Abstract
The peripartum period is associated with the onset of behaviors that shelter, feed and protect young offspring from harm. The neural pathway that regulates caregiving behaviors has been mapped in female rats and is conserved in mice. However, rats rely on late gestational hormones to shift their perception of infant cues from aversive to attractive, whereas laboratory mice are “spontaneously” maternal, but their level of responding depends on experience. For example, pup-naïve virgin female mice readily care for pups in the home cage, but avoid pups in a novel environment. In contrast, pup-experienced virgin mice care for pups in both contexts. Thus, virgin mice rely on experience to shift their perception of infant cues from aversive to attractive in a novel context. We hypothesize that alterations in immediate early gene activation may underlie the experience-driven shift in which neural pathways (fear/avoidance versus maternal/approach) are activated by pups to modulate context-dependent changes in maternal responding. Here we report that the effects of sodium butyrate, a drug which allows for an amplification of experience-induced histone acetylation and gene expression in virgins, are comparable to the natural onset of caregiving behaviors in postpartum mice and induce postpartum-like patterns of immediate early gene expression across brain regions. These data suggest that pups can activate a fear/defensive circuit in mice and experience-driven improvements in caregiving behavior could be regulated in part through decreased activation of this pathway.
Keywords: maternal experience, histone acetylation, immediate early gene expression
Introduction
Mothering involves complex behavioral modifications aimed at ensuring the survival of progeny, which include the provision of warmth, food, transport, tactile contact and protection for their offspring. These behavioral modifications are robust, energetically demanding and unique to the maternal state (Numan and Woodside, 2010). For the most part, the behavioral transition from a non-maternal to maternal state is unidirectional and stable even under challenging circumstances (Ayers et al., 2016; Kenny et al., 2014; McLeod et al., 2007; Orpen and Fleming, 1987).
Mothering behaviors typically first emerge around the time of parturition such that the onset of maternal care is synchronized with the birth of one’s own offspring (Numan, 2015). New mothers are attracted to infant cues and readily care for their offspring (Olazabal et al., 2013). However, the continued care of young until weaning is ultimately dependent on the experience of interacting with infants (Bridges, 1975; Numan, 2015; Numan and Stolzenberg, 2009; Numan, 2003; Stolzenberg and Champagne, 2016). Further, conspecifics that have not been exposed to pregnancy hormones (such as fathers or other kin) participate in the rearing of young in some species and humans appear to be emancipated from hormonal control as fathers and/or non-biological parents provide high levels of care toward infants (Numan, 2003). Thus, other mechanisms, which do not involve hormonal changes during late gestation, must be capable of activating maternal neural pathways to regulate caregiving behaviors.
We have proposed that in the absence of pregnancy hormone exposure experience with pups alone may drive plasticity in maternal neural circuits by increasing the acetylation of histone proteins within gene promoters, thus regulating transcriptional programs within medial preoptic area (MPOA) neurons that ultimately function to regulate maternal responses (Stolzenberg and Champagne, 2016; Stolzenberg et al., 2012, 2014). This hypothesis is based on the idea that the hormonal events of late gestation (namely estradiol stimulation) affect estrogen receptor mediated transcription through alterations in histone acetylation involving the recruitment of the transcriptional coactivator and histone acetyltransferase enzyme cyclic AMP response element binding protein (CREB) binding protein (CBP) (Duong et al., 2006; Gagnidze et al., 2013; Kamei et al., 1996; Sharma et al., 2012). In support of this idea, administration of the HDACi drug sodium butyrate, which allows for increased histone acetylation, increased the expression of candidate genes known to be involved in maternal responsiveness within the MPOA and reduced the amount of pup exposure required for the experience-driven onset of maternal care (Stolzenberg et al., 2012, 2014). However the extent to which HDAC inhibition induces a maternal state that is comparable to that of a postpartum female is presently unclear. Further, given that the administration of the HDACi is systemic, it is not clear how this drug affects neural pathways downstream from MPOA, which are involved in the regulation of maternal care. The present study is aimed at addressing these two issues.
A convincing body of work has demonstrated that regardless of whether the maternal state is induced by hormonal priming or repeated pup exposure; maternal behavior is associated with a conserved neural circuit. In particular, functional neuroanatomical work indicates that MPOA projections to the ventral tegmental area (VTA) must remain intact for mothering behavior to occur (Numan and Numan, 1991; Numan and Smith, 1984; Numan et al., 2009). Subsequent regions downstream from VTA activation such as the nucleus accumbens (NA) and ventral pallidum (VP) also must receive inputs from the MPOA to regulate maternal care (Numan et al., 2005). This circuit is conserved in males, and across several species (Akther et al., 2014; Lee and Brown, 2002; Zhong et al., 2014). Whereas caregiving behaviors emerge under certain conditions that permit infant activation of this conserved maternal neural pathway, non-parental responses toward infant conspecifics are induced by hypothalamic pathways known to regulate anxiety/escape/attack behaviors (Sheehan et al., 2000; Tachikawa et al., 2013). For example, non-maternal rats show significantly more expression of the neural activity marker, c-Fos, in the anterior hypothalamic nucleus (AHN) in response to pup stimuli (Sheehan et al., 2000). The AHN gets olfactory information about pups from the medial amygdala (meA) and it projects to the periaqueductal gray (PAG). Lesions of the meA, AHN or PAG all facilitate maternal behavior in non-maternal rats and the fact that disconnection of the meA and AHN circuitry stimulates the onset of maternal care suggests that these two regions function in a serial circuit to block caregiving behavior (Numan and Sheehan, 1997; Sheehan et al., 2001; Sukikara et al., 2006).
In the current series of experiments we investigated the effects of HDACi treatment in virgin female and naturally postpartum mice on caregiving responses in a familiar and novel environment. Given that pregnancy hormones facilitate the onset of caregiving behaviors by increasing attraction toward infant odors (Fleming et al., 1989; Fleming and Luebke, 1981; Fleming and Rosenblatt, 1974; Kinsley and Bridges, 1990; Lonstein et al., 2015) we also asked whether HDACi treatment produced effects on how female mice respond to social odors. Finally, we examined the expression of immediate early genes (IEGs) in two neural pathways that respond to infant stimuli using an experimental design in which maternal state was not dependent on the amount of pup experience, but rather the environmental context the subject was tested in. We chose to focus on two regulatory IEGs, Fos and Egr1, whose products act as transcription factors to regulate later responding genes involved in synaptic strengthening. In addition, we chose to examine the effector IEG Arc, which is directly involved in the regulation of neuronal activity-dependent synaptic plasticity (Bramham et al., 2010; Guzowski et al., 2001; Lyford et al., 1995). Arc was also examined because its expression is particularly sensitive to context (Klebaur et al., 2002; Link et al., 1995; Ramírez-Amaya et al., 2005; Vazdarjanova et al., 2002).
Methods and Materials
Subjects and drug treatment
All mice were C57BL/6J nulliparous adult females (60+ days of age) naive to pups (except for their own littermates). Sodium butyrate (SB; Sigma-Aldrich) was dissolved in sterile drinking water and was administered at a dose of 8mg/mL in the drinking water. Control mice received standard drinking water. Drinking water containing sodium butyrate was given ad libitum beginning at least 24 hours prior to the first 2-hour experience and continued throughout testing. Daily drinking water was monitored for all animals. All mice were single housed during this time. A separate group of C57BL/6J females, drinking normal water, served as foster dams that provided stimulus pups. Although the average litter size in our colony is 5-6 pups, 3 stimulus pups were used for testing. Mice were housed on a 12-hour reverse light cycle and given food and water ad libitum. Behavioral testing began one hour into the dark phase of the light/dark cycle under dim red light. All procedures were in compliance with the University of California, Davis Institutional Animal Care and Use Committee.
Behavioral Procedures
Home cage tests
Maternal behavior testing was conducted as described previously (Stolzenberg et al., 2012, 2014). Briefly, during the dark phase of the light/dark cycle, 3 stimulus pups (0-5 days old, mixed sex) were scattered in the home cage. Latency to sniff, retrieve the first pup back to the nest, retrieve all 3 stimulus pups back to the nest and lick and hover over pups in the nest were recorded during the first 15 minutes of the test. During the last 15 minutes of the first hour of testing, frequency of licking, crouching, and hovering over pups was recorded every 15 seconds.
