in mouse blood and mouse seroreactivity, broken down by total number of fed larvae per mouse, number of fed infected larvae, and feeding status of infected larvae, are shown in Table 1. Overall, we documented infection, based on detection of B. miyamotoi DNA, in blood from 13 (57%) of 23 mice exposed to a single infected larva and from 5 (83%) of 6 mice exposed to two infected larvae feeding simultaneously. Increased probability of tick feeding to result in host infection after exposure to multiple versus single infected ticks was previously reported for nymphal I. scapularis ticks infected with the Lyme disease spirochetes, B. burgdorferi s.s. and B. mayonii (Eisen, 2018). In this study, all 18 PCR-positive mice also demonstrated seroreactivity to B. miyamotoi. Moreover, 7 (64%) of the 11 remaining mice exposed to one or two infected larvae but without detectable B. miyamotoi DNA in their blood had evidence of spirochete exposure by serology (Fig. 1). None of 5 mice exposed only to non-infected larvae had reactive sera.
Table 1.
Transmission of B. miyamotoi to experimental hosts by transovarially-infected larval I. scapularis ticks.
| Female tick source for the larvae | Total no. larvae fed per mouse | No. B. miyamotoi-infected larvae fed per mouse | Feeding status of infected larvae | No. experimental mouse hosts | Evidence of infection with or exposure to B. miyamotoi in micea | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| B. miyamotoi DNA detected in mouse blood 10 d after larval attachment | Mouse serum reactive to B. miyamotoi 8 wk after larval feed | |||||||
|
|
|
|||||||
| No. infected mice | Percent of mice infected | No. seroreactive mice | Percent of mice seroreactive | |||||
| MN17-30/MN17-52 | 1 | 1 | Minimal/partial blood meal | 9 | 5 | 55.6 | 6 | 66.7 |
| MN17-30 | 1 | 1 | Complete blood meal | 8 | 4 | 50.0 | 7 | 87.5 |
| MN17-30 | 2 | 1 | Complete blood meal | 6 | 4 | 66.7 | 6 | 100 |
| MN17-30 | 2 | 2 | One with complete blood meal; One with minimal/partial blood meal |
3 | 2 | 66.7 | 3 | 100 |
| MN17-30 | 2 | 2 | Both with complete blood meal | 3 | 3 | 100 | 3 | 100 |
All individual mice with evidence of B. miyamotoi DNA in their blood 10 d after larval attachment also were seroreactive 8 wk after the larval feed.
Among 8 mice exposed to a complete blood meal by a single-feeding transovarially B. miyamotoi-infected larva, 4 (50%) had B. miyamotoi DNA in their blood and 7 (88%) had serologic evidence of B. miyamotoi exposure. The 50% probability of detecting B. miyamotoi DNA in the blood of experimental hosts after exposure to single-feeding transovarially B. miyamotoi-infected larval I. scapularis ticks reported here (Table 1) is similar to our previous study on single-feeding B. miyamotoi-infected nymphs, where B. miyamotoi DNA was recorded from 22 (73%) of 30 experimental hosts after a complete nymphal blood meal (Breuner et al., 2017). Another interesting observation was that minimal or partial blood meals by single-feeding transovarially B. miyamotoi-infected larvae also resulted in approximately half of experimental hosts developing infections detectable by examination of blood for presence of B. miyamotoi DNA (Table 1). This is perhaps not surprising as we previously showed that B. miyamotoi-infected I. scapularis nymphs can transmit spirochetes during partial blood meals (Breuner et al., 2017).
Borrelia miyamotoi was cultured from two mice which tested positive for B. miyamotoi DNA in their blood, on the same day blood was placed into culture. This recovery of live spirochetes provides additional evidence of active infection in mice resulting from the bite of transovarially B. miyamotoi-infected larval ticks. Spirochetes were first detected by microscopy 15 d post-inoculation.
In conclusion, our study found that single-feeding, transovarially B. miyamotoi-infected I. scapularis larvae can effectively transmit this relapsing fever spirochete to experimental hosts not only when allowed to feed to repletion but also when taking partial blood meals. We previously demonstrated that transmission of B. miyamotoi can occur within the first 24 h of attachment by infected I. scapularis nymphs but that the probability of transmission increases with every day the ticks are allowed to remain attached (Breuner et al., 2017). It would be worthwhile to similarly assess how the probability of transmission by infected larvae changes with tick attachment duration.
A final consideration is that public health messaging for risk of exposure to I. scapularis-borne human pathogens, including Lyme disease spirochetes and the causative agents of anaplasmosis and babesiosis, focuses primarily on bites by nymphal ticks and secondarily by female ticks (Eisen et al., 2017; Eisen and Eisen, 2018). It now appears that hard tick-borne relapsing fever may be an exception in this respect: our experimental demonstration of transmission of B. miyamotoi by larval ticks supports the idea put forth in epidemiological studies (Molloy et al., 2015; Fiorito et al., 2017) that transovarially-infected larvae are involved as vectors of this relapsing fever spirochete to humans in the United States. Pathogen transmission by I. scapularis larvae is especially concerning in the Northeast, where the seasonal timing of peak larval activity (summer) is distinct from that of both peak nymphal activity (late spring) and peak activity of female ticks (fall and early spring). Larval bites therefore occur during a time of year when people not only spend a lot of time outside but also may be less vigilant in their personal tick protection measures if they perceive it as a “safer” period with regards to tick-borne illness.
