Abstract
Uterine leiomyomas (ULM) grow under high oxidative stress due to a hypoxic microenvironment and defects in redox metabolism. AKT is one major pathway activated by reactive oxygen species (ROS)that maintains ULM growth and survival. We previously reported thatAKT inactivated by AKT inhibitors can significantly induce cellular senescence in ULM cells. Since some miRNAs are induced by AKT inhibitors in an ROS-dependent manner, we proposed that these miRNAs may modulate AKT function and cellular senescence in ULM. We therefore established ex vivo models of three dimensional ULM spheroid culture system to study the role of miRNAs in cellular senescence. Four miRNAs, miR-29b, miR-181a, miR-182and miR-200c,were found to induce cellular senescence in primary ULM and MM spheroid cultures when stably overexpressed. MiR-181aandmiR-182 were found to repress AKT3 and CCND2, respectively. Correspondingly, RNAi of AKT3 or CCND2 also induced cellular senescence and G0/G1 arrest. Thus, miR-181a and miR-182 may drive cellular senescence in ULM by repressing AKT3 and CCND2 activity, respectively. We further demonstrated that senescent ULM cells can be effectively removed by BH3 mimietics ABT263, which provides a new therapeutic venue for the treatment of ULM. Our findings suggest that miRNAs are potent modulators in regulating ROS-AKT-cell cycle axis in uterine leiomyoma.
Keywords: Leiomyoma, senescence, miR-181a, miR-182, AKT3, CCND2
INTRODUCTION
Uterine leiomyomas (ULM) are smooth-muscle tumors of the myometrium that occur in 77% of women in the United States 1.ULM are generally slow growing tumors driven by sex steroid hormones and growth factors. Due to poor vasculature and excessive collagen production, ULMs grow in an unfavorable hypoxic microenvironment 2, 3. Such hypoxic conditions increase levels ofreactive oxygen species (ROS)4, 5.Recently, we demonsrated that ULM has dysfunctional MnSOD activity leading to accumulation of ROS which can activate AKT signaling6. AKT may play a major role in maintaining ULM growth and survivalunderoxidative stress6, 7.Previousstudieshave shown that inactivation of AKT by the AKT inhibitor MK2206 in ULM cells enhancesROS production and induces cellular senescenceatlow doses8 or cell death at high doses7. Therefore a balance of ROS levels and AKT activity is vital for ULM growth and survival. The underlyingcellular and molecular mechanisms remain to be fully characterized and deserve further investigation. Deciphering these molecular mechanisms can be valuable for the development of targeted therapies.
MiRNAs, which are small non-coding RNAs, are early response molecules to cellular stresses. It has been shown that a subset of miRNAs can be induced by oxidative stress9, 10.Induced miRNAs react to oxidative stress through modulating expression of their target genes at the levels of transcription and translation. These target genes may redirect the cells from proliferation to cellular arrest topreventfurther cellular damage11. Since hypoxia and increased ROS production in cells can elicit an adaptive response that involves activation of the AKT pathway6 and induction of miRNAs that serve as downstream effectors and upstream regulators of AKT activity12, identification of the key miRNAs that are induced under these conditions will help elucidate the functional relationship between miRNAsandstress response in ULM. ULM are under increased oxidative stress and AKT serves as a major pathway for ULM cell growth and survival. Inactivation of AKT by AKT inhibitors can induce cellular senescence. Many miRNAs can be induced by ROS and we speculated that these miRNAs may modulate AKT function and cellular senescence in ULM. Review of the literature and our previous studies demonstrated that a subset of miRNAs were dysregulated with oxidative exposure. In this study, we studied the miRNAs induced by ROS and AKT inhibitor. We demonstrated that miR-181a and miR-182 repress ATK3 and cyclin D2 and function to promote senescence.
MATERIALS AND METHODS
Collection of tissue samples and culture of primary cells
Fresh surgical specimens of uterine leiomyoma (ULM)andmyometrium (MM) were obtained from consented premenopausal women undergoing hysterectomy at the Northwestern University Prentice Women’s Hospital (Chicago, IL, USA) and the protocol for acquisition of specimens was approved by Northwestern University’s Institutional Review Board (IRB). Study tissue pool include 28 cases with mean age of 44.9 years old (range of 37–55 years old) and tumor size of 9.2±2.7 cm. Among them, 46.4% (13/28) were African American, 32.1% (9/28) were Caucasion, and 21.4% (6/28) othe ethnic group (Supplementary Table 1). Subjects included in the study were not taking hormonal contraceptives or had hormonal treatment for at least 3months. Tissue was minced into small pieces and digested with collagenase A and DNAase (Sigma Aldrich) for 5hrs on a 37 °C tissue shaker. The digested material was filtered to obtain a single-cell suspension. The primary cells were cultured in smooth muscle cell basal medium (SmBM; Clonetics, Lonza Group, Switzerland) at 37°C and 5% CO2 atmosphere no longer than 10 days.
Three dimensional leiomyoma spheroid cultures
Primary cells were suspended and seeded in6-well (coated with 1.5% agarose gel) or 96-well ultra low attachment culture plates(Corning). Cells were cultured with mesenchymal stem cell medium (Lonza, cat#190632 or cat#PT-3001), and incubated at 37°C in the humidified incubator with 5% CO2 for at least 48 hrs for formation of spheroids. The spheroids were evaluated with the inverted microscope. Spheroid cultureswere maintained for up to 5–7 days and further processed for β-galactosidase staining.
Treatment of Cells
Primary ULMandMMcellsor spheroids were treated with 0 (DMSO), 2 and 5μM MK-2206 (Selleckchem) for 24hrs; with 0(3DW),100, 200μMParaquat (PQ) (Sigma-Aldrich)in serum-free medium for 6hrs; with 0(DMSO),5μMABT263 and ABT199 (provided by AbbVie Inc, North Chicago, IL)for 0, 2, 4 and 6 days.
siRNA transfection
ULM and MM cells inmonolayerswere transfected withON-TARGET plusSMART poolsiRNAs (GE Dharmacon) for CCND2 and AKT3usingLipofectamineRNAiMAX (ThermoFisher Scientific) according to the manufacturer’s instructions. A non-targetingsiRNA, (siCTR; GE Dharmacon) was used in parallel as a control for each assay.After 48 hrs, cells were harvestedorfixed for further analysis. For spheroids, cells transfected with test and control siRNAsin monolayer cultures first and then transferred to three dimensional culture for spheroid formation.
Quantitative real-time RT-PCR
Total RNA was isolated using the mirVanaTM RNA Isolation Kit (Ambion, Austin, TX). The detailed method was described previously 13. Then cDNA was prepared by a Mir-X™ miRNA First Strand Synthesis Kit (Clontech, Mountain View, CA). The entire sequence of mature miRNA was used as miRNA-specific, 5’ primer (Suppl Table 2). The 3’ primer for qPCR is the mRQ 3’ Primer supplied with the kit. For real-time PCR, cDNA was synthesized by PowerUp™ SYBR® Green Master Mix (Life Technologies) using The Applied Biosystems® StepOnePlus™ Real-Time PCR Systems with sequence specific miRNA primers. Relative mRNA levels were calculated using the 2-△△Ct method. The miRNA levels were normalized to U6. Results were obtained from at least threeindependent experiments performed in triplicate.