T-maze pup retrieval test
The walls and floors of the T-maze apparatus (67.3×11.4×8.3 cm) were clear Plexiglas and the vertical runway measured 48.3 cm in length and opened into a horizontal runway that measured 67.3 cm in length. An 11.4 cm × 12.7 cm goal box was attached to the end of the vertical runway which could be closed off from the rest of the T-maze by a clear Plexiglas guillotine door. At the start of the retrieval test, each female was placed into the goal box of the T-maze with her nest material. Three stimulus pups were placed on the horizontal arm of the T-maze. After a 10-minute habituation period, the Plexiglas door was removed and the 15-minute pup retrieval test began. During the 15-minute retrieval test, latency to emerge (all four paws) from the goal box, latency to sniff the first pup, and latency to retrieve all 3 stimulus pups to the goal box were recorded. The test ended after 15 minutes, or when the female had retrieved all 3 pups to the goal box.
Olfactory preference
Mice were habituated to a Noldus Phenotyper (45 cm × 45 cm) test box for 10 minutes with 3 small (38/25 mm) plastic disposable weigh boats filled with clean bedding. Mice were briefly returned to the home cage while 3 new plastic weigh boats each containing 15ml of pup soiled, male soiled or clean bedding were placed in the test box. Pup soiled bedding (nesting material) was obtained from the dirty cages of novel donor females. Male bedding was taken from the dirty cages of sexually experienced mice. The order the odor stimuli were placed in was counterbalanced. Time spent sniffing each odor was manually coded from each video file using (Noldus Observer XT 12) software by an observer blind to group and condition. Sniffing was defined as the nose being directly over or in direct contact with bedding.
Olfactory discrimination
An odor habituation-dishabituation task was used to test whether there were differences in olfactory abilities between groups (Yang and Crawley, 2009; Zou et al., 2015). Male urine was collected from multiple sexually experienced adult male mice and pooled into a single tube. Pup urine was collected from pups aged 4-7 days old by holding each pup on its back and collecting urine in a microcentrifuge tube. Aliquots were used immediately or stored at −20°C. On the day of testing, mice were repeatedly exposed to volatile odors from water or urine. Ten μl of water or urine was dropped onto a piece of filter paper taped to a small plastic weigh boat and inverted onto a clean empty wire cage lid. Each odor was presented for 3 consecutive 2-minute trials with a 1-minute inter-trial interval. Mice received a total of 9 odor trials. The order of the odors was the same for each mouse; water was presented for the first 3 trials, followed by 3 presentations of male urine, followed by 3 presentations of pup urine. The total number of seconds the mouse spent sniffing each odor was recorded using a stopwatch.
Quantification of mRNA by real time PCR
Mice were briefly anesthetized with isoflurane and euthanized by cervical dislocation. Brains were immediately removed, frozen, and later sectioned (120 microns) on a cryostat and frost-mounted onto slides. The MPOA (Bregma 0.37 to −0.35), AHN (Bregma −0.59 to −1.67), meA (Bregma −1.31 to −2.03), VTA (Bregma −2.69 to −3.51), and PAG (−3.07 to −3.87) were dissected out using a blunted 15.5 gauge needle using coordinates from the Franklin and Paxinos Mouse Brain Atlas. Total RNA was isolated with Qiazol reagent (Qiagen) and purified with an RNeasy® Plus Micro Kit (74004; Qiagen, Valencia, CA) as well as the optional DNase digestion (Qiagen 129046). A Nanodrop™ Spectrophotometer was used to determine the quality and quantity of the RNA. Samples in which the 260/280 ratio was lower than 1.8 were omitted from further analyses (33 samples of 285). The cDNA templates were prepared using an Applied Biosystems cDNA Synthesis Kit (4368813) according to the manufacturer’s protocol. Quantitative real-time PCR was performed using the ABI Viia7 real-time PCR system. The PCR products of interest were detected using TaqMan® Gene Expression assays from (Applied Biosystems, Carlsbad, CA) (Table 1). All samples were normalized to beta-2 microglobulin (B2M). There were no statistically significant differences in the expression of the endogenous control gene between treatment groups. Target and endogenous control genes were measured in triplicate for each cDNA sample during each real-time run to avoid intra-sample variance. All genes of interest were analyzed with Viia7 Applied Biosystems software using the comparative cycle thresholds (delta delta CT) method.
Table 1.
Taqman primers used for qPCR reactions.
| Abbreviation | Gene Name | RefSeq | Assay ID |
|---|---|---|---|
| Fos | FBJ osteosarcoma oncogene | NM_010234.2 | Mm00487425_m1 |
| Egr1 | early growth response 1 | NM_007913.5 | Mm00656724_m1 |
| Arc | activity regulated cytoskeletal-associated protein | NM_001276684 | Mm01204954_g1 |
| B2m | Beta-2-microglobulin | NM_009735.3 | Mm00437762_m1 |
Statistical analysis
The frequencies of home cage maternal care observations (licking, crouching, total pup contact) were analyzed by mixed two-way ANOVAs (Treatment × Test Day), with repeated measures on the second factor. Survival analyses were used to analyze all pup retrieval latency data (Jahn-Eimermacher et al., 2011). These methods take into account that some subjects did not retrieve pups during the 15-minute test and censor those data. A Cox Proportional Hazard Model was used to analyze the latency to initiate and complete pup retrieval in the home cage using experience (test day), HDACi treatment and reproductive status as covariates. Cumulative incidence plots depict the effects of each covariate on behavior. Latency to retrieve pups in the T-maze was measured in a single test with the hypothesis that group differences would emerge during this task; therefore we used the Mantel-Cox log-rank test to statistically compare survival curves. These data are plotted using Kaplan–Meier survival curves, in which the fraction of mice that have retrieved pups at each time point is calculated using the product limit (Kaplan-Meier) method. In addition, hazard ratio and confidence intervals are reported for each pairwise comparison. The hazard ratio, which is calculated from all the data in the survival curve, indicates the rate at which one group retrieves pups compared to the other.
Other latency data in which all of the subjects completed the task in the duration of the test were analyzed using two way ANOVAs with (Group × Odorant) or (Reproductive Status × HDACi treatment) as factors. The olfactory discrimination test involves sequential testing and therefore a mixed two way ANOVA was used (Group × Trial) with repeated measures on the second factor.
Relative gene expression data were analyzed using two way ANOVAs (Group × Context). Significant interaction effects between group and context were followed by post-hoc analyses between virgin mice and all other groups within each context. Since we planned to compare gene expression between groups within each context, we conducted this post-hoc analysis in some cases where a significant interaction was not detected. For instances where the same data were used for more than one comparison, we adjusted our alpha levels using the Bonferroni method based on the number of comparisons. All statistical tests were two tailed. The Cox Proportional Hazard Model was analyzed using XLSTAT (Addinsoft, NY). All other data were analyzed using GraphPad Prism 7 software (GraphPad, Inc., La Jolla, CA).
Design for Experiment 1
Mice were randomly assigned to the following groups: Postpartum (n=8), Postpartum + HDACi (n=8), Virgin (n=11), and Virgin + HDACi (n=10). Virgin female mice were cohabitated with a male of the same strain for 2 weeks to induce pregnancy. All females were single-housed thereafter. For all HDACi treated females, sodium butyrate treatment began at least 1 day before testing (Stolzenberg et al., 2012, 2014). All virgin mice were tested for maternal care responses with 3 pups obtained from a lactating donor female. Due to random distribution of the pups, it is unlikely that females were exposed to the same pups more than once. For all postpartum mice, behavioral testing began on the day of birth (postnatal day 0). Whereas pup exposure was limited to the duration of behavioral testing (2 hours for 2 consecutive days, followed by a 15 minute pup retrieval test on day 3) for all virgin mice, all postpartum mice remained with their own offspring for a full 72 hours. Thus, virgin and postpartum mice were not matched in terms of pup exposure. This experimental design allowed for a direct comparison of the artificial induction of maternal behavior in virgin mice with the natural onset and maintenance of caregiving behaviors in postpartum female mice. All postpartum mice, with the exception of 1 subject, gave birth to at least 3 healthy pups that could be used as stimuli for experimental testing. In the one instance where 3 viable biological offspring could not be used, foster pups of the same age were used for the first test and remained in the cage for the duration of the study. Some postpartum mice gave birth to more than 3 pups. In these cases, litters were culled to 3 pups on postnatal day 0.
