Abstract
Erythema ab igne (EAI) is a cutaneous finding caused by prolonged heat exposure and is characterized by a reticular, brownish-pigmented, often telangiectatic dermatosis. The eruption is reminiscent of livedo reticularis which is typically seen in the setting of a number of rheumatologic conditions, most prominently vasculitis. Identification of key features distinguishing EAI from livedo reticularis can aid in the diagnosis of EAI and correct elucidation of the underlying etiology. Our patient presented with heating-pad induced EAI in the setting of chronic pain. Only 6 other pediatric cases of EAI associated with heat sources for chronic pain are reported1–6. Our case highlights the need for awareness of this pathognomonic skin eruption in children with chronic pain conditions to help avoid an extensive workup for vasculitis.
Patient Case
A 16 year old female with a complex medical history was admitted to the hospital for an expedited evaluation of worsening skin changes, dyspnea, chest pain, headaches, decreased sensation in her left leg, constipation, hypertension, and abdominal pain. The patient had a history of nephrolithiasis status-post multiple surgeries, spinal cord syrinx, hypertension, ovarian torsion and scoliosis. Her symptoms started 4 months earlier with left lower back and left lower quadrant pain accompanied by nausea. Her pain was alleviated by over the counter medications, chronic use of heating pads (including overnight exposure) and hot showers. She denied allodynia or autonomic changes associated with her pain. She described an aching in her legs that was exacerbated by physical activity, reminiscent of claudication. Three months prior to presentation, the patient noticed a lacy skin eruption on her abdomen and back with mild tenderness. She was switched from amlodipine to furosemide for her hypertension prior to the onset of her skin eruption but experienced no other new drug exposures. One week prior to admission the patient noticed decreased sensation on her left leg. An ultrasound was performed and did not reveal a deep venous thrombus.
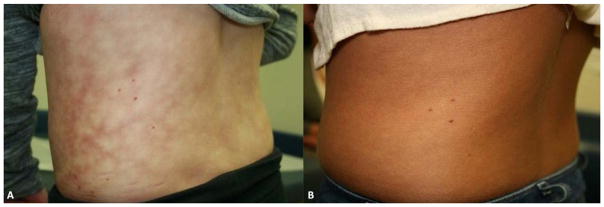
On admission, she was afebrile, tachycardic and hypertensive with a pulse of 128 beats per minute and blood pressure of 133/87 mmHg (98th percentile systolic and 97th percentile diastolic). Her exam was notable for decreased sensation to light touch on her left leg from the mid-thigh to mid-calf. She also had a lacey, mottled brown patch on the trunk and groin concerning for livedo reticularis (Figure 1A). She reported her pain to be 7/10 in the left lower quadrant for which nalbuphine was administered. A pelvic ultrasound did not demonstrate ovarian torsion. Initial laboratory testing revealed a normal complete blood cell count, creatinine kinase, lactate dehydrogenase, uric acid, erythrocyte sedimentation rate and partial thromboplastin time. A chest radiograph did not reveal evidence for acute cardiopulmonary process.
Figure 1. Patient’s Skin Eruption Before and After Heating Pad Use.
A) The patient had a lacey, mottled skin eruption on the trunk, characteristic of EAI.
B) The reticulated patch significantly improved after 6 months of heat avoidance to the affected area.
Rheumatology was consulted for concern for vasculitis given her livedo-like skin changes, abdominal pain, and hypertension. The differential included Takayasu arteritis or polyarteritis nodosa (PAN), systemic lupus erythematosus (SLE), anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis and anti-phospholipid antibody syndrome (APS). Testing for SLE, including anti-nuclear antibody (ANA) profile and anti-histone antibody were negative except for a mildly elevated ANA titer (1:80). Testing for APS was negative and testing for ANCA-associated vasculitis was also negative. CT-angiograms of the chest, abdomen, pelvis and neck were all normal.