Western blotting
Cultured cells were harvested and lysed on ice in RIPA lysis and extraction buffer with protease and phosphatase inhibitors (Thermo Fisher Scientific).The concentration was determined by using BCA Protein Assay kit (Thermo Fisher Scientific).Total protein (30 μg) was separated by SDS-PAGE and electro-transferred onto polyvinylidene fluoride membrane (Invitrogen, Grand Island, NY). The membranes were blocked with 5% BSA in TBST, then incubated with primary antibodies overnight at 4°C. The specific horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse secondary antibody (Bio-Rad, Hercules, CA) was used to blot the target proteins (Suppl Table 3), and the secondary antibody was detected by an enhanced chemiluminescence ECL detection kit (Bio-Rad, Hercules, CA).
β-Galactosidase staining
Senescence-associated β-Gal stainingwas done as previously described 8. In brief, monolayer cells were seeded in 6-well plates overnight. After appropriate treatments (siRNA, lentiviral transfection, MK2206, PQ), the cells were fixed with 2% formaldehyde and 0.2% glutaraldehyde in PBS at room temperature for 15 minutes. After washing with PBS, cells were stained with staining solution containing 1 mg/mL X-gal and incubated in a CO2-free incubator at 37°C for 16 hrs. Blue cells were counted under the microscope and statistically analyzed. Five randomly selected fieldswerecaptured,and the percentage of cells that were positive for β-galactosidase was calculated and averaged.For spheroids, cells were transfected with miRNA lentivirus for 48 hrs and then change medium, after 5 days, all the spheroids were collected in tubes, then fixed withformaldehyde and 0.2% glutaraldehyde in PBS at room temperature for 15 minutes. After washing with PBS, cells were stained with staining solution containing 1 mg/mL X-gal and incubated in a CO2-free incubator at 37°C for 3 hrs.The reactions were terminated when the cells were stained blue-green.Spheroids were resuspended in 100μL 70% glycerinum,and seeded onto glass slide,then visualized under an inverted bright-field microscope.
Lentiviral packaging and transduction
Lentiviralpre-miRNA constructs and viral particles were prepared as previously described 14. Briefly, lentiviral vectors with and without pre-miRNAs were cotransfected with psPAX2 and pMD2G vectors into HEK293T cells. Supernatants were collected at 24hrs and 48hrs after transfection and stored in −80°C. For transduction, 5×104 cells were plated in each well of the six-wellplates along with 2 mL of medium without antibiotics. After 48 hrs’incubation, the medium wasremoved and replaced with 1 mLmedium containing lentivirus. 48hrslater, fresh medium containing 1μg /mL puromycin was added to each well.
Analysis of cell cycle by flow cytometry
Cells were serum-starved for 24 hrs before treatment. Control and treated cells were harvested using 0.25% Trypsin, and washed once with cold PBS. Thepelletswere resuspended in ice-cold 70% ethanol and stored at −20°C. Samples were incubated with 20mg/mL propidiumiodide0.1% Triton X-100 staining solution with 0.1 mg/mLRNase A. Cell-cycle distribution was determined using the BDBiosciencesFACSCanto II Analyzer. At least 20,000 cells werecollected. For these studies, all experiments were repeated three times.
WST-1 viability assay
Primary ULM and MM cells were cultured in 96-well plates. At the end oftreatments, WST-1 (BioVision) was added to each well (1:10 dilution)for4hrs at 37°C, and absorbance was read at 420 nm with referencewavelength set at 650 nm. Data were analyzed following the manufacturer’smanual.
3’UTR of AKT3 vector construction and luciferase reporter assay
The 3’untranslated region (UTR) of human AKT3containingtwo putative miR-181abinding sites was amplified from human genomic DNA andcloned into psiCHECK2 vector (Promega, Madison, WI,USA) using the XhoI and NotIsites.The psiCHECK2-AKT3-mut construct (replacing the nucleotide at the miR-181a ‘seed’ sequencebinding sites 15,Suppl Table 2) was mutated by using the QuickchangeXL Mutagenesis Kit (Stratagene, La Jolla, CA)16. For the luciferase assay, HEK293T cells were seeded at 1×104cells per well in a 96-well plate. 24 hrslater,cells were transfectedwith 100 ng ofpsiCHECK2 control, psiCHECK2-AKT3-WT, or psiCHECK2-AKT3-mutplasmid, and for each well, 20nM of miR-181a mimic or miR-NC wasco-transfected with the reporter construct as indicated. 24hrs later, luciferase activity was measured using the Dual-Luciferase Reporter System (Promega).
Statistical analysis
Results arepresented as mean ± SEM.GraphPad Prism was used for statistical analysis. Student’s t-test (two groups) and ANOVA (multiple groups) was used to determine significance. P<0.05 was considered statistically significant, and levels of significance were presented at *P < 0.05, **P< 0.01 and ***P < 0.001, respectively. Data from each patient were considered as an independent experiment.
RESULTS
Oxidative stress-induced miRNA expression in ULM and matched myometrium (MM).
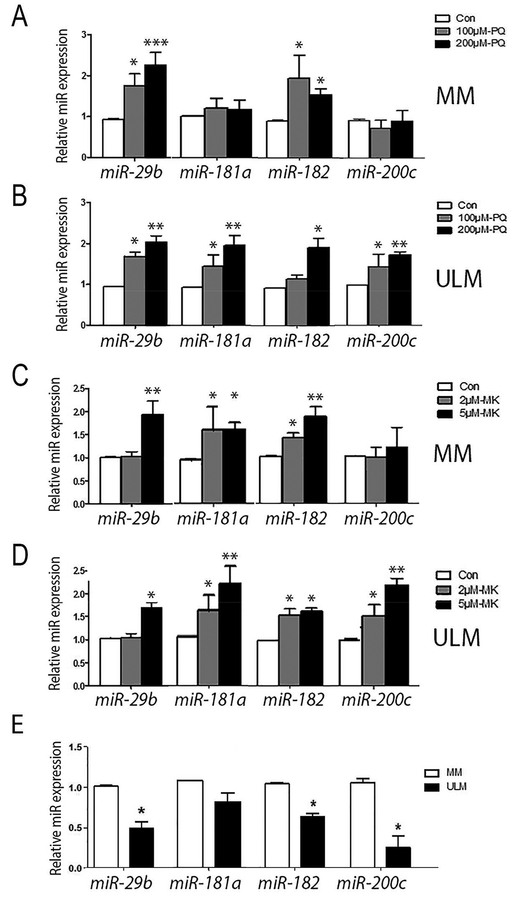
Among those miRNAs regulated by oxidative stress (45 miRNAs were upregulated and 24 downregulated (Suppl Table 4)), we chose 11 miRNAs that were also highly dysregulated in ULM17, 18, including let-7c, miR-22, miR-29b, miR-96, miR-99a, miR-145, miR-155, miR-181a, miR-182, miR-195, miR-200a, and miR-200c. In order to determine which miRNAswere induced by both ROS and AKT inhibitors, primary ULM cells and MM cells were treated with the superoxide anion generator, paraquat (PQ) as well as the AKT inhibitor, MK2206. Among three independent cases,miRNAs(miR-29b, miR-181a, miR-182 and miR-200c) were repeatedly induced by both PQ and MK2206 in primary ULM cellswhich was dose dependent (Figure 1B and 1D). Similar results were observed in some but not all MMcells (Figure 1A and 1C). The findings show that these specific miRNAs are inducible under oxidative stress orAKTinactivation.