Design for Experiment 2
The results of Experiment 1 indicate that differences in home cage maternal behavior between virgin and postpartum mice are eliminated by day 2 of testing. Interestingly, whereas these 4 groups behave similarly in the home cage environment, differences in pup retrieval are present in a novel context. One possibility is that although their behavior remains the same, the sensory cues from pups are perceived differently in these groups, with perhaps increased salience in all groups but the virgin mice. Given the importance of olfaction, we tested whether there were group differences in preference for pup odors over male odors in a separate cohort of mice. This experimental design allowed us to investigate the extent to which virgin female mice might prefer pup odors at the time when they would have been tested for pup retrieval on the T-maze (24- hours after the last pup experience), without the confound of additional exposure to pup odors during the T-maze test. Mice were randomly assigned to the following groups: Virgin (n= 8), Virgin + HDACi (n= 10), Postpartum (n= 9), Postpartum + HDACi (n=7). Virgin mice received a 2-hour exposure to pups for 2 consecutive days. All postpartum mice remained with their pups from postnatal days 0-2. Pups were culled at the end of the 2-hour exposure session on day 2 and all subjects were placed in clean cages. On test day 3, all mice were tested in the olfactory preference test and 24-hours later on test day 4, all mice were tested for olfactory discrimination in the habituation-dishabituation test.
Design for Experiment 3
The results of Experiments 1-2 informed the design of Experiment 3. The purpose of experiment 3 was to uncover how exposure to pups in two distinct contexts impacts immediate early gene expression in neural regions that respond to infant stimuli. Typically, experiments that have examined differences in neural activity measured by IEG expression based on maternal behavior have focused on using subjects that show different levels of maternal responding based on behavioral state: maternal or non-maternal. Here we have the unique opportunity to investigate animals that have the same behavioral state: maternal, but whose level of maternal responding varies as a result of context. Mice were assigned to 3 groups and tested in either the home cage (Virgin, n= 9; Virgin + HDACi, n= 9; Postpartum, n= 10) or in the T-maze context (Virgin, n= 12; Virgin + HDACi, n= 9; Postpartum, n= 9). In this experiment, postpartum females were roughly matched with virgins in terms of pup exposure. Pups were removed the morning of postnatal day 0 after a brief pup retrieval test. All mice received 2 hours of pup exposure for 2 consecutive days, and on test day 3 mice were presented with 3 stimulus pups in either the home cage or the novel T-maze. Precisely 30 minutes following the presentation of pups, all mice were euthanized.
Design for Experiment 4
The results of Experiment 3 show that context exclusively impacts IEG expression within the meA and PAG. One possibility is that IEG expression in these regions represents an aversion to the novel T-maze rather than an aversion to pups in this context. Experiment 4 was conducted to examine the effects of the novel T-maze context alone on IEG expression in the meA and PAG regions of pup-experienced virgin mice treated with or without an HDACi. Virgin mice were assigned to 4 groups and exposed to either the home cage (control = 5, HDACi = 5) or T-maze context (control = 5, HDACi = 4) in the absence of pup stimuli 24 hours following the last pup exposure (2h/2days). In the home cage context, all mice were exposed to a “mock” stimulus, which consisted of opening the cage lid and rustling the bedding in the cage corners so as to simulate the placement of pups into the cage. All mice were euthanized precisely 30 minutes following T-maze context exposure or mock stimulus exposure in the home cage.
Results
Experiment 1: Effects of HDAC inhibition on maternal behavior in the home cage and novel T-maze
Latency to initiate pup retrieval in the home cage was significantly affected by experience (X2 (3) = 25.95, p< 0.0001) and reproductive status covariates (X2 (3) = 23.59, p< 0.0001) (Fig. 1B-C). However, HDACi treatment had no effect on this behavioral measure (X2 (3) = 2.07, p= 0.15). Thus, postpartum mice were significantly faster to retrieve pups compared with virgin mice, regardless of HDACi treatment. All mice were faster to retrieve pups on test day 2 compared with test day 1. Similarly, experience (X2 (3) = 19.80, p< 0.0001) and reproductive status (X2 (3) = 10.06, p= 0.002) had significant effects on latency to retrieve all pups (Fig. 1D-E).
Figure 1. Caregiving behaviors in the home cage vary by group and day.
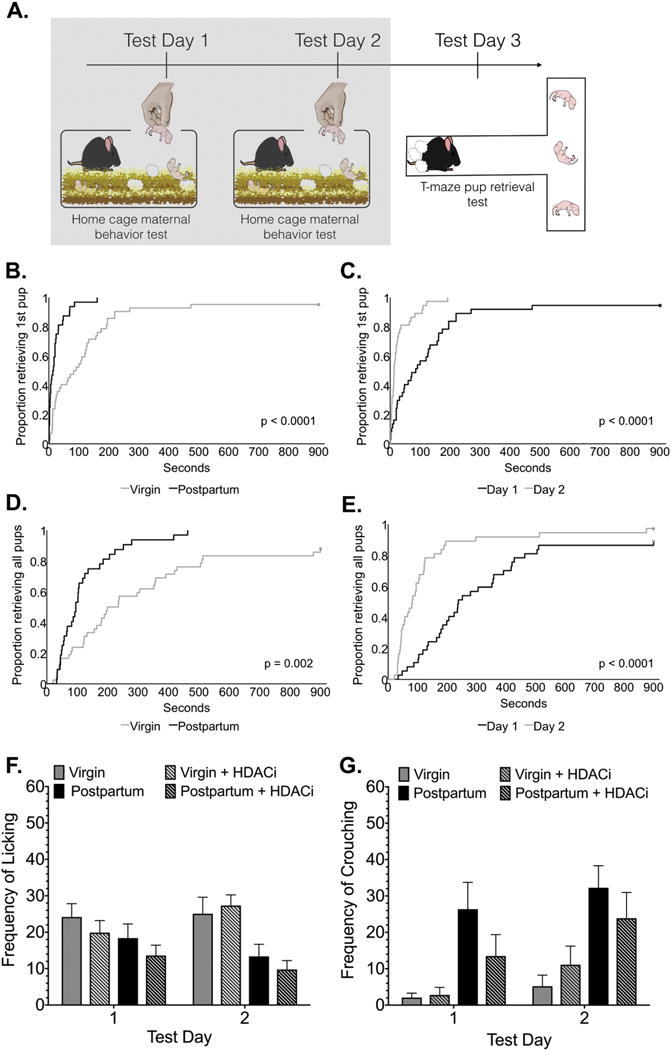
(A) Timeline for Experiment 1, the shaded box indicates the data that are shown in this figure. All mice were tested for caregiving responses in the home cage on Test Days 1-2. (B-E) Cumulative incidence plots depict the significant effects of reproductive status and experience covariates on pup retrieval latencies. Plots show the proportion of animals completing the retrieval tasks (retrieving first or last pup) at each time point on the X-axis in the home cage. (F-G) Frequency observations (Mean ± SE) of maternal care in the home cage are illustrated during a 15-minute test (60 total observations). Although the total frequency of observations with pups did not vary between groups, there was a main effect of group on the frequency of pup licking and main effects of both group and day on crouching behavior.
Whereas no significant differences between groups in total contact with pups were observed, there were significant differences in how postpartum and virgin mice spent time with pups during behavioral testing (Fig. 1F-G). A two-way mixed ANOVA (group × day) with a repeated measure on the second factor revealed significant main effects of group on licking (F(3,33) = 4.07, p= 0.014, η2=0.2), whereas there were significant main effects of group (F(3,33) = 8.98, p=0.0002, η2= 0.33) and test day (F(3,33) = 6.13, p= 0.02, η2= 0.04) on crouching behavior.
In the novel T-maze, all mice emerged from the goal box with similar latencies, however postpartum mice were faster to sniff the first pup (main effect of reproductive status F(1,33) = 5.69, p= 0.02, η2= 0.13) (Fig. 2B-C). Both reproductive status (F(1,33) = 9.29, p= 0.004, η2= 0.17) and HDACi treatment (F(1,33) = 4.35, p= 0.047, η2= 0.08) had an impact on the number of pups retrieved to the goal box in the novel T-maze. These factors significantly interacted as well (F(1,33) = 6.18, p= 0.0181, η2= 0.12) (Fig. 2D). Post-hoc analyses revealed that virgin mice retrieved significantly fewer pups than postpartum mice (adjusted p=0.0016, d= 1.68), postpartum mice treated with HDACi (adjusted p= 0.0036, d=1.52) and HDACi treated virgin mice (adjusted p= 0.006, d= 1.23). HDACi treatment did not affect the retrieval behavior of postpartum mice. Virgin mice treated with the HDACi were not significantly different from postpartum mice.
Figure 2. Maternal responses in the novel T-maze.