Neurology was consulted given her decreased left lower extremity sensation and history of a syrinx. No focal neurologic deficits were noted on examination. Magnetic resonance imaging/angiography (MRI/MRA) of the brain were performed which were unremarkable, except for the incidental finding of a small pars intermedia cyst that was felt to be non-contributory. At this point, given the negative diagnostic tests for both possible neurologic and rheumatologic etiologies, dermatology was consulted.
Physical examination by dermatology noted a non-blanching, non-tender, coarse, hyperpigmented, reticulated patch involving the lower back and wrapping around the left flank. Similar eruption was not noted on the remainder of skin examination. The distribution corresponded to that of the heating pad the patient had been using for her pain and the lacy hyperpigmented appearance was consistent with a diagnosis of EAI.
Due to the lack of systemic inflammation, normal imaging, and benign cutaneous eruption, an underlying vasculitis was excluded and the patient was diagnosed with localized amplified musculoskeletal pain syndrome (AMPS). She was advised to discontinue heating pads and referred to the AMPS clinic where she was successfully treated with a multi-disciplinary approach including psychological counseling and physical and occupational therapy. Her EAI slowly improved and resolved within 6 months (Figure 1B). Her pain resolved as well and she was fully functional, attending school fulltime.
Discussion
We present a patient with a lacy, reticulated, hyperpigmented patch on the lower back initially concerning for livedo reticularis in the setting of multiple incongruent neurologic and systemic complaints. Due to concerns for polyarteritis nodosa (PAN), a full rheumatologic and neurologic evaluation was requested and was not indicative of an underlying occult process. Dermatologic evaluation of the eruption led to a final diagnosis of EAI and the remaining systemic symptoms led to a final diagnosis of AMPS.
EAI is a benign cutaneous finding characterized by asymptomatic, geographic, brown, reticulated patch due to chronic exposure to an external heat source. The exact pathophysiology is unknown but repeated exposure to submaximal infrared heat is proposed to lead to persistent reticulated erythema due to local hemostasis and vasodilation. Overtime, red blood cells extravasate into the dermis from dilated blood vessels and are degraded in the dermis leaving hemosiderin. The hemosiderin causes the brown reticulated appearance of EAI. A skin biopsy is not necessary for diagnosis. Most frequent causes of EAI in children include radiant heaters7, laptops computers8, hot water bottles4, and heat packs2. There are six reports in the English literature of children developing EAI after direct application of heat sources such as heating pads and water bottles to the skin for abdominal cramping and pain1–6..
The skin eruption of EAI can commonly be mistaken for livedo reticularis, a violaceous, often symmetric, partially blanching, reticular eruption usually on the legs that reflects an underlying change in cutaneous blood flow 9. Livedo reticularis can be classified as physiologic, known as cutis marmorata, or pathologic. Cutis marmorata represents a normal vasospastic response upon exposure to the cold and resolves with re-warming. Pathologic livedo reticularis does not resolve with rewarming and can be divided into congenital and acquired causes, the latter of which is the result of vasospasm, reduced intravascular flow, vessel-wall pathology or vessel obstruction 9. Table 1 outlines the differential diagnosis of EAI and its potential mimickers. Potential etiologies include neoplasms, neurologic disorders, connective tissue disorders, occlusive vascular disease and vasculitides10, 11. Panniculitis12, infections13, and drug exposures (amantadine and memantine)14, 15 may also result in skin eruptions mimicking EAI. Adolescents and young adults with eating disorders have been reported to use heating pads to reduce feelings of coldness and alleviate the sensation of fullness after eating 3, 16–18. Therefore anorexia nervosa and bulimia must be considered in patients with a skin eruption mimicking EAI. Lastly, livedo reticularis when associated with rheumatologic conditions, such as APS, is a poor prognostic factor 19. Therefore, identification of the underlying etiology of pathologic livedo reticularis is warranted to prevent morbidity and mortality.
Table 1.