Acknowledgments
We thank Robert Prose of the Centers for Disease Control and Prevention (CDC) for technical assistance, Jeannine Petersen of the CDC for helpful discussions, and Jenna Bjork of the Minnesota Department of Health for providing source material to start tick colonies.
Footnotes
Disclaimer
The findings and conclusions of this study are by the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner NE, Dolan MC, Replogle AJ, Sexton C, Hojgaard A, Boegler KA, Clark RJ, Eisen L. Transmission of Borrelia miyamotoi sensu lato relapsing fever group spirochetes in relation to duration of attachment by Ixodes scapularis nymphs. Ticks Tick-borne Dis. 2017;8:677–681. doi: 10.1016/j.ttbdis.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L. Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick-borne Dis. 2018;9:535–542. doi: 10.1016/j.ttbdis.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L. The blacklegged tick, Ixodes scapularis: an increasing health concern. Trends Parasitol. 2018;34:295–309. doi: 10.1016/j.pt.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Kugeler KJ, Eisen L, Beard CB, Paddock CD. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319–335. doi: 10.1093/ilar/ilx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito TM, Reece R, Flanigan TP, Silverblatt FJ. Borrelia miyamotoi polymerase chain reaction positivity on a tick-borne disease panel in an endemic region of Rhode Island. A case series. Infect Dis Clin Pract. 2017;25:250–254. [Google Scholar]
- Graham CB, Pilgard MA, Maes SE, Hojgaard A, Eisen RJ. Paired real-time PCR assays for detection of Borrelia miyamotoi in North American Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) Ticks Tick-borne Dis. 2016;7:1230–1235. doi: 10.1016/j.ttbdis.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CB, Maes SE, Hojgaard A, Fleshman AC, Sheldon SW, Eisen RJ. A molecular algorithm to detect and differentiate human pathogens infecting Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) Ticks Tick-borne Dis. 2018;9:390–403. doi: 10.1016/j.ttbdis.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojgaard A, Lukacik G, Piesman J. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti, with two different multiplex PCR assays. Ticks Tick-borne Dis. 2014;5:349–351. doi: 10.1016/j.ttbdis.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Kingry LC, Anacker M, Pritt B, Bjork J, Respicio-Kingry L, Liu G, Sheldon S, Boxrud D, Strain A, Oatman S, Berry J, Sloan L, Mead P, Neitzel D, Kugeler KJ, Petersen JM. Surveillance for and discovery of Borrelia species in US patients suspected of tickborne illness. Clin Infect Dis. 2018;66:1864–1871. doi: 10.1093/cid/cix1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, Barbour A, Fish D. Human Borrelia miyamotoi infection in the United States. N Engl J Med. 2013;368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Barbour AG, Platonov AE, Brancato J, Lepore T, Dardick K, Mamula M, Rollend L, Steeves TK, Diuk-Wasser M, Usmani-Brown S, Williamson P, Sarksyan DS, Fikrig E, Fish D the Tick Borne Diseases Group. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the Northeastern United States. Emerg Infect Dis. 2014;20:1183–1190. doi: 10.3201/eid2007.131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and humans. Clin Microbiol Infect. 2015;21:631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbow ML, Christe M, Rutti B, Brossard M. Absence of acquired resistance to nymphal Ixodes ricinus ticks in Balb/C mice, developing cutaneous reactions. J Parasitol. 1994;80:81–87. [PubMed] [Google Scholar]
- Molloy PJ, Telford SR, III, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med. 2015;163:91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- Nelder MP, Russell CB, Sheehan NJ, Sander B, Moore S, Li Y, Johnson S, Patel SN, Sider D. Human pathogens associated with the blacklegged tick Ixodes scapularis: a systematic review. Parasit. Vectors. 2016;9:265. doi: 10.1186/s13071-016-1529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Maupin GO, Campos EG, Happ CM. Duration of adult female Ixodes dammini attachment and transmission of Borrelia burgdorferi with description of a needle aspiration isolation method. J Infect Dis. 1991;163:895–897. doi: 10.1093/infdis/163.4.895. [DOI] [PubMed] [Google Scholar]
- Rollend L, Fish D, Childs JE. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick-borne Dis. 2013;4:46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- Soares CAG, Zeidner NS, Beard CB, Dolan MC, Dietrich G, Piesman J. Kinetics of Borrelia burgdorferi infection in larvae of refractory and competent tick vectors. J Med Entomol. 2006;43:61–67. doi: 10.1093/jmedent/43.1.61. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE. Biology of Ticks. Vol. 2. Oxford University Press; New York, NY, USA: 1993. [Google Scholar]
- Stafford KC., III . An Integrated Guide for Homeowners, Pest Control Operators, and Public Health Officials for the Prevention of Tick-Associated Disease. The Connecticut Agricultural Experiment Station; New Haven, CT, USA: 2007. Tick Management Handbook. Bulletin no. 1010. [Google Scholar]
- Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci U S A. 2004;101:18159–18164. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvendijk G, Coipan C, Wagemakers A, Fonville M, Ersöz J, Oei A, Földvári G, Hovius J, Takken W, Sprong H. Larvae of Ixodes ricinus transmit Borrelia afzelii and B. miyamotoi to vertebrate hosts. Parasit. Vectors. 2016;9:97. doi: 10.1186/s13071-016-1389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]