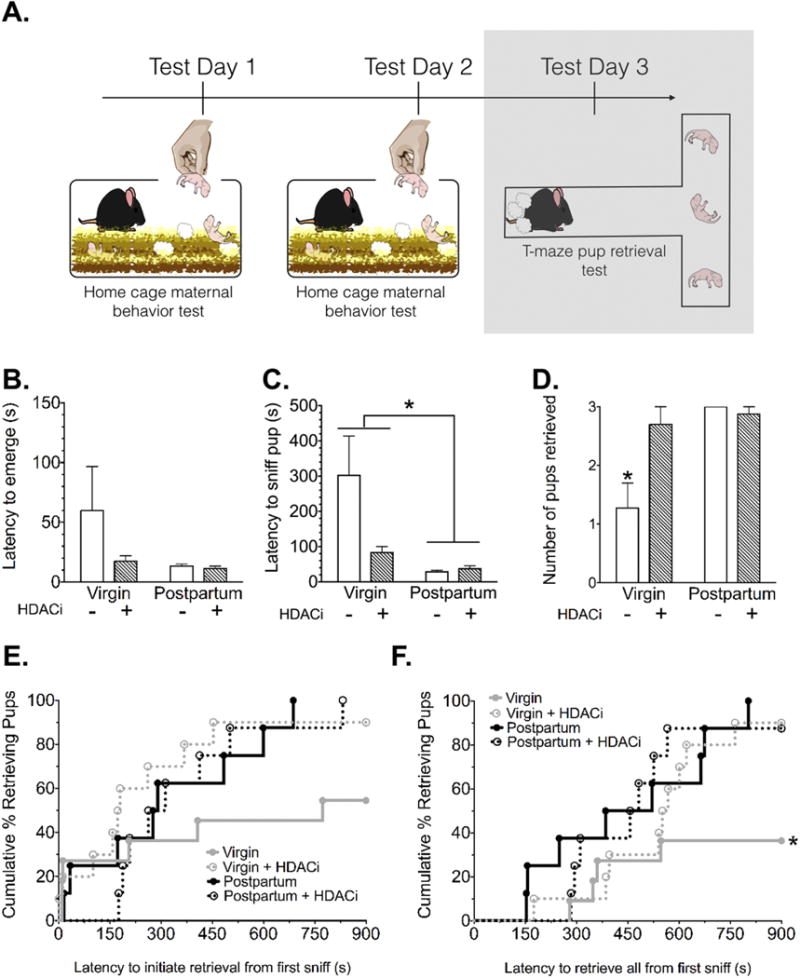
(A) Timeline for Experiment 1, the shaded box indicates the data that are shown in this figure. (B) There were no significant differences between groups in latency to emerge from the goal box and enter the maze after removal of the goal box door at the start of the test. (C) Virgin mice were significantly slower to sniff the first pup during the 15-min retrieval test (*Significant main effect of reproductive status, p= 0.02). (D) Virgin control mice retrieved significantly fewer pups by the end of the retrieval test (*Significantly different from all other groups, Bonferroni adjusted p<0.007). (E) Kaplan-Meier survival curves depict the percentage of mice retrieving the first and (F) last pup during each time point along the X-axis (*Significantly different from the postpartum group, Bonferroni adjusted p=0.03).
Based on the significant difference in latency to sniff the first pup, retrieval latencies in the T-maze were calculated from the start of pup interaction (first nose-to-pup contact). Survival curves for latency to initiate pup retrieval in the T-maze were not significantly different (Fig. 2E). However, latencies to complete pup retrieval differed significantly between groups (X2 (3) = 8.80, p= 0.03) (Fig. 2F), with virgin mice significantly slower to retrieve compared with postpartum mice (X2 (1) = 6.97, adjusted p= 0.03) (Table 2).
Table 2.
Hazard ratios, calculated from all the data in the survival curves for T-maze pup retrieval behavior, indicate the rate at which one group retrieves pups compared to the other.
| Comparison | Hazard Ratio [95%CI] | Adjusted p-value |
|---|---|---|
| Virgin + HDACi vs. Virgin | 3.011 [0.9882, 9.174] | 0.21 |
| Postpartum + HDACi vs. Postpartum | 0.9162 [0.3267, 2.569] | 3.507 |
| Postpartum vs. Virgin | 5.266 [1.534, 18.07] | 0.033* |
| Postpartum vs. Virgin + HDACi | 1.330 [0.4982, 3.552] | 2.276 |
Experiment 2: Effects of HDAC inhibition on preference for pup odors
Olfactory preference tests were conducted in a separate cohort of mice to investigate whether an odor preference would have existed at the time of T-maze testing (24 hours after the second day of pup experience). There were no differences between groups in time spent sniffing male versus pup odorants, latency to sniff male versus pup odorants or number of visits to male versus pup-soiled bedding cups. All female mice, regardless of reproductive status or HDACi treatment, spent more time investigating male and pup-soiled bedding over clean bedding (effect of odor stimulus: F(2,84) = 27.55, p< 0.001, η2= 0.37) (Fig. 3B).
Figure 3. Maternal preference for olfactory cues from social stimuli.
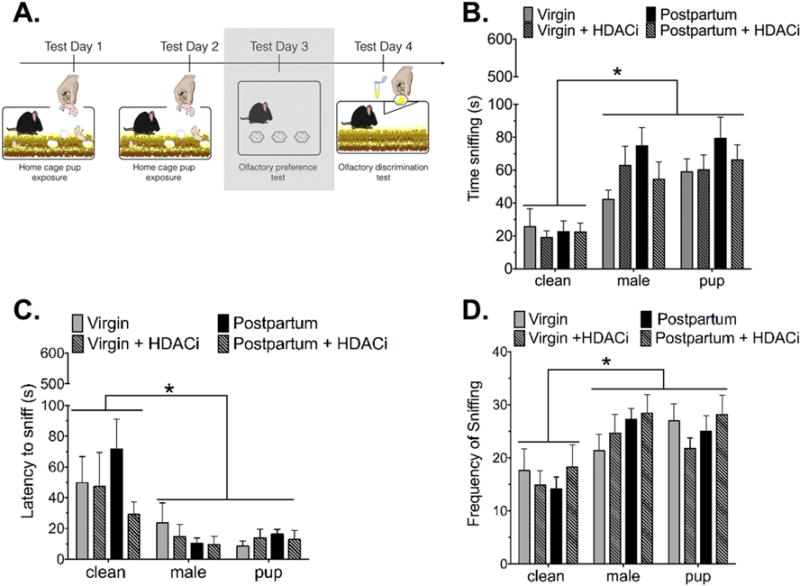
(A) Timeline for Experiment 2, the shaded box indicates the data that are shown in this figure. (B) All groups of mice spent significantly more time sniffing male-soiled or pup-soiled bedding compared to clean bedding. Within a particular odor stimulus, there were no differences between groups. (C) There were no significant differences between groups in latency to sniff a particular stimulus. All mice were faster to sniff pup or male-soiled bedding compared to clean bedding. (D) All mice visited male and pup-soiled bedding cups more frequently than clean bedding cups. Data are expressed as Mean ± SE.
*Significantly different from male or pup-soiled bedding stimulus, p<0.0001
Similarly, all female mice were faster to sniff male or pup-soiled bedding when compared with clean bedding (main effect of odor stimulus: F(2,84) = 11.67, p<0.0001, η2= 0.2) and visited the male and pup-soiled bedding cups more frequently than the clean bedding (main effect of odor stimulus: F(2,84) = 11.61 p<0.0001, η2= 0.2) (Fig. 3C-D).
To rule out the possibility that females could not distinguish between pup-soiled and male-soiled bedding we conducted an olfactory habituation-dishabituation test to determine whether mice could discriminate between male and pup odors. A two-way repeated measures ANOVA revealed a significant main effect of trial (F(8,240)= 70.38, p< 0.0001, η2= 0.62). All groups spent significantly more time sniffing the male urine (trial 3 versus 4, p< 0.001, d= 3.61) or pup urine (trial 6 versus 7, p<0.001, d= 2.08) on the first presentation (Fig. 4B).
Figure 4. Maternal discrimination of olfactory cues from social stimuli.