Differential Diagnoses of Erythema Ab Igne and Its Mimickers
| Etiology | Examples of Conditions |
|---|---|
| Autoimmune/Connective tissue diseases | Dermatomyositis, Sjögren syndrome, Vasculitides (e.g. polyartertis nodosa), Systemic lupus erythematosus |
| Environmental (e.g. heat) exposures | Chronic pain, Eating disorders, Electronic use |
| Hematologic/Hypercoagulable | Antiphospholipid antibody syndrome, Deep venous thrombosis, Thrombotic thrombocytopenic purpura |
| Infections | Mycoplasma pneumonia, Brucella, Parvovirus B19, Rheumatic fever, Endocarditis |
| Medication Exposures | Amantadine, Bismuth, Memantine, Minocycline |
| Neurologic | Reflex sympathetic dystrophy, Encephalitis, Susac’s syndrome |
| Physiologic/Benign | Cutis marmorata |
In our patient, the livedo reticularis-like eruption was most concerning for PAN, a primary small and medium vessel vasculitis characterized by lethargy, various cutaneous findings including livedo reticularis, and other constitutional symptoms.20 Our patient’s hypertension, skin findings and possible peripheral neuropathy were all symptoms pointing towards a diagnosis of PAN. Confirmation of the diagnosis is needed with either a biopsy demonstrating necrotizing inflammation of a medium vessel or demonstrable angiographic abnormalities. After consultation with dermatology, repeated and chronic exposure history of heating pads, and a negative workup for underlying vasculitis including a normal CT-angiography, the patient was diagnosed with a chronic non-inflammatory musculoskeletal pain condition known as amplified musculoskeletal pain syndrome (AMPS)
Our patient had a number of concerning symptoms, including a challenging reticulated skin eruption concerning for livedo reticularis necessitating costly and invasive diagnostic testing to rule out underlying vascular pathology. This increased healthcare utilization and over-medicalization of children with chronic pain is common in AMPS21, 22. Increased awareness of the distinctive and puzzling presentation of EAI among medical care providers and its consideration in patients with chronic pain is of utmost importance and may avoid unnecessary and expensive medical testing.
Conclusions
EAI can resemble livedo reticularis which may be due to vasculitides, infections, thromboses, or infections. Differentiating between these can avoid an expensive workup. Our patient presented with heating-pad induced EAI in the setting of chronic pain. Our case highlights the need for an increased suspicion of this pathognomonic skin finding in children with chronic pain conditions.
Acknowledgments
Funding sources: No funding was secured for this study. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers T32-AR007442 (Gmuca) and K23-AR059749 (Weiss). Dr. Sherry is supported by the Snider Family. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AMPS
amplified musculoskeletal pain syndrome
- ANA
anti-nuclear antibody
- ANCA
anti-neutrophil cytoplasmic antibody
- APS
anti-phospholipid antibody syndrome
- CT
computed tomography
- GFR
glomerular filtration rate
- MRI
magnetic resonance imaging
- PAN
polyarteritis nodosa
- SLE
systemic lupus erythematosus
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Dizdarevic A, Karim OA, Bygum A. A reddish brown reticulated hyperpigmented erythema on the abdomen of a girl. Erythema ab igne, also known as toasted skin syndrome, caused by a heating pad on the abdomen. Acta derm Venereol. 2014;94(3):365–367. doi: 10.2340/00015555-1722. [DOI] [PubMed] [Google Scholar]