Figure 1:
The selected miRNA expression by real-time quantitative RT-PCR in primary myometrial (MM) and leiomyoma (ULM) cells induced by oxidative stress (PQ) and AKT inhibitor (MK) (n=3). A-B. The relative miRNA expression in MM (A) and ULM (B) cells treated by control (Con, open box, DMSO), paraquat (PQ, 100μM, gray box and 200μM, black box). C-D. The relative miRNA expression in MM (A) and ULM (B) cells treated by control (open box, DMSO), MK2206 (MK, 2μM, gray box and 4μM, black box). E. Relative expression of the selected miRNAs between primary myometrium (MM) and leiomyoma (ULM) were illustrated. Small T bar stands for standard error. *p<0.05; **p<0.01, ***p<0.001.
Establish stable miRNA expression in primary ULM and MMcells
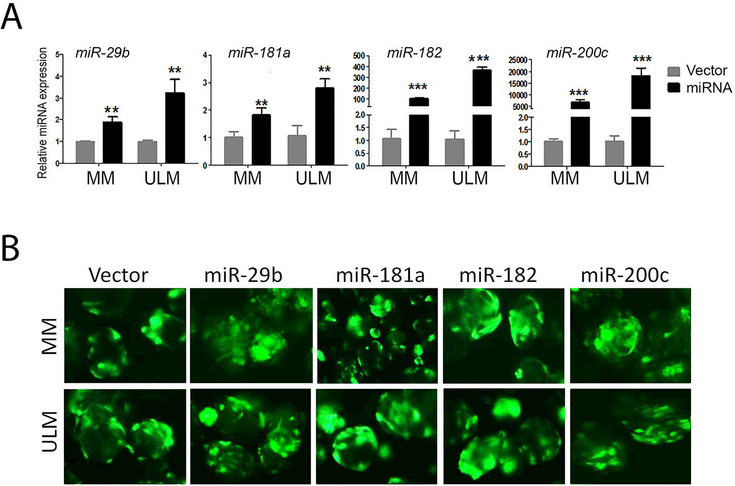
To evaluate the functional role of ROS/MK2206-induced miRNAs in ULM and MM, stable miRNA expression was established usinglentiviral constructs whichcontainpre-miRNAs of miR-29b, miR-181a, miR-182 ormiR-200c, by method described previously14. The efficacy of lentiviral infection was illustrated by bright and diffuse green fluorescence in over 80% of culture cells (Suppl Figure 1). To confirm miRNA overexpression in primary culture ULM and MM cells, miRNA expression was examined by miRNA RT-PCR (Figure 2A). Of note, the fold increase of miRNA expression varied in different miRNA constructs, suggestive of different efficacy in miRNA maturation processes. To evaluate the miRNA function in primary ULM and myometrial cells, we overexpressed miRNAs in the spheroid cultures. AftermiRNA overexpression was confirmed, the primary cells in monolayers were cultured as spheroids. Upon spheroid formation after 3–5days,stable lentiviral infection was confirmed by the presence of green fluorescence (Figure 2B).
Figure 2:
Stable miRNA expression established by transduction of miRNA lentivirus in primary myometrial (MM) and leiomyoma (ULM) cells. A. MiRNA (listed above the bars) expression was examined and quantified by real-time RT-PCR (see Methods) in primary culture MM and ULM cells in vector control and miRNA lentiviral transduction. Small T bar stands standard error. B. Fluorescent photomicrographs illustrated the efficacy of control and each of four miRNA lentiviral infection (green fluorescent color) in primary three dimensional spheroid culture in MM and ULM cells. *p<0.05; **p<0.01; ***p<0.001.
Overexpression of stress-induced miRNAs resulted in cellular senescence in ULM and MM
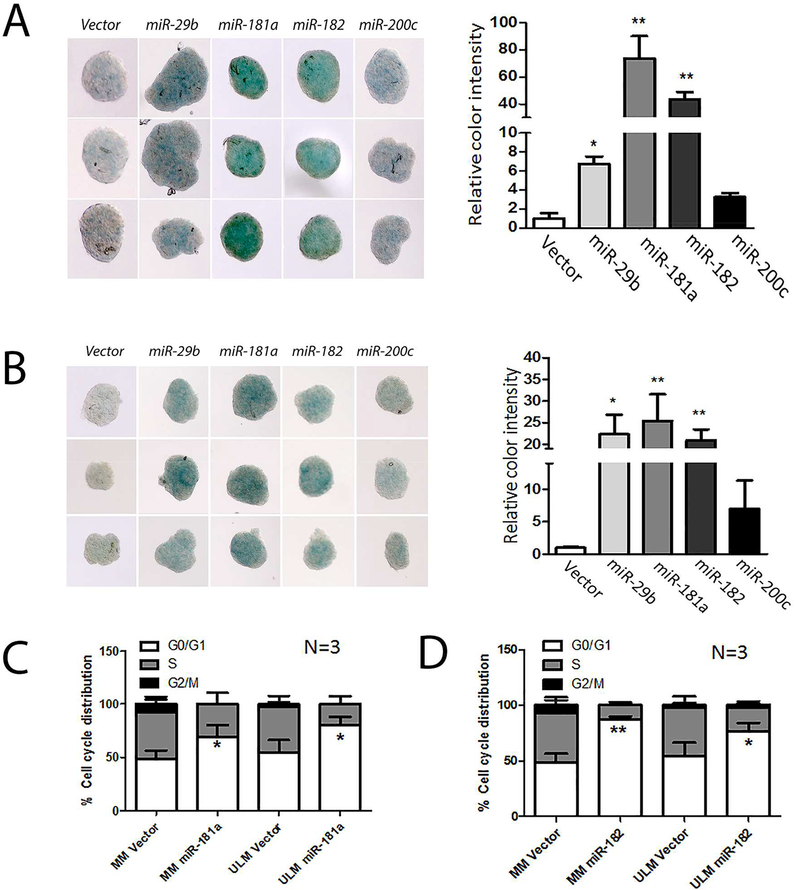
To determine whether the selected miRNAs induced senescence, ULM and MM cells overexpressing themiRNAsandcultured as spheroids were fixed and stained for SA-β-galactosidase. As illustrated in Figure 3A and 3B, 3D spheroid MM and ULM showed global senescence pattern and significant increase of cellular senescence was observed in both ULM and MM spheroids with overexpressing miR-29b, miR-181a, miR-182, and miR-200c compared to vector controls. The level of senescence in matched MM was relatively lower than in ULM (Figure 3A and 3B). Of note, miR-181a and miR-182 overexpression resulted in an intense and diffusive pattern of β-galactosidase stainingin ULM. miR-29b overexpression could also increase senescence but not miR-200c (Figure 2A and 2B). These data were confirmedin three cases (Supplementary Figure 2).The monolayer of ULM and MM cells with miRNA overexpression were also examined by β-galactosidase stainingand were found to be positive(Supplementary Figure 3). In the 2D cultures of primary MM and LM cells, senescence were evaluated in individual cells which showed that miR-181a and miR-182 overexpression resulted in nearly 1.5 fold increase of cellular senescence as detected by β-Gal staining (Supplementary Figure 3B,C). Senescent cells are usually arrested in G0 phase of the cell cycle. Cell cycle analysis showed that about 50% of cells were in G0/G1phasein primary culture (day 5–7) of both myometrial and leiomyoma cells (Figure 3C and 3D). In contrast, the same primary culture of ULM and MM cells with overexpression of miR-181a and miR-182had significantly high percentagesof G0/G1 cells (69.43% in MM and 79.9%in LM with overexpression of miR-181a; 86.96% in MM and 76.84% in LM with overexpression of miR-182;p<0.05). These findings further support that miR-181aand miR-182overexpression can drive smooth muscle cells into cellular arrest or senescence.