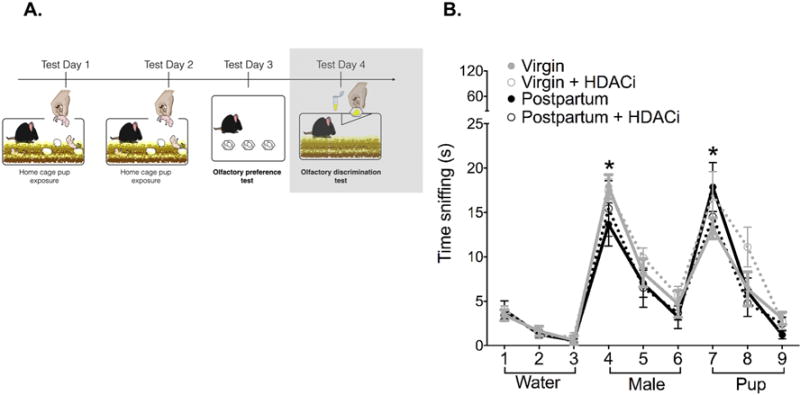
(A) Timeline for Experiment 2, the shaded box indicates the data that are shown in this figure. Mice in all groups were able to discriminate between clean, male and pup-soiled bedding. Data are expressed as Mean ± SE.
*Significantly different from the preceding trial (all groups), Bonferroni adjusted p<0.0001
Experiment 3: Effects of context on immediate early gene expression in response to pups
In the MPOA there were significant main effects of group (F2,43 = 7.45, p=0.001, η2= 0.13) and context (F1,43 = 53.90, p< 0.0001, η2= 0.47) on relative Fos mRNA expression (Fig. 5B). Post-hoc analyses revealed that virgin mice had significantly higher Fos expression than both postpartum (adjusted p=0.033, d = 1.09) and virgin + HDACi groups (adjusted p= 0.002, d = 1.85) in the T-maze. There was no significant difference between postpartum and virgin + HDACi groups. Egr1 expression in the MPOA followed a similar pattern. There were main effects of group (F2,43 = 9.40, p= 0.0004, η2= 0.21) and context (F1,43 = 20.44, p< 0.0001, η2= 0.23), as well as a significant interaction of these effects (F2,43 = 4.824, p= 0.02, η2= 0.09) on relative expression of Egr1. In the T-maze, but not home cage context, virgin mice had the highest Egr1 expression compared with both postpartum (adjusted p= 0.0003, d = 1.48) and virgin + HDACi (adjusted p< 0.0001, d = 1.7) groups. Arc expression varied by context, not group (F1,42 = 6.35, p= 0.01, η2= 0.13).
Figure 5. Immediate early gene expression in response to 30 min of pup exposure in the home cage or novel T-maze.
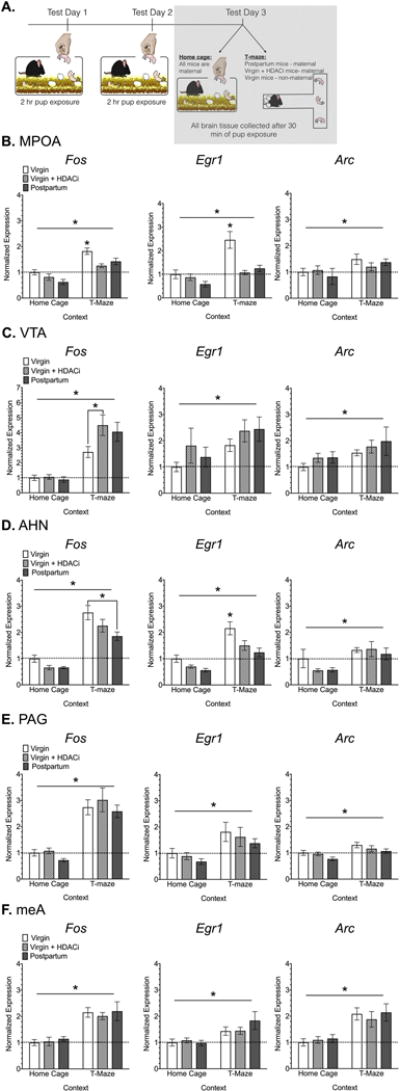
(A) Timeline for Experiment 3, the shaded box indicates the data that are shown in this figure. Normalized Fos, Egr1 and Arc mRNA expression in regions of the maternal (B-C) and defensive (D-F) neural pathways. Data are expressed as Mean ± SE. Abbreviations: AHN- anterior hypothalamic nucleus, MeA- medial amygdala, MPOA- medial preoptic area of the hypothalamus, PAG- periaqueductal grey, VTA- ventral tegmental area. Horizontal bars indicate a significant main effect of context. *Significantly different from all other groups in a particular context, unless otherwise noted with bars, Bonferroni adjusted p<0.05.
Overall, IEG expression in the VTA was primarily influenced by context. There was a significant main effect of experimental context (F1,47 = 56.93, p< 0.0001, η2= 0.5) on Fos expression. A planned comparison revealed that untreated virgin mice had significantly lower Fos expression in the T-maze when compared with virgin mice that received HDACi treatment (adjusted p= 0.0134, d= 1.14) (Fig. 5C). There were significant effects of context, but not group for both Egr1 (F1,47 = 6.31, p= 0.01, η2= 0.1) and Arc expression F1,45 = 5.78, p= 0.02, η2= 0.1).
In the AHN, Fos expression varied by group (F2,49 = 5.55, p= 0.006, η2= 0.07) and context (F1,49 = 90.24, p< 0.0001, η2= 0.59) (Fig. 5D). In the T-maze, virgin mice had significantly higher expression than postpartum mice (adjusted p= 0.0032, d= 1.23). Similar effects were seen for Egr1 expression (main effect of group F2,46 = 8.77, p= 0.006, η2= 0.16) and context (F1,46 = 40.70, p< 0.001, η2= 0.38). Virgin mice had significantly higher Egr1 expression than both postpartum (p= 0.006, d= 1.47) and virgin + HDACi mice (p= 0.0227, d= 1.02) in the T-maze context. Arc expression varied only by context in this region (F1,49 = 11.77, p= 0.001, η2= 0.18).
Context also played a significant role in relative Fos expression in the PAG (F1,49 = 86.91, p< 0.0001, η2= 0.63), with expression significantly higher in the T-maze versus home cage context (Fig. 5E). Although less robust, there was also a significant main effect of context on Egr1 (F1,49 = 12.27, p= 0.001, η2= 0.19). Arc expression varied by group (F2,49 = 3.34, p= 0.04, η2 = 0.09) and context (F1,49 = 14.97, p= 0.0003, η2= 0.21). Virgin mice showed significantly higher Arc expression in the PAG when compared to postpartum mice (adjusted p= 0.02, d = 0.81). Finally, within the meA, expression of all 3 IEGs varied by context, but not by group (Fos: F1,34 = 46.68, p< 0.0001, η2= 0.57; Egr1: F1,34 = 14.53, p= 0.0006, η2= 0.23; Arc: F1,34 = 25.70, p< 0.0001, η2= 0.42) (Fig. 5F).
Experiment 4: Effects of on immediate early gene expression in the meA and PAG in the absence of pups
IEG expression in the PAG did not vary significantly by context or by group when female mice were tested in the absence of pup stimuli (Fig. 6B). IEG expression in the meA, however, was significantly affected by the T-maze context, even in the absence of pups. Relative Fos expression (F1,15 = 222.5, p< 0.0001, η2= 0.93), Egr1 expression (F1,15 = 51.55, p< 0.0001, η2= 0.73) and Arc expression (F1,15 = 16.78, p= 0.001, η2= 0.48), were all significantly higher in the T-maze versus home cage context (Fig. 6C). There were no significant effects of group on IEG in the meA.
Figure 6. Immediate early gene expression in the home cage or novel T-maze in the absence of pups.