- 2.Steadmon MJ, Riley KN. Erythema ab igne: a comeback story. J Pediatr. 2013;163(6):1789. doi: 10.1016/j.jpeds.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Docx MK, Simons A, Ramet J, Mertens L. Erythema ab igne in an adolescent with anorexia nervosa. Int J Eat Disord. 2013;46(4):381–383. doi: 10.1002/eat.22075. [DOI] [PubMed] [Google Scholar]
- 4.Tighe MP, Morenas RA, Afzal NA, Beattie RM. Erythema ab igne and Crohn’s disease. Arch Dis Child. 2008;93(5):389. doi: 10.1136/adc.2008.137968. [DOI] [PubMed] [Google Scholar]
- 5.Goldman JL, Nopper AJ, Myers AL. Picture of the month--quiz case. Erythema ab igne. Arch Pediatr Adolesc Med. 2012;166(2):185–186. doi: 10.1001/archpediatrics.2011.762a. [DOI] [PubMed] [Google Scholar]
- 6.Mucklow ES, Freeman NV. Pancreatic ascites in childhood. Br J Clin Pract. 1990;44(6):248–251. [PubMed] [Google Scholar]
- 7.Brzezinski P, Ismail S, Chiriac A. Radiator-induced erythema ab igne in 8-year-old girl. Revista chilena de pediatria. 2014;85(2):239–240. doi: 10.4067/S0370-41062014000200015. [DOI] [PubMed] [Google Scholar]
- 8.Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126(5):e1227–1230. doi: 10.1542/peds.2010-1390. [DOI] [PubMed] [Google Scholar]
- 9.Rose AE, Sagger V, Boyd KP, Patel RR, McLellan B. Livedo reticularis. Dermatol Online J. 2013;19(12):20705. [PubMed] [Google Scholar]
- 10.Sajjan VV, Lunge S, Swamy MB, Pandit AM. Livedo reticularis: A review of the literature. Indian Dermatol Online J. 2015;6(5):315–321. doi: 10.4103/2229-5178.164493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engeholm M, Leo-Kottler B, Rempp H, Lindig T, Lerche H, Kleffner I, et al. Encephalopathic Susac’s Syndrome associated with livedo racemosa in a young woman before the completion of family planning. BMC Neurol. 2013;13:185. doi: 10.1186/1471-2377-13-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borgia F, De Pasquale L, Cacace C, Meo P, Guarneri C, Cannavo SP. Subcutaneous fat necrosis of the newborn: be aware of hypercalcaemia. J Paediatr Child Health. 2006;42(5):316–318. doi: 10.1111/j.1440-1754.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- 13.Treister-Goltzman Y, Peleg R. Erythema ab igne. Am J Trop Med Hyg. 2015;92(3):476–476. doi: 10.4269/ajtmh.14-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsner P, Schliemann S. Erythema ab igne as an occupational skin disease (BK 5101) J Dtsch Dermatol. 2014;12(7):621–622. doi: 10.1111/ddg.12303. [DOI] [PubMed] [Google Scholar]
- 15.Xu LY, Liu A, Kerr HA. Livedo reticularis from amantadine. Skinmed. 2011;9(5):320–321. [PubMed] [Google Scholar]
- 16.Dessinioti C, Katsambas A, Tzavela E, Karountzos V, Tsitsika AK. Erythema Ab Igne in Three Girls with Anorexia Nervosa. Pediatr Dermatol. 2016;33(2):e149–150. doi: 10.1111/pde.12770. [DOI] [PubMed] [Google Scholar]
- 17.Beneke J, Koerner M, de Zwaan M. Erythema ab igne in a patient with bulimia nervosa. Psychother Psychosom Med Psychol. 2014;64(5):197–199. doi: 10.1055/s-0033-1361141. [DOI] [PubMed] [Google Scholar]
- 18.Fischer J, Rein K, Erfurt-Berge C, de Zwaan M. Three cases of erythma ab igne (EAI) in patients with eating disorders. Neuropsychiatr. 2010;24(2):141–143. [PubMed] [Google Scholar]
- 19.Sangle SR, D’Cruz DP. Livedo Reticularis: An Enigma. Isr Med Assoc J. 2015;17(2):104–107. [PubMed] [Google Scholar]
- 20.Woo P, Laxer RM, Sherry DD. Pediatric Rheumatology in Clinical Practice. Springer; London: 2007. [Google Scholar]
- 21.Kaufman EL, Tress J, Sherry DD. Trends in Medicalization of Children with Amplified Musculoskeletal Pain Syndrome. Pain Med. 2016 doi: 10.1093/pm/pnw188. [DOI] [PubMed] [Google Scholar]
- 22.Tian F, Guittar P, Bout-Tabaku S. Chronic Pain in Children Seen at a Rheumatology Clinic: Healthcare Utilization Patterns [abstract] Arthritis Rheumatol. 2016;68(suppl 10) [Google Scholar]