Figure 3:
miR181a and miR182 overexpression resulted in cellular senescence and arrest at G0–1 status in primary myometrial (MM) and leiomyoma (ULM) cells. A-B. Cellular senescence detected by global β-galactosidase stain (left panels) in 3-dimensional spheroid culture cells of ULM (A) and MM (B) in vector control and four miRNA overexpression (indicated above). The relative expression of β-galactosidase (right panels) was measured by the intensity of blue color detected by Image J software. C-D. Cell cycle analysis of percentage of MM and ULM cells with vector control, miR-181a (C) and miR-182 overexpression in G0/G1 (open box), S (gray box) and G2/M (black box) phases. Small T bar stands for standard error. *p<0.05; **p<0.01. The results were calculated in three cases (n=3).
MiR-181a represses AKT3 in fibroids
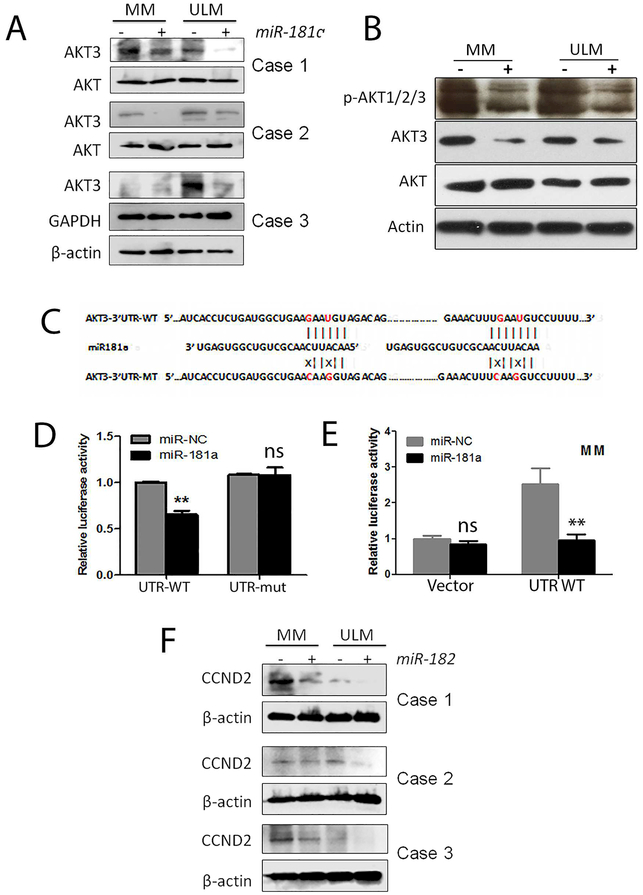
Findings of miR-181a and miR-182overexpression driving cellular senescence prompted us to investigate the underlying mechanism.We speculatedthat some miR-181atarget genes may play an important role in protecting ULM cells from ROS mediated DNA damage and drive ULM cell into senescence. Among the target genes predicted to be targeted by miR-181aby PicTar and Target Scan, AKT3 was one of the top predicted target genes. AKT activity is critical for ULM cell survival and proliferation. AKT3 was moderately expressed in both MM and ULM, but varied from case to case (Figure 4A). Transient transfection of miR-181a in MM and ULM cells consistently reduced AKT3 expression (Figure 4A). miR-181a mediated repression of phosphorelated AKT were also observed in MM and ULM cells (Figure 4B). Cilicon analysis revealed that there are two highly reserved miR-181a-5p binding sites at 3’UTR of AKT3 (Figure 4C). To confirm that miR-181a-5p specifically repress AKT3 expression, two miR-181a-5p binding sites at 3’UTR of AKT3 were mutated by CRISPR/CAS9 techniques at 3rd and 6th nucleotides complementary to miR-181a-5p seed sequence (Figure 4C). Luciferase activity was blocked in wild type 3’UTR, but not in mutant 3’UTR, by miR-181a-5p in HEK293 cell line (Figure 4D). Consistently, miR-181a overexpression can reduce the luciferase activity when transfection of luciferase with AKT3 3’UTR in myometrial cells (Figure 4E). These results indicate that miR-181a-5p can specifically inhibit AKT3 expression.
Figure 4:
Analysis of miR181a repression of AKT3 and miR182 repression of CCND2 in myometrial (MM) and leiomyoma (ULM) cells. A. Western blot analysis of AKT3 expression in MM and ULM cells (n=3) with (+) and without (−) miR-181a overexpression. Total AKT, GAPDH and β-actin were used as loading controls. B. Western blot analysis of phosporalated AKT1/2/3, AKT3, total AKT and Actin expression in MM and ULM cells (n=3) with (+) and without (−) miR-181a overexpression. C. Two miR-181a binding site sequences at 3’UTR of AKT3 with introduced mutant nucleotides (in red) were prepared. D. Luciferase activity in AKT3 3’UTR sequence without (left, UTR-WT) and with (right, UTR- mut) mutant binding sites of miR-181a was examined when treated with miRNA mimic control (miRNC) and miR-181a in HEK293T cell line. E. Luciferase activity in AKT3 3’UTR sequence with binding sites of miR-181a was examined when treated with miRNA mimic control (miR-NC) and miR-181a in myometrial cells. F. Western blot analysis of CCND2 expression in MM and ULM cells (n=3) with and without miR-182 overexpression. β-actin was used as loading control. β-actin was used as loading control. Small T bar stands for standard error. *p<0.05; **p<0.01.