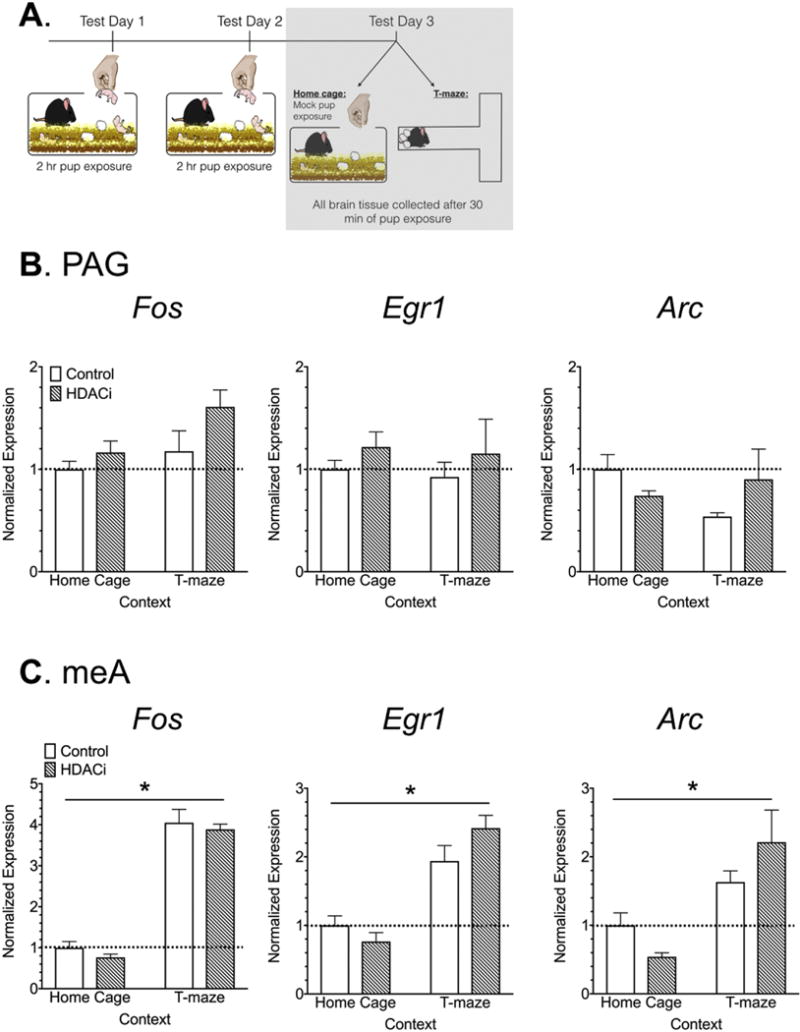
(A) Timeline for Experiment 4, the shaded box indicates the data that are shown in this figure. (B-C) Normalized Fos, Egr1 and Arc mRNA expression in the PAG and meA. Data are expressed as Mean ± SE.
Discussion
Four important conclusions emerge from the results of the present study. First, HDAC inhibitor treatment potentiates the impact of maternal experience on subsequent maternal behavior in virgin mice. Within 72 hours of HDACi treatment behavioral differences in maternal responding between virgin and postpartum mice were eliminated. Second, the effects of HDACi treatment are not mediated by an increased interest or attraction toward pup odors. Further, attraction toward pup odors appears to be unrelated to maternal performance on the T-maze because there were no significant differences in preference for pup over male-soiled bedding between virgin and postpartum mice. Third, activation of the maternal neural circuit differs based on the context in which female mice display pup retrieval behavior and both maternal and defensive neural circuits seem to be activated to a greater extent in the T-maze. Fourth, whereas HDACi treatment is linked to higher IEG expression in the VTA, which lies downstream from the MPOA in the maternal neural circuit, and lower IEG expression in the AHN, which lies downstream from the MPOA as part of the defensive circuit, IEG expression within the MPOA was highest in virgin mice. This result suggests a complex role for the MPOA in the regulation of these two systems.
The fact that HDACi treatment potentiated the effects of sub-threshold maternal experience (2h/2days) on maternal responsiveness in the novel T-maze replicates our previous findings (Stolzenberg et al., 2012, 2014). The present study extends this work to indicate that differences in pup retrieval between virgin and naturally parturient female mice are abolished by HDACi treatment within 48 hours of the first pup exposure. Note that we have previously reported that HDACi treatment does not affect circulating levels of estradiol and progesterone in virgin mice, thus gonadal hormones are not likely involved in stimulating this postpartum-like level of maternal responding in virgins (Stolzenberg et al., 2012). Consistent with our previous reports, postpartum and virgin mice tend to spend similar amounts of time with pups, but sometimes engage in different behaviors. For example, postpartum (lactating) mice spend more time crouching over pups, whereas non-lactating virgins tend to lick the pups more frequently.
Given that olfactory information is critical for the onset of maternal care in mice and the olfactory bulbs are sites of pup-experience induced plasticity during maternal learning in mice, we hypothesized that HDACi treatment might affect maternal behavior by increasing responsiveness toward pup odors (Canavan et al., 2011; Gandelman et al., 1971; Sato et al., 2010). In contrast with our prediction, we found that although all females can readily distinguish between pup and male odors, there were no preferences in any group for one odorant over the other. Instead all mice preferred either male or pup-soiled bedding to clean bedding. One possibility is that perhaps pup odor alone was not a strong enough cue to promote a behavioral preference (Okabe et al., 2013). Another possibility is that olfactory preferences require more experience to be established, even for postpartum females. For example, Agrati and colleagues report that only multiparous female rats show an olfactory preference for pup over male odors (Agrati et al., 2008).
The present series of experiments examine caregiving behaviors in both a familiar (home cage) and a novel (T-maze) environment. In contrast to the home cage environment, caring for pups in the novel T-maze context is a challenge for neophobic mice. Therefore a critical question is whether differences in basal anxiety impact behavioral responsiveness in the T-maze. Although we found no significant differences in latency to enter the maze between groups, postpartum mice were faster to make an initial contact with a pup in the horizontal arm. This finding may be associated with a reduction in anxiety that is widely reported in postpartum females (Lonstein, 2007). Note that whereas HDACi treatment eliminated the difference in number of pups retrieved on the T-maze between virgin and postpartum mice, it did not impact the latency to initiate pup contact. This finding is consistent with our previous work, which indicated that HDACi treatment had no affect on anxiety-like behavior in either the novel T-maze or elevated plus maze environments (Stolzenberg et al., 2012).
Whereas the experience of interacting with pups seems to allow pups to activate maternal responses in the presence or absence of pregnancy hormone stimulation, the extent to which pups activate the same underlying neural mechanisms in these two conditions is presently unclear. It should be noted that Rosenblatt documented a slow, gradual transition from a non-maternal to a maternal state as a result of continuous and repeated pup exposure in sensitized rats, whereas parturient rats immediately transition to the maternal state upon the first pup exposure (Rosenblatt, 1967). Similarly, although virgin mice are relatively responsive to pups on the first exposure, the present work again suggests that repeated exposure is necessary to produce caregiving behaviors on par with a newly parturient female (Stolzenberg and Rissman, 2011). For example, on the day of birth parturient mice display caregiving behaviors consistently, even if the environment is novel and potentially threatening (unpublished findings), whereas naïve virgin mice are unresponsive even if they have received HDACi (Orpen and Fleming, 1987) (although whether a higher dose of HDACi could have produced an immediate onset has not been tested). Importantly, the dependence of HDACi effects on at least 2 hours of maternal experience may be related to its mechanism of action within the brain. Although sodium butyrate is capable of affecting non-HDAC substrates, its widespread inhibitory effects on class I and class IIa HDAC enzymes (1,2,3,4,5,7,8,9) have been well established (Morris and Monteggia, 2013; Xu et al., 2007). HDACs are typically described as having inhibitory affects on gene transcription, thus administration of sodium butyrate and other HDACi drugs are associated with increased gene expression (Eberharter and Becker, 2002; Peserico and Simone, 2010; Shahbazian and Grunstein, 2007; Vettese‐Dadey et al., 1996; Wang et al., 2009; Xu et al., 2007). Despite the theoretically global effects of such drug administration, HDACi treatment tends to affect small subsets of genes (Van Lint et al., 1996; Wang et al., 2009). Perhaps surprisingly, HDAC enzymes 1-3 are enriched in promoter regions of actively transcribed genes and positively correlated with active gene transcription (mRNA transcripts) and increased acetylation rather than gene repression or silenced chromatin (Wang et al., 2009). Therefore, HDACs may play a role in resetting chromatin to a quiescent state after gene activation, rather than silencing gene transcription. These data suggest that the effects of HDACi treatment may depend on which genes are poised for activation given the signaling context of a particular cell at a particular time. With respect to the potential mechanisms through which HDACi treatment amplified the experience-driven transition to the maternal state in the present study, perhaps it prevented the re-setting of chromatin that occurs following pup removal at the end of each 2-hour exposure, allowing expression programs induced by pup exposure to remain active for a longer period of time.
In terms of hormonal mechanisms of action, the hormonal changes associated with late pregnancy are thought to prime maternal neural circuits for immediate responses toward pups (Numan, 2006; Numan and Sheehan, 1997). For example, administration of estradiol to rats that are experiencing progesterone withdrawal induces Fos protein expression in the maternal neural circuit even in the absence of pup exposure (Sheehan and Numan, 2002). Ligand-bound estrogen receptors function as transcription factors capable of turning on novel, estrogen-regulated genes as well as poising them for future activation (Duong et al., 2006). Thus, transcriptional alterations within the maternal neural circuit may already be turned on in advance of pup exposure (Sheehan and Numan, 2002). Results of the current study show that HDACi treatment did not facilitate maternal care in postpartum mice, which could be due to this prior activation of gene transcription programs. It is also possible that the behavioral tests used in the present study were not sensitive enough to detect any behavioral differences due to a ceiling effect.