While miR-182 has several direct and indirect target genes involved in DNA repair, several cell cycle regulators are also regulated by miR-182, including p21 and CCND219. When miR-182 was overexpressed in MM and ULM cells, upregulation of p21 was observed in some but not all cases (data not shown). In contrast, miR-182 overexpression consistently represses CCND2 expression in all MM and LM cells tested (Figure 4F). Taken together, ROS induced miR-181a and miR-182result in significant downregulation of AKT3 and CCND2.
Silencing of AKT3 and CCND2 expression drives ULM cell into cellular senescence and cycle arrest.
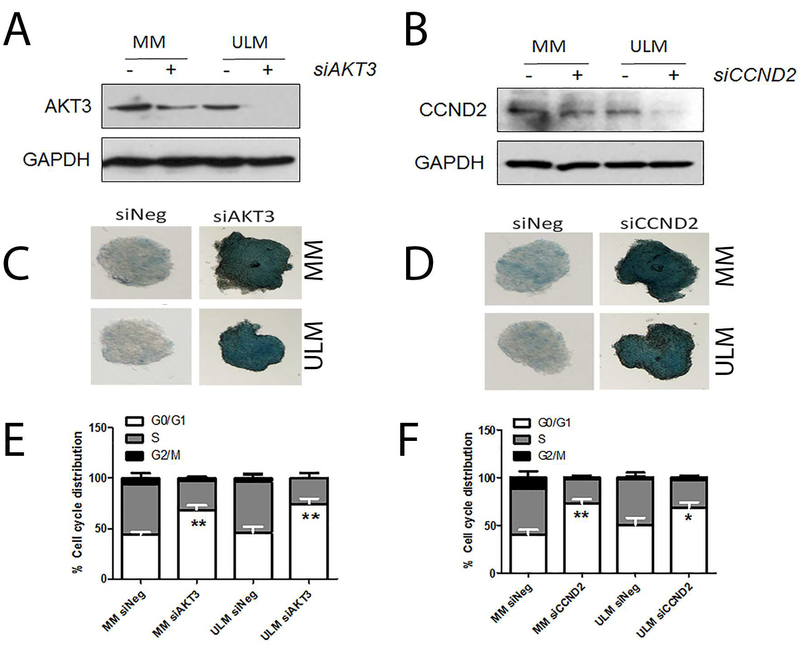
To further evaluate the functional role of AKT3 in cultured ULM and MM cells, AKT3 expression was silenced by siRNA. Upon AKT3 silencing (Fig 5A), levels of β-galactosidase increased, demonstrating an increase in senescent cells in ULM and MM spheroids (Fig 5C). Moreover, AKT3 silencing increased the percentage of cells in G0/G1 of the cell cycle (Fig 5E). Similarly, silencing CCND2(Fig 5B), showed increases in β-galactosidase staining and the number of cells in the G0/G1 phase(Fig 5D, F). These data suggest that, in particular, miR181a and miR-182serve as regulators of AKT3 and CCND2 that eventually drive cells into permanent arrest/senescence.
Figure 5:
Silencing AKT3 and CCND2 expression by siRNA resulted in cellular senescence in myometrium (MM) and leiomyoma (ULM). A-B. Western blot analysis of AKT3 and CCND2 expression with and without siRNA treatment in MM and ULM cells. GAPDH is protein loading controls. C-D. Cellular senescence was detected by global β-galactosidase stain in 3-diemensional spheroid culture of ULM and MM in siRNA of AKT3 (C) and CCND2 (D). E-F. Cell cycle analysis of percentage of MM and ULM cells with vector control, siRNA of AKT and CCND2 treatments in G0/G1 (open box), S (gray box) and G2/M (black box) phases. Small T bar stands for standard error. *p<0.05; **p<0.01.
BH3 mimietics are effectively in reducing viability of senescent cells in ULM.
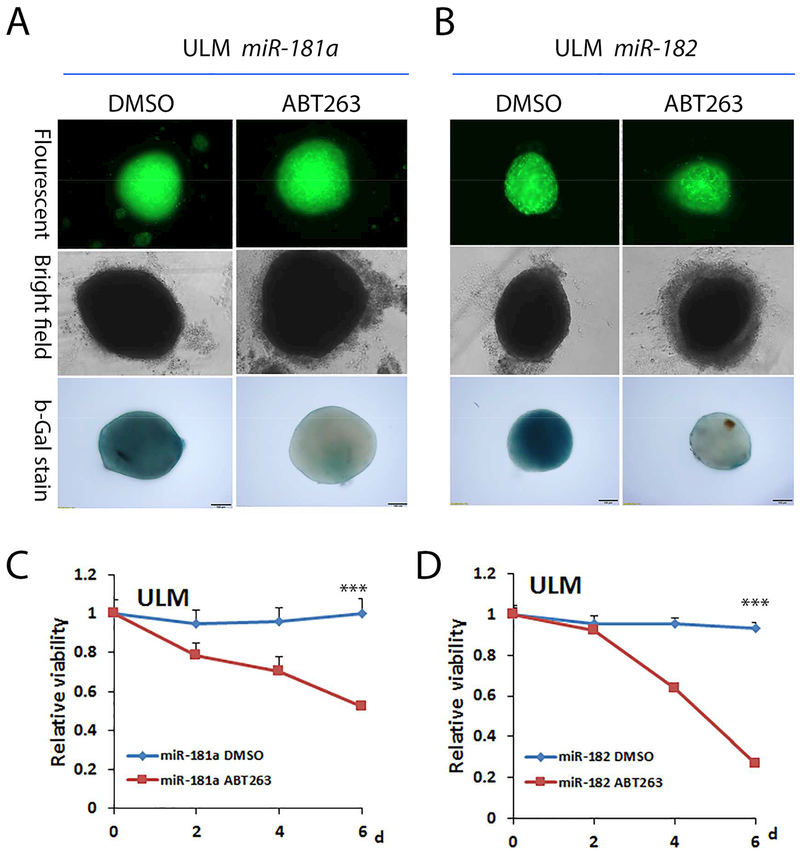
Recent studies showed that a novel class of therapeutic molecules, BH3-mimetics, can reduce or clear senescent cells through targeting at bcl-2 family 20. ABT263 is one of such compounds developed by Abbvie. To evaluate whether ABT263 can be used in reduction or removal of senescent cells in ULM, we examined the level of cellular senescence and cell viability in ULM cells. miR-181a and miR-182 was overexpressed by transduction of miRNA lentivirus in fresh collected primary ULM cells. Cells were transferred to spheroid culture and miRNA overexpression were evaluated by fluorescent scope (Figure 6A and B). Then ULM spheroids with miRNA overexpression were treated with control (DMSO) and senolytic compound (ABT263). After ABT263 treatment for four days, there was significant increase of cell death under light microscope, demonstrating a ring of diassociated cell debris around spheroids (Figure 6A and 6B). These spheroids were then fixed briefly and further examined by β-galactosidase stain. Notabley spheroids treated by ABT263 showed minimal β-galactosidase positive cells in comparison to DMSO control (Figure 6A and 6B). These findings indicate that ABT263 can effectly kill senescence ULM cells induced by miR-181a and miR-182. To further confirm the findings, the viability of ULM cells with miRNA overexpression were examined liquid base assay. As showed in Figure 6 C and D, significantly reduced cell viability could only be seen in ULM cells with miR-181a or miR-182 overexpression when treated by ABT263. The findings suggest that highly senescent ULM cells can be effectively removed by senolytic therapies such as ABT263.