We predicted that significant differences in IEG expression would emerge in the T-maze, but not in the home cage. This prediction was based on the behavioral findings from Experiment 1, which indicated that group differences in pup retrieval had significantly diminished by test day 2, but were robust in the novel T-maze on test day 3. Overall, the gene expression data support this prediction. First, for mice exposed to pups in the home cage, there were no significant differences between groups in IEG expression in any brain region examined. Second, for all brain regions, expression of all 3 IEGs was significantly affected by context, with higher expression occurring in the novel T-maze. Third, differences between groups with respect to IEG expression within the T-maze context were only identified for Fos and Egr1 in the MPOA and AHN. We also identified a significant increase in Fos expression as a result of HDACi treatment within the VTA in virgin female mice. Given that context almost exclusively impacted IEG expression in the meA and PAG, we examined whether exposure to the novel T-maze environment alone, regardless of pup exposure, was sufficient to induce expression in these regions. The results of Experiment 4 suggest that the novel T-maze context alone is not sufficient to induce IEG expression in the PAG. Thus, pup stimuli seem necessary to drive the context-dependent changes in IEG expression illustrated in Figure 5E. In contrast, novel context alone was able to drive IEG expression in the meA.
Given the well-established role of MPOA projections to the VTA in the promotion of proactive pup retrieval responses, we predicted that virgin mice, who tend to retrieve fewer pups (if any) on the T-maze, would have significantly less IEG expression in both the MPOA and VTA. Conversely, regions of the brain that are associated with the defensive circuit regulating non-maternal responses should show the highest IEG activation in virgin mice, which do not respond to pups in the T-maze. Our data partially support this prediction. For example, virgin female mice have a significantly lower Fos response to pups in the T-maze compared with HDACi treated females in the VTA, and a significantly greater Fos and Egr1 response to pups in the T-maze compared with both HDACi treated and postpartum mice in the AHN. However, both Fos and Egr1 expression is significantly higher in the MPOA of virgin mice compared to other groups. Although the latter finding is quite surprising, it is consistent with some reports in the literature. For example, several studies report that MPOA significantly responds to pup exposure as measured by IEG expression, regardless of whether or not caregiving behaviors occur (Calamandrei and Keverne, 1994; Geissler et al., 2013; Kuroda et al., 2007; Sheehan et al., 2000; Tachikawa et al., 2013). In contrast inhibitory regions like AHN are only elevated in non-maternal animals (Sheehan et al., 2000; Tachikawa et al., 2013). Thus, the increased Fos response seen here may be indicative of an increased sensitivity of the MPOA to pup-related sensory cues in virgin mice more generally. A related hypothesis is that Fos expression was highest in virgin mice because these mice are presumably still learning, whereas maternal experiences have already been consolidated in the other groups. In support of this idea note that Fos expression is higher during training compared with acquisition and its expression is actually attenuated during repeated learning experiences (Anokhin and Rose, 1991; Bertaina-Anglade et al., 2000). One critical caveat is that these data lack cellular resolution. Therefore a missing piece of the puzzle is whether overlapping or distinct cell populations are activated by pup cues between groups in these contexts. Future work will need to resolve this issue.
Highlights.
Histone deacetylase inhibitor treatment amplified the effects of maternal experience, inducing caregiving behaviors in virgin mice that are indistinguishable from postpartum mice.
Experienced female mice do not prefer the smell of pup odors to male odors, regardless of whether experience occurred following pregnancy hormone and/or histone deacetylase inhibitor exposure.
Immediate early gene expression in response to pup stimuli varies throughout maternal and fear/defensive neural pathways based on the context in which pup exposure occurs.
Histone deacetylase inhibitor treatment induced a similar pattern of Fos gene expression in the MPOA, AHN and VTA in response to pup stimuli in pup-experienced virgin mice compared to un-treated postpartum mice.
Acknowledgments
The authors thank Marc Crepeau for his outstanding technical assistance.
Funding: This work was supported by the National Institute of Child Health and Human Development [1R01HD087709-01A1] and the University of California, Davis Provost’s Fellowship for first year graduate students.
Abbreviations
- MeA
medial amygdala
- PAG
periaqueductal grey. Horizontal bars indicate a significant main effect of context
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflicts of Interest: The authors declare no conflicts of interest.
References
- Agrati D, Fernández-Guasti A, Ferreira A. The reproductive stage and experience of sexually receptive mothers alter their preference for pups or males. Behavioral neuroscience. 2008;122:998. doi: 10.1037/a0012585. [DOI] [PubMed] [Google Scholar]
- Akther S, Fakhrul AA, Higashida H. Effects of electrical lesions of the medial preoptic area and the ventral pallidum on mate-dependent paternal behavior in mice. Neuroscience letters. 2014;570:21–25. doi: 10.1016/j.neulet.2014.03.078. [DOI] [PubMed] [Google Scholar]
- Anokhin KV, Rose SP. Learning-induced Increase of Immediate Early Gene Messenger RNA in the Chick Forebrain. European Journal of Neuroscience. 1991;3:162–167. doi: 10.1111/j.1460-9568.1991.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Ayers LW, Asok A, Blaze J, Roth TL, Rosen JB. Changes in dam and pup behavior following repeated postnatal exposure to a predator odor (TMT): A preliminary investigation in long-evans rats. Developmental psychobiology. 2016;58:176–184. doi: 10.1002/dev.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaina-Anglade V, Tramu G, Destrade C. Differential learning-stage dependent patterns of c-Fos protein expression in brain regions during the acquisition and memory consolidation of an operant task in mice. European Journal of Neuroscience. 2000;12:3803–3812. doi: 10.1046/j.1460-9568.2000.00258.x. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A. The Arc of synaptic memory. Experimental brain research. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS. Long-term effects of pregnancy and parturition upon maternal responsiveness in the rat. Physiology & Behavior. 1975;14:245–249. doi: 10.1016/0031-9384(75)90028-1. [DOI] [PubMed] [Google Scholar]
- Calamandrei G, Keverne EB. Differential expression of Fos protein in the brain of female mice dependent on pup sensory cues and maternal experience. Behav Neurosci. 1994;108:113–120. doi: 10.1037//0735-7044.108.1.113. [DOI] [PubMed] [Google Scholar]
- Canavan S, Mayes L, Treloar H. Changes in Maternal Gene Expression in Olfactory Circuits in the Immediate Postpartum Period. Frontiers in Psychiatry. 2011;2 doi: 10.3389/fpsyt.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong V, Licznar A, Margueron R, Boulle N, Busson M, Lacroix M, Katzenellenbogen BS, Cavailles V, Lazennec G. ERalpha and ERbeta expression and transcriptional activity are differentially regulated by HDAC inhibitors. Oncogene. 2006;25:1799–1806. doi: 10.1038/sj.onc.1209102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberharter A, Becker PB. Histone acetylation: a switch between repressive and permissive chromatin. EMBO reports. 2002;3:224–229. doi: 10.1093/embo-reports/kvf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Cheung U, Myhal N, Kessler Z. Effects of maternal hormones on ‘timidity’and attraction to pup-related odors in female rats. Physiology & behavior. 1989;46:449–453. doi: 10.1016/0031-9384(89)90019-x. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Luebke C. Timidity prevents the virgin female rat from being a good mother: emotionality differences between nulliparous and parturient females. Physiol Behav. 1981;27:863–868. doi: 10.1016/0031-9384(81)90054-8. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Rosenblatt JS. Maternal behavior in the virgin and lactating rat. Journal of comparative and physiological psychology. 1974;86:957. doi: 10.1037/h0036414. [DOI] [PubMed] [Google Scholar]
- Gagnidze K, Weil ZM, Faustino LC, Schaafsma SM, Pfaff DW. Early histone modifications in the ventromedial hypothalamus and preoptic area following oestradiol administration. Journal of neuroendocrinology. 2013;25:939–955. doi: 10.1111/jne.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandelman R, Zarrow M, Denenberg VH, Myers M. Olfactory bulb removal eliminates maternal behavior in mouse. Science. 1971 doi: 10.1126/science.171.3967.210. [DOI] [PubMed] [Google Scholar]
- Geissler DB, Schmidt HS, Ehret G. Limbic brain activation for maternal acoustic perception and responding is different in mothers and virgin female mice. Journal of Physiology-Paris. 2013;107:62–71. doi: 10.1016/j.jphysparis.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genesArc, c-fos, and zif268. Journal of Neuroscience. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn-Eimermacher A, Lasarzik I, Raber J. Statistical analysis of latency outcomes in behavioral experiments. Behavioural brain research. 2011;221:271–275. doi: 10.1016/j.bbr.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Kenny SL, Wright LD, Green AD, Mashoodh R, Perrot TS. Expression of maternal behavior and activation of the bed nucleus of the stria terminalis during predatory threat exposure: Modulatory effects of transport stress. Physiology & behavior. 2014;123:148–155. doi: 10.1016/j.physbeh.2013.08.024. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Bridges RS. Morphine treatment and reproductive condition alter olfactory preferences for pup and adult male odors in female rats. Developmental psychobiology. 1990;23:331–347. doi: 10.1002/dev.420230405. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Ostrander MM, Norton CS, Watson SJ, Akil H, Robinson TE. The ability of amphetamine to evoke arc (Arg 3.1) mRNA expression in the caudate, nucleus accumbens and neocortex is modulated by environmental context. Brain research. 2002;930:30–36. doi: 10.1016/s0006-8993(01)03400-x. [DOI] [PubMed] [Google Scholar]
- Kuroda KO, Meaney MJ, Uetani N, Fortin Y, Ponton A, Kato T. ERK-FosB signaling in dorsal MPOA neurons plays a major role in the initiation of parental behavior in mice. Molecular and Cellular Neuroscience. 2007;36:121–131. doi: 10.1016/j.mcn.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Lee AW, Brown RE. Medial preoptic lesions disrupt parental behavior in both male and female California mice (Peromyscus californicus) Behavioral neuroscience. 2002;116:968. doi: 10.1037//0735-7044.116.6.968. [DOI] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proceedings of the National Academy of Sciences. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS. Regulation of anxiety during the postpartum period. Frontiers in neuroendocrinology. 2007;28:115–141. doi: 10.1016/j.yfrne.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Lévy F, Fleming AS. Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Hormones and behavior. 2015;73:156–185. doi: 10.1016/j.yhbeh.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- McLeod J, Sinal C, Perrot-Sinal T. Evidence for non-genomic transmission of ecological information via maternal behavior in female rats. Genes, Brain and Behavior. 2007;6:19–29. doi: 10.1111/j.1601-183X.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Monteggia LM. Unique functional roles for class I and class II histone deacetylases in central nervous system development and function. Int J Dev Neurosci. 2013;31:370–381. doi: 10.1016/j.ijdevneu.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev. 2006;5:163–190. doi: 10.1177/1534582306288790. [DOI] [PubMed] [Google Scholar]
- Numan M. Neurobiology of social behavior. Academic Press; 2015. [Google Scholar]
- Numan M, Numan MJ. Preoptic-brainstem connections and maternal behavior in rats. Behavioral neuroscience. 1991;105:1013. doi: 10.1037//0735-7044.105.6.1013. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, Smith CD. Medial preoptic area interactions with the nucleus accumbens–ventral pallidum circuit and maternal behavior in rats. Behavioural brain research. 2005;158:53–68. doi: 10.1016/j.bbr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Numan M, Sheehan TP. Neuroanatomical circuitry for mammalian maternal behavior. Annals of the New York Academy of Sciences. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in neuroendocrinology. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS, Dellevigne AA, Correnti CM, Numan MJ. Temporary inactivation of ventral tegmental area neurons with either muscimol or baclofen reversibly disrupts maternal behavior in rats through different underlying mechanisms. Behavioral neuroscience. 2009;123:740. doi: 10.1037/a0016204. [DOI] [PubMed] [Google Scholar]
- Numan M, Woodside B. Maternity: neural mechanisms, motivational processes, and physiological adaptations. Behavioral neuroscience. 2010;124:715. doi: 10.1037/a0021548. [DOI] [PubMed] [Google Scholar]
- Numan MI, T R. The neurobiology of parental behavior. Springer-Verlag; New York: 2003. [Google Scholar]
- Okabe S, Nagasawa M, Kihara T, Kato M, Harada T, Koshida N, Mogi K, Kikusui T. Pup odor and ultrasonic vocalizations synergistically stimulate maternal attention in mice. Behavioral neuroscience. 2013;127:432. doi: 10.1037/a0032395. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Pereira M, Agrati D, Ferreira A, Fleming AS, Gonzalez-Mariscal G, Levy F, Lucion AB, Morrell JI, Numan M, Uriarte N. Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neurosci Biobehav Rev. 2013;37:1875–1892. doi: 10.1016/j.neubiorev.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Orpen BG, Fleming AS. Experience with pups sustains maternal responding in postpartum rats. Physiol Behav. 1987;40:47–54. doi: 10.1016/0031-9384(87)90184-3. [DOI] [PubMed] [Google Scholar]
- Peserico A, Simone C. Physical and functional HAT/HDAC interplay regulates protein acetylation balance. Biomed Research International. 2010;2011 doi: 10.1155/2011/371832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. Journal of Neuroscience. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–1513. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- Sato A, Nakagawasai O, Tan-No K, Onogi H, Niijima F, Tadano T. Influence of olfactory bulbectomy on maternal behavior and dopaminergic function in nucleus accumbens in mice. Behavioural brain research. 2010;215:141. doi: 10.1016/j.bbr.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- Sharma D, Handa RJ, Uht RM. The ERbeta ligand 5alpha-androstane, 3beta,17beta-diol (3beta-diol) regulates hypothalamic oxytocin (Oxt) gene expression. Endocrinology. 2012;153:2353–2361. doi: 10.1210/en.2011-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan T, Numan M. Estrogen, progesterone, and pregnancy termination alter neural activity in brain regions that control maternal behavior in rats. Neuroendocrinology. 2002;75:12–23. doi: 10.1159/000048217. [DOI] [PubMed] [Google Scholar]
- Sheehan T, Paul M, Amaral E, Numan M, Numan M. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience. 2001;106:341–356. doi: 10.1016/s0306-4522(01)00286-x. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Cirrito J, Numan MJ, Numan M. Using c-Fos immunocytochemistry to identify forebrain regions that may inhibit maternal behavior in rats. Behavioral neuroscience. 2000;114:337. doi: 10.1037//0735-7044.114.2.337. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Champagne FA. Hormonal and non-hormonal bases of maternal behavior: the role of experience and epigenetic mechanisms. Hormones and behavior. 2016;77:204–210. doi: 10.1016/j.yhbeh.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Rissman EF. Oestrogen-independent, experience-induced maternal behaviour in female mice. J Neuroendocrinol. 2011 doi: 10.1111/j.1365-2826.2011.02112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, Stevens JS, Rissman EF. Experience-facilitated improvements in pup retrieval; evidence for an epigenetic effect. Horm Behav. 2012;62:128–135. doi: 10.1016/j.yhbeh.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, Stevens JS, Rissman EF. Histone deacetylase inhibition induces long-lasting changes in maternal behavior and gene expression in female mice. Endocrinology. 2014;155:3674–3683. doi: 10.1210/en.2013-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukikara M, Mota-Ortiz S, Baldo M, Felicio L, Canteras N. A role for the periaqueductal gray in switching adaptive behavioral responses. Journal of Neuroscience. 2006;26:2583–2589. doi: 10.1523/JNEUROSCI.4279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachikawa KS, Yoshihara Y, Kuroda KO. Behavioral transition from attack to parenting in male mice: a crucial role of the vomeronasal system. Journal of Neuroscience. 2013;33:5120–5126. doi: 10.1523/JNEUROSCI.2364-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint C, Emiliani S, Verdin E. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene expression. 1996;5:245–253. [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. Journal of Neuroscience. 2002;22:10067–10071. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettese-Dadey M, Grant P, Hebbes T, Crane-Robinson C, Allis C, Workman J. Acetylation of histone H4 plays a primary role in enhancing transcription factor binding to nucleosomal DNA in vitro. The EMBO journal. 1996;15:2508–2518. [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, Zhao K. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–1031. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Parmigiani R, Marks P. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Current protocols in neuroscience. 2009:8.24.21–28.24.12. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Liang M, Akther S, Higashida C, Tsuji T, Higashida H. c-Fos expression in the paternal mouse brain induced by communicative interaction with maternal mates. Molecular brain. 2014;7:66. doi: 10.1186/s13041-014-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Wang W, Pan YW, Lu S, Xia Z. Methods to measure olfactory behavior in mice. Current protocols in toxicology. 2015:11.18.11–11.18.21. doi: 10.1002/0471140856.tx1118s63. [DOI] [PMC free article] [PubMed] [Google Scholar]