Figure 6:
Treatment of ABT263, a senelytical compound, resulted in cell death of senescent cells in leiomyoma (ULM) cells. A. Phenotype analysis of spheroids of primary ULM cells with miR-181a and miR-182 overexpression followed by ABT263 treatment. Top panel: strong green fluorescence demonstrating miRNA expression induced by lentivirus; Mid panel: bright microscopic examination demonstrating a ring of diassociated cell debris in ABT263 treated shperids; lower panel: The relative β-galactosidase stain in ULM spheroids treated by control (DMSO) and test (ABT263). C an D. Relative cell viability measurement in primary culture leiomyoma (ULM) cells at 0–6 days. ULM cells with miR-181a (C) or miR-182 (D) overexpression were treated by DMSO (blue line) and ABT263 (red line). ***p<0.001.
DISCUSSION
Leiomyomas require pro-survival mechanisms to adapt to an unfavorable hypoxic microenvironment. Emerging evidence point to AKT as a major survival pathwayin ULM6, 7.Activation of AKT signaling in ULM is in part caused by oxidative stress due to hypoxia and defects of ROS metabolism6. Inhibiting the AKT pathway results in a dynamic and transient regulation of specific pathways that ultimately navigate cells into various downstream fates, including decreased proliferation and increased cellular senescence. Direct inactivation of AKT by AKT inhibitors (MK2206) in ULM resulted in significant cellular senescence in vitro 8. The mechanisms of AKT-mediated cellular senescence are unclear in ULM. Previous studies showed that AKT can either induce o or prevent senescence depending on the cellular context. In cells with RAS mutations, activation of AKT can significantly suppress RAS induced senescence and promote malignant tumor cell growth21. In ULM and other cell types, increase of ROS exposure can trigger senescence in vitro8, but elevated oxidative exposure also activates AKT signaling through depleting PTEN functions 6. It has been reported that in glioblastoma cells with PTEN mutations, AKT may directly drive cellular senescence 22. These findings suggest that AKT may act differently during senescence depending on cell types and functional status. Our findings indicate that miRNAs are importantmembers of the ROS-AKT axis during cellular senescence.
MiRNAs are important regulators of cellular response to ROS and oxidative DNA damage. A subset of miRNAs can be induced by ROS exposure 8, 23. ROS-induced miRNAs play a major role in protecting cellsfromDNA damage24, mitochondria dysfunction 25.Since MK2206-induced cellular senescence occurs inaROS-dependent manner 8, we proposed that some ROS induced miRNAs may modulate AKT activity and cell cycle genes. We found a subset of miRNAs can be induced by both ROS or AKT inhibitor MK2206 in primary ULM and matched myometrial cells. Overexpression of these miRNAs (miR-29b, miR-181a, miR-182, andmiR-200c) significantly increased cellular senescence and cell cycle arrest at G0/G1 in both ULM and myometrial cells (Figure 3). Among them, miR-181aand miR-182 overexpression resulted in a strong induction of cellular senescence in all cases of ULM and myometrial cells, observed in both monolayer and spheroid cultures (Figure 3, Suppl Figure 3).
MiR-182 targets multiple pathways. It was shown to negatively regulate BRCA114, 26 and positively regulate P21(CDKN1A) in cells with normal p53 status 27. In addition, miR-182can regulate cell cycle in response to DNA damage 24. A recent study showed miR-182 overexpression induced G1-phase arrest via inhibition of AKT/FOXO3a signaling 28. Interestingly, Cyclin D2 (CCND2) is a major cell cycle regulator downstream of AKT. AKT promotes CCND2 activity through its negative regulation of GSK-3β 29. CCND2and CDK4/6 form a complex to promote cell proliferation at G1/S transition 30. The findings of miR-182 repression of CCND2in ULM in this study (Figure 4) and other cell types 19 suggest ROS induced G1-phase arrest or senescence in ULM is related to downregulation of CCND2 by miR-182.
MiR-181a is recognized as a senescence associated miRNA and is modulated by ROS 31and highly expressed in senescent cells 32, 33. MiRNA expression profiling revealed continuous changes in the expression of miR-181a during the whole senescence process34. Importantly, pathway analysis revealed many miR-181a target genes are related to replicative senescence and cell cycle progression, both G1/S and G2/M cell cycle phase transitions and telomere maintenance35. We found miR-181ais significantly upregulated in ULM when exposed to ROS. Silicon target gene analysis revealed that several functional genes in the AKT pathway may be directly regulated bymiR-181a, including IGF2BP2 and AKT3. IGF2BP2 was recently confirmed to be miR-181atarget 36. In this study, we demonstrated that AKT3, a AKT subunit overexpressed in ULM, is the target gene ofmiR-181a. Two conservative binding sites of miR-181a at the 3’UTR of AKT were identified. Introducing miR-181a overexpression in ULM cells in vitro resulted in AKT3 downregulation. When generating specific mutations of miR-181a binding sites, miR-181a repression of AKT3 was lost. Collectively, these findings confirmed that AKT3 is the direct target of miR-181a (Figure 5). This along with its upstream target of IGF2BP2 make miR-181a a potent negative regulator for AKT pathway. Taken together, ROS-induced miR-181amay promote cellular senescence through its negative regulation of cell cycle and AKT pathway genes.
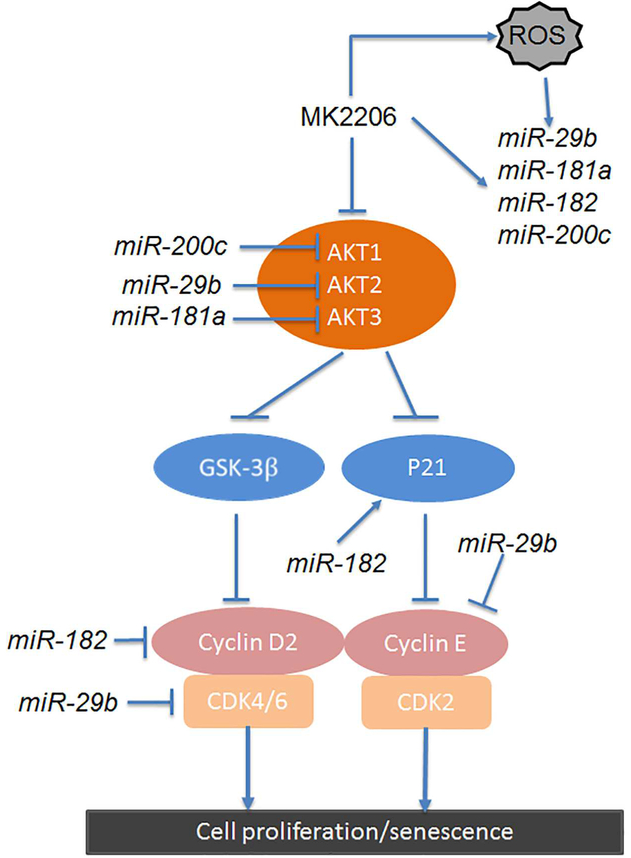
MiR-29b and miR-200c are two stress-responding miRNAs in regulation of cell proliferation and remodeling signaling. These two miRNAs express in low level in primary ULM17, 37–40 and can be induced by ROS and AKT inhibitors and also drive ULM cell into senescence. Based on current study and published data, the selected miRNAs can directly or indirectly regulate many functional genes in AKT pathway and they are summarized in Figure 7. These findings support that in an environment of oxidative stress, miRNAs and activation of AKT and its downstream effectors are intricately connected and balanced in order for leiomyoma cellsto grow and survive. If the balance is interrupted, oxidative stress can promote cellular senescence or cell death. Understanding these molecular mechanisms in ULM will help to develop targeted therapies to control ULM growth. One of challenge for such efforts is that the senescent cells may produce the undesignable effects by the acquisttion of a senescence-associated secretory phenotype (SASP). SASP may promote non-senescent tumor cell growth 41. Apparently, clearance of senescent cells is desiable. Recent studies discovered that ABT263, an anti-bcl-2 family compound, can effectively kill sesnecent cells 20. In out pilot test, when treated with ABT263 in the senescent ULM cells induced by in vitro passage and miR-182 overexpression, significant cell death of senescent cells was observed (Figure 6). This preliminary results provide a great potential for our future study in which a combined senescence and senolytic approach in treating ULM.
Figure 7:
Sketch diagram illustrated the target genes by the selected miRNAs in ROS-AKT-cyclinsenescence pathway.
In summary, we identified a subset of miRNAs which were inducible by both oxidative stress and AKT inactivation. Overexpression of these miRNAs resulted in significant cellular senescence in ULM in vitro and also in ex vivo system. Our finding suggest that under oxidative stress, an interaction of the stress-induced miRNAs and AKT activation are the major cellular and molecular events for cell survival and growth.
Supplementary Material
A subset of oxidative stress-induced miRNAs involve in AKT signaling in uterine leiomyoma
Overexpression of miR-181a and miR-182 resulted in cellular senescence in leiomyoma through repression of AKT3 and CCND2, respectively.
Silencing of AKT3 and CCND2 drive leiomyoma cell into senescence and cycle arrest.
Application of our newly developed 3D leiomyoma spheroids can provide a quick and reliable Ex Vivo model for cytopathologic and functional analysis.
BH3 mimietics can effectively reduce the viability of miRNA mediated senescent cells in leiomyoma.
Acknowledgement
We thank Drs. Serdar Bulun, Debabrata Chakravarti and Ping Yin for their valuable scientific comments and technical supports. Mrs. Stacy Ann Kujawa for consenting patients and providing all fresh samples for the study. All immunostains and histology were performed in Pathology Core Facility. This study was supported by NIH P01HD57877, NSFC grant 81572785 and China Scholarship Council.
Footnotes
Conflict of interest: Authors have nothing to disclose.
References:
- [1].Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM: High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003, 188:100–7. [DOI] [PubMed] [Google Scholar]
- [2].Pollard PJ, Briere JJ, Alam NA, Barwell J, Barclay E, Wortham NC, Hunt T, Mitchell M, Olpin S, Moat SJ, Hargreaves IP, Heales SJ, Chung YL, Griffiths JR, Dalgleish A, McGrath JA, Gleeson MJ, Hodgson SV, Poulsom R, Rustin P, Tomlinson IP: Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet 2005, 14:2231–9. [DOI] [PubMed] [Google Scholar]
- [3].Mayer A, Hockel M, Wree A, Leo C, Horn LC, Vaupel P: Lack of hypoxic response in uterine leiomyomas despite severe tissue hypoxia. Cancer Res 2008, 68:4719–26. [DOI] [PubMed] [Google Scholar]
- [4].Azimi I, Petersen RM, Thompson EW, Roberts-Thomson SJ, Monteith GR: Hypoxia-induced reactive oxygen species mediate N-cadherin and SERPINE1 expression, EGFR signalling and motility in MDA-MB-468 breast cancer cells. Sci Rep 2017, 7:15140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT: Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A 1998, 95:11715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vidimar V, Gius D, Chakravarti D, Bulun SE, Wei JJ, Kim JJ: Dysfunctional MnSOD leads to redox dysregulation and activation of prosurvival AKT signaling in uterine leiomyomas. Sci Adv 2016, 2:e1601132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sefton EC, Qiang W, Serna V, Kurita T, Wei JJ, Chakravarti D, Kim JJ: MK-2206, an AKT Inhibitor, Promotes Caspase-Independent Cell Death and Inhibits Leiomyoma Growth. Endocrinology 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xu X, Lu Z, Qiang W, Vidimar V, Kong B, Kim JJ, Wei JJ: Inactivation of AKT induces cellular senescence in uterine leiomyoma. Endocrinology 2014, 155:1510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Magenta A, Greco S, Gaetano C, Martelli F: Oxidative stress and microRNAs in vascular diseases. International journal of molecular sciences 2013, 14:17319–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P: Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reproductive biology and endocrinology : RB&E 2009, 7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D: ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid Med Cell Longev 2016, 2016:3565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu M, Mo YY: The Akt-associated microRNAs. Cell Mol Life Sci 2012, 69:3601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xu X, Ayub B, Liu Z, Serna VA, Qiang W, Liu Y, Hernando E, Zabludoff S, Kurita T, Kong B, Wei JJ: Anti-miR182 reduces ovarian cancer burden, invasion, and metastasis: an in vivo study in orthotopic xenografts of nude mice. Molecular cancer therapeutics 2014, 13:1729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu Z, Liu J, Segura MF, Shao C, Lee P, Gong Y, Hernando E, Wei JJ: MiR-182 overexpression in tumourigenesis of high-grade serous ovarian carcinoma. J Pathol 2012, 228:204–15. [DOI] [PubMed] [Google Scholar]
- [15].Li X, Zhang J, Gao L, McClellan S, Finan MA, Butler TW, Owen LB, Piazza GA, Xi Y: MiR-181 mediates cell differentiation by interrupting the Lin28 and let-7 feedback circuit. Cell death and differentiation 2012, 19:378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wei JJ, Wu X, Peng Y, Shi G, Basturk O, Yang X, Daniels G, Osman I, Ouyang J, Hernando E, Pellicer A, Rhim JS, Melamed J, Lee P: Regulation of HMGA1 expression by microRNA-296 affects prostate cancer growth and invasion. Clinical cancer research : an official journal of the American Association for Cancer Research 2011, 17:1297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, Mittal K, Soteropoulos P, Wei JJ: A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer 2007, 46:336–47. [DOI] [PubMed] [Google Scholar]
- [18].Zavadil J, Ye H, Liu Z, Wu J, Lee P, Hernando E, Soteropoulos P, Toruner GA, Wei JJ: Profiling and functional analyses of microRNAs and their target gene products in human uterine leiomyomas. PLoS One 2010, 5:e12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yan D, Dong XD, Chen X, Yao S, Wang L, Wang J, Wang C, Hu DN, Qu J, Tu L: Role of microRNA-182 in posterior uveal melanoma: regulation of tumor development through MITF, BCL2 and cyclin D2. PLoS One 2012, 7:e40967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D: Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 2016, 22:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kennedy AL, Morton JP, Manoharan I, Nelson DM, Jamieson NB, Pawlikowski JS, McBryan T, Doyle B, McKay C, Oien KA, Enders GH, Zhang R, Sansom OJ, Adams PD: Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol Cell 2011, 42:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee JJ, Kim BC, Park MJ, Lee YS, Kim YN, Lee BL, Lee JS: PTEN status switches cell fate between premature senescence and apoptosis in glioma exposed to ionizing radiation. Cell Death Differ 2011, 18:666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li G, Luna C, Qiu J, Epstein DL, Gonzalez P: Alterations in microRNA expression in stress-induced cellular senescence. Mech Ageing Dev 2009, 130:731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Krishnan K, Steptoe AL, Martin HC, Wani S, Nones K, Waddell N, Mariasegaram M, Simpson PT, Lakhani SR, Gabrielli B, Vlassov A, Cloonan N, Grimmond SM: MicroRNA-182–5p targets a network of genes involved in DNA repair. RNA 2013, 19:230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang L, Huang H, Fan Y, Kong B, Hu H, Hu K, Guo J, Mei Y, Liu WL: Effects of downregulation of microRNA-181a on H2O2-induced H9c2 cell apoptosis via the mitochondrial apoptotic pathway. Oxid Med Cell Longev 2014, 2014:960362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moskwa P, Buffa FM, Pan Y, Panchakshari R, Gottipati P, Muschel RJ, Beech J, Kulshrestha R, Abdelmohsen K, Weinstock DM, Gorospe M, Harris AL, Helleday T, Chowdhury D: miR-182-mediated downregulation of BRCA1 impacts DNA repair and sensitivity to PARP inhibitors. Mol Cell 2011, 41:210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu Y, Qiang W, Xu X, Dong R, Karst AM, Liu Z, Kong B, Drapkin RI, Wei JJ: Role of miR-182 in response to oxidative stress in the cell fate of human fallopian tube epithelial cells. Oncotarget 2015, 6:38983–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xu X, Wu J, Li S, Hu Z, Xu X, Zhu Y, Liang Z, Wang X, Lin Y, Mao Y, Chen H, Luo J, Liu B, Zheng X, Xie L: Downregulation of microRNA-182–5p contributes to renal cell carcinoma proliferation via activating the AKT/FOXO3a signaling pathway. Mol Cancer 2014, 13:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mirzaa G, Parry DA, Fry AE, Giamanco KA, Schwartzentruber J, Vanstone M, Logan CV, Roberts N, Johnson CA, Singh S, Kholmanskikh SS, Adams C, Hodge RD, Hevner RF, Bonthron DT, Braun KPJ, Faivre L, Riviere JB, St-Onge J, Gripp KW, Mancini GM, Pang K, Sweeney E, van Esch H, Verbeek N, Wieczorek D, Steinraths M, Majewski J, Consortium FC, Boycot KM, Pilz DT, Ross ME, Dobyns WB, Sheridan EG: De novo CCND2 mutations leading to stabilization of cyclin D2 cause megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome. Nat Genet 2014, 46:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sherr CJ: D-type cyclins. Trends Biochem Sci 1995, 20:187–90. [DOI] [PubMed] [Google Scholar]
- [31].Magenta A, Dellambra E, Ciarapica R, Capogrossi MC: Oxidative stress, microRNAs and cytosolic calcium homeostasis. Cell Calcium 2016, 60:207–17. [DOI] [PubMed] [Google Scholar]
- [32].Mancini M, Saintigny G, Mahe C, Annicchiarico-Petruzzelli M, Melino G, Candi E: MicroRNA-152 and −181a participate in human dermal fibroblasts senescence acting on cell adhesion and remodeling of the extra-cellular matrix. Aging (Albany NY) 2012, 4:843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Markopoulos GS, Roupakia E, Tokamani M, Vartholomatos G, Tzavaras T, Hatziapostolou M, Fackelmayer FO, Sandaltzopoulos R, Polytarchou C, Kolettas E: Senescence-associated microRNAs target cell cycle regulatory genes in normal human lung fibroblasts. Exp Gerontol 2017, 96:110–22. [DOI] [PubMed] [Google Scholar]
- [34].Yentrapalli R, Azimzadeh O, Kraemer A, Malinowsky K, Sarioglu H, Becker KF, Atkinson MJ, Moertl S, Tapio S: Quantitative and integrated proteome and microRNA analysis of endothelial replicative senescence. J Proteomics 2015, 126:12–23. [DOI] [PubMed] [Google Scholar]
- [35].Rippo MR, Olivieri F, Monsurro V, Prattichizzo F, Albertini MC, Procopio AD: MitomiRs in human inflamm-aging: a hypothesis involving miR-181a, miR-34a and miR-146a. Exp Gerontol 2014, 56:154–63. [DOI] [PubMed] [Google Scholar]
- [36].Wu L, Song WY, Xie Y, Hu LL, Hou XM, Wang R, Gao Y, Zhang JN, Zhang L, Li WW, Zhu C, Gao ZY, Sun YP: miR-181a-5p suppresses invasion and migration of HTR-8/SVneo cells by directly targeting IGF2BP2. Cell Death Dis 2018, 9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zavadil J, Ye H, Liu Z, Wu J, Lee P, Hernando E, Soteropoulos P, Toruner GA, Wei J-J: Profiling and functional analyses of microRNAs and their target gene products in human uterine leiomyomas. PloS one 2010, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chuang TD, Khorram O: Mechanisms underlying aberrant expression of miR-29c in uterine leiomyoma. Fertil Steril 2016, 105:236–45 e1. [DOI] [PubMed] [Google Scholar]
- [39].Marsh EE, Steinberg ML, Parker JB, Wu J, Chakravarti D, Bulun SE: Decreased expression of microRNA-29 family in leiomyoma contributes to increased major fibrillar collagen production. Fertil Steril 2016, 106:766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chuang TD, Panda H, Luo X, Chegini N: miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. Endocr Relat Cancer 2012, 19:541–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Coppe JP, Desprez PY, Krtolica A, Campisi J: The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010, 5